Environmental monitoring is commonly used in food and other end-consumer product manufacturing facilities1,2 and has gained traction as a method to determine the presence of pathogens that typically indicate fecal presence.3 In addition, some healthcare systems have instituted environmental monitoring for bacteria and viruses to improve hygiene and mitigate biosecurity risks, particularly with bacterial strains known to be resistant to antibiotics.4 The use of on-farm environmental monitoring of viral pathogens has increased in popularity with the growing pressure from diseases like porcine epidemic diarrhea virus (PEDV). Environmental swabs have been shown to be effective when detecting viruses within feed manufacturing environments5 and within swine farms. Specifically, environmental sampling has been used to monitor the eradication of PEDV in feed mills6 and swine farms.7 Feed and feed manufacturing facilities have increased scrutiny because PEDV has been shown to be transmitted through contaminated feed ingredients.8-11 Therefore, fast and reliable methods to monitor for PEDV, such as environmental sampling, provide an avenue to prevent infections.
Despite documented use within several industries, there is a lack of information regarding the applicability of environmental monitoring within swine facilities. Specifically, there is uncertainty about how the results of environmental sampling can be applied to modify biosecurity practices during an outbreak. Therefore, this case report evaluates the presence of PEDV within a farm currently experiencing a PEDV outbreak. Additionally, this case report evaluates the use of environmental sampling to make real-time biosecurity changes to prevent transmission of the virus to a susceptible animal within the infected herd or to other susceptible herds.
Case summary
Initial investigation
The opportunity to evaluate the impact of environmental monitoring arose when the Kansas State Swine Teaching and Research Center (KSTRC; Figure 1) experienced an outbreak of PEDV in Spring 2019. The facility includes sow, nursery, and finisher housing separated into different barns for each phase and maintains a 160-head batch-farrow sow herd with additional group housing for nursery, growing, and finishing pigs. On March 8, 2019, a group of weaned pigs with scours was observed. Over the course of the next two days, diarrhea was observed within the gestation barn. Fecal samples submitted to the Kansas State University Veterinary Diagnostic Laboratory (KSU VDL) confirmed the presence of PEDV at the facility.
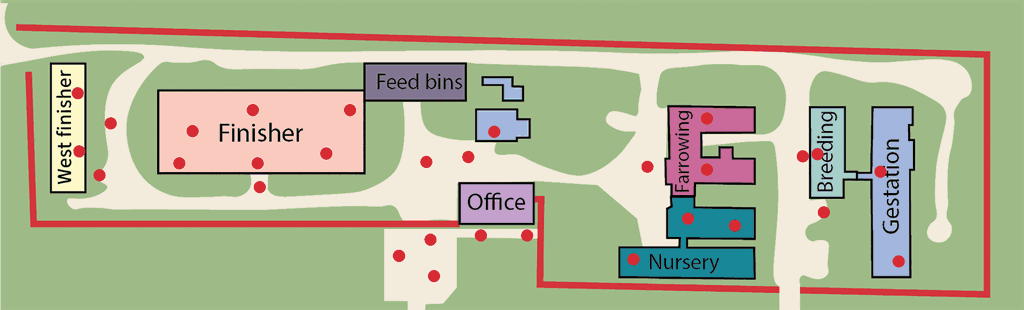
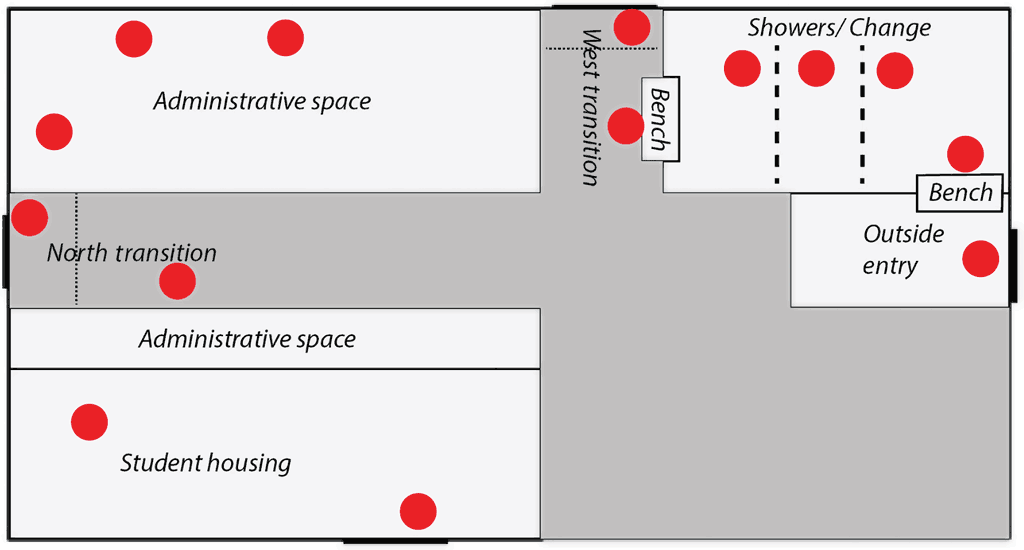
Pre-outbreak biosecurity procedures included a fenced perimeter buffer zone with limited vehicle and personnel access, off-site quarantine, porcine reproductive and respiratory syndrome testing of new gilts for 8 weeks prior to farm entry, and the requirement that delivered supplies were from pig-free areas of origin. Personnel and visitor entry were restricted with visitor policies posted and a visitor log kept. Employees and visitors were allowed to enter the farm if they had previous pig contact but were required to shower prior to entry if the contact was within the previous 24 hours. Initial entry requirements included the use of a Danish bench system to establish a clear line between the perimeters. Outside footwear was not permitted to cross the bench and all entrants were required to don provided coveralls and boots once through the shower. Showering upon entry was only required in situations where prior exposure to pigs, livestock facilities, processing plants, or laboratories handling known pathogens or diagnostic samples had occurred. The area prior to crossing the Danish bench was considered dirty and the showers and changing rooms acted as an intermediary between the dirty and transition zone, which was within the main office area (Figure 2). The office area contained 2 different access points to the outside paths leading to different barns; the only requirement for moving between barns was to wash boots and change gloves.
There was typically a greater level of foot traffic in and out of the KSTRC from students and researchers than would be found on a typical swine operation of this size. However, health had historically been good at the facility. Prior to this outbreak, there had been limited environmental monitoring done at the site.
Environmental swabbing
Environmental swabs were collected 2, 4, 6, 8, 12, and 16 weeks after initial PEDV diagnosis. Samples were collected by swabbing a surface area of approximately 20 cm × 20 cm with a 10 cm × 10 cm cotton gauze square soaked in 5 mL of phosphate-buffered saline with a 7.2 pH as described by Griener.12 Sampling locations are designated by red circles on Figures 1 and 2, and were collected from a total of 5 zones: 1) on- and off-farm vehicles, including feed delivery trucks, tractors, employee vehicles, and areas outside the farm perimeter (vehicles/outside perimeter); 2) direct pig contact surfaces including pen flooring, pen walls, feeders, and waterers (pig contact); 3) non-pig contact surfaces within one of the barn areas including employee walkways, work areas, feed storage, and in-barn transition zones (non-pig contact inside); 4) non-pig contact surfaces including walkways and work areas outside of the barn (non-pig contact outside); and 5) surfaces in the main office building including laundry areas, change rooms and shower areas, and transition zones upon entering and exiting the building (transition zones; Figure 2). Analysis of PEDV was conducted by KSU VDL using quantitative real-time polymerase chain reaction (PCR) with an upper cycle threshold (Ct) limit of 45.
Environmental swabbing results
A reduction in the number of positive samples over time was observed for multiple zones, particularly within transition areas and areas outside of barns as shown in Table 1. Two weeks following the initial diagnosis, 44% of samples obtained from vehicles/outside perimeter (worker’s vehicles, on-site student housing, and near the entry bench) tested positive for PEDV. At the same timepoint, 81% of the transition zone areas (including the shower/changing area and main office) as well as 66% of samples from non-pig contact areas inside the barns were positive for PEDV.
| Weeks after initial diagnosis | ||||||
|---|---|---|---|---|---|---|
| Zone, % positive (No. positive/Total No. samples) | 2 | 4 | 6 | 8 | 12 | 16 |
| Vehicles/outside perimeter* | 44% (4/9) | 13% (1/8) | 0% (0/1) | 25% (1/4) | 0% (0/2) | 0% (0/3) |
| Transition zones† | 81% (13/16) | 21% (3/14) | 29% (4/14) | 44% (4/9) | 0% (0/6) | 0% (0/7) |
| Non-pig contact outside‡ | 66% (4/6) | 50% (2/4) | 25% (1/4) | NS | 0% (0/4) | 0% (0/5) |
| Non-pig contact inside§ | 100% (12/12) | 80% (4/5) | 88% (8/9) | 75% (3/4) | 100% (4/4) | 80% (4/5) |
| Pig contact¶ | NS | 100% (2/2) | 100% (2/2) | 100% (4/4) | 100% (4/4) | 75% (3/4) |
* Included on- and off-farm vehicles, feed delivery trucks, tractors, employee vehicles, and areas outside the farm perimeter.
† Included laundry areas, changing rooms, shower areas, and transition zones upon entering or exiting the main office building.
‡ Outside surfaces such as walkways and work areas not in direct contact with pigs.
§ Inside surfaces such as walkways, work areas, feed storage areas, and in-barn transition zones not in direct contact with pigs.
¶ Included pen flooring, pen walls, feeders, and waterers.
PEDV = porcine epidemic diarrhea virus; PCR = polymerase chain reaction; NS = not sampled.
A reduction in the number of positive samples was observed beginning on week 4 at all locations except pig contact surfaces. There was a 29% reduction in positive results from vehicle samples, a 60% reduction in positive samples seen in transition zones, a 16% reduction in positive samples from non-pig contact areas outside of barns, and a 20% reduction in positive samples from non-pig contact areas within barns. At this timepoint, environmental monitoring of pig-contact areas was initiated, which remained 100% positive until the final collection (16 weeks). These reductions were not consistent throughout the entire data collection period, but upon the final collection at 16 weeks post infection, samples collected from vehicles/outside perimeter, within transition zones, and in non-pig contact areas outside of barns had been consistently negative for the 4 weeks prior.
Implementing biosecurity changes
As environmental swabbing results were reported, biosecurity protocols were modified to prevent the spread of the virus within the facility and to contain it within the farm. Problem areas were noted, especially locations that had multiple positive samples across different timepoints. Specific areas of concern were on-site vehicles, including those that were being used to transport or dispose of waste or carcasses, transition zones in barns and within the main office, and areas within the main office that were part of the clean area in the biosecurity plan.
Immediately after receiving the positive PEDV diagnosis, employees were required to use new coveralls when entering a new area or room, and all nonessential entry into the farm was halted. Students and faculty who would typically be visiting the facility for research or class were not allowed onto the farm. Essential employees were assigned to specific areas; either working exclusively in the finishing rooms, farrowing and nursery areas, or the breeding and gestation barns. Since a small amount of virus has the potential to infect large quantities of feed, there was concern surrounding the feed delivery protocol that was in place when the outbreak first occurred. To mitigate this risk, the driver began bringing the truck to the perimeter barrier and transferring the feed to an intermediary truck that remained within the perimeter. This was done to minimize the risk of transmission from the farm to the feed mill and other off-farm areas.
Following the week 4 testing, the main office and shower/entrance areas were disinfected multiple times per day, and clearly visible transition zones or swing benches were placed in barns where they were not already present. The entrance protocol was modified to require clean gloves and boot covers to be worn from vehicles to the entrance bench, clean scrubs to be worn past the showers, and boots were required to stay in specific barns.
The laundry area was moved from an area adjacent to the showers to a section considered to be dirty at week 6. This was done to minimize contamination of the shower area from the laundry.
Discussion
The results of this study were evaluated by separating samples into those that had PEDV RNA detected by PCR and those that did not. This was done due to variation in equipment shapes, sizes, and surface types, which influences the quantity of virus in the samples and therefore, the Ct value. Because directly comparing Ct values between zones is confounded by the surface materials, results are reported as the percentage of positive samples collected from each zone.
All pig-contact surfaces had detectable PEDV RNA through week 12, after which there was a 25% reduction in the number of positive samples observed. However, a positive PCR result indicated the presence or absence of viral RNA and did not indicate whether the sample was from infectious or inactivated, noninfectious RNA. Therefore, these data alone do not indicate the success or failure of the disinfection procedures used.
While the feed mill remained negative for the duration of the outbreak, the virus was found in areas within the farm that were not initially observed to have detectable PEDV RNA, indicating that the initial changes to the biosecurity protocols were not successful in limiting viral spread within the facility. This led to further enhancements to the protocol, including requiring gloves and boot covers to be worn by all entrants from their vehicles to the farm entrance bench, and instituting a captive boot system in which boots are used in and do not leave that specific barn. The farm was divided into 3 main areas (finishing, farrowing and nursery, and breeding and gestation) and employees working within those zones were prohibited from comingling throughout the day.
Farm entrants donned scrubs after passing through the entrance, put on clean coveralls over their scrubs prior to entering a barn, and then removed the coveralls and left them in a dirty laundry collection area prior to returning to the main office. Dirty laundry was transported in a biosecure manner to the laundry area when necessary. Boot covers were worn while walking between the main office and the barns and were changed prior to entering the main office through either transition zone.
Workers play a huge role in any facility’s biosecurity, and employee compliance is essential for a successful biosecurity plan. Demonstrating tangible metrics surrounding the cleanup effort after a disease outbreak can serve as an informational and motivational tool for employees. After each timepoint within the data collection period, results were reported back to employees which allowed for problem areas to be identified and addressed. Sharing data with the employees also aided in developing solutions to recurring issues; for example, the laundry area for the facility was originally located directly next to the showers. The suggestion of adding a laundry area within the dirty area of the facility at week 6 resulted in the elimination of positive results within the shower area at the next data collection timepoint. While some areas had continued PEDV RNA presence, there was a marked improvement in areas of high concern. Although there are no data to support this, the improvement is likely due to reduced viral shedding or improved employee practices. As the outbreak progressed, some previously negative areas became positive for PEDV RNA, which could be attributed in part to employee complacency. Without environmental data for these timepoints, there is no tangible way to measure or rectify the increase.
While this case shed important light on the use of biosecurity practices to prevent pathogen spread during an outbreak, it is important to note that several limitations exist. A more robust use of environmental monitoring would prove useful with a greater sample size, more refinement in sampling location, and a specific consideration of surface types. The learnings from this have led to the development of the K-State Feed Safety Sampling Resources at www.ksufeed.org, where there are standard operating procedures for how to prepare for sampling of viral pathogens, how to collect environmental samples, and how to calculate the necessary number of samples based on the severity of the pathogen of interest and the probability of the pathogen being introduced through feed. While this project would have benefited from these materials being available, it was the project itself that led to their development.
In closing, environmental monitoring was an important tool in managing this disease outbreak. In this circumstance, results from environmental monitoring swabs allowed for the real-time adaptation of biosecurity practices to address the greatest areas of risk. There are several considerations when selecting sampling locations and frequency, but consistent environmental monitoring during an outbreak allows for dynamic decisions to minimize disease spread.
Implications
Under the conditions of this study:
- Environmental monitoring was an important tool in managing this disease outbreak.
- Environmental monitoring identified areas in the farm with poor biosecurity.
- Biosecurity adjustments made resulted in fewer contaminated surfaces.
Acknowledgments
Conflict of interest
None reported.
Disclaimer
Scientific manuscripts published in the Journal of Swine Health and Production are peer reviewed. However, information on medications, feed, and management techniques may be specific to the research or commercial situation presented in the manuscript. It is the responsibility of the reader to use information responsibly and in accordance with the rules and regulations governing research or the practice of veterinary medicine in their country or region.
References
1. Rigarlsford JF. Microbial monitoring of cleaning and disinfection in food plants. In: Mead GC, ed. Microbiological analysis of red meat, poultry and eggs. Woodhead Publishing; 2007:148-164.
2. Archibald F. The presence of coliform bacteria in Canadian pulp and paper mill water systems – A cause for concern? Water Qual Res J. 2000;35:1-22. https://doi.org/10.2166/wqrj.2000.001
*3. Baylis C, Uyttendaele M, Joosten H, Davies A. The Enterobacteriaceae and their significance to the food industry. ILSI Europe Report Series. December 2011. Accessed April 2021. https://ilsi.eu/wp-content/uploads/sites/3/2016/06/EP-Enterobacteriaceae.pdf
4. Palmore TN, Henderson DK. Managing transmission of carbapenem-resistant Enterobacteriaceae in healthcare settings: A view from the trenches. Clin Infect Dis. 2013;57(11):1593-1599. https://doi.org/10.1093/cid/cit531
5. Muckey MB. Evaluation of the surface sanitation to prevent biological hazards in animal food manufacturing. MS Thesis. Kansas State University; 2016.
6. Huss AR, Schumacher LL, Cochrane RA, Poulsen E, Bai J, Woodworth JC, Dritz SS, Stark, CR, Jones CK. Elimination of porcine epidemic diarrhea virus in an animal feed manufacturing facility. PLoS One. 2017;12(1):e0169612. https://doi.org/10.1371/journal.pone.0169612
7. Jang G, Park J, Lee C. Successful eradication of porcine epidemic diarrhea in an enzootically infected farm: a two-year follow-up study. Pathogens. 2021;10(7):830. https://doi.org/10.3390/pathogens10070830
8. Dee S, Neill C, Singrey A, Clement T, Cochrane R, Jones C, Patterson G, Spronk G, Christopher-Hennings J, Nelson E. Modeling the transboundary risk of feed ingredients contaminated with porcine epidemic diarrhea virus. BMC Vet Res. 2016;12(1):51. https://doi.org/10.1186/s12917-016-0674
9. Schumacher LL, Woodworth JC, Jones CK, Chen Q, Zhang J, Gauger, PC, Stark CR, Main RG, Hesse RA, Tokach MD, Dritz SS. Evaluation of the minimum infectious dose of porcine epidemic diarrhea virus in virus-inoculated feed. Am J Vet Res. 2017;77(10):1108-1113. https://doi.org/10.2460/ajvr.77.10.1108
10. Pasick J, Berhane Y, Ojkic D, Maxie G, Embury‐Hyatt C, Swekla K, Handel K, Fairles J, Alexandersen S. Investigation into the role of potentially contaminated feed as a source of the first‐detected outbreaks of porcine epidemic diarrhea in Canada. Transbound Emerg Dis. 2014;61(5):397-410. https://doi.org/10.1111/tbed.12269
11. Dee S, Clement T, Schelkopf A, Nerem J, Knudsen D, Christopher-Hennings, J, Nelson E. An evaluation of contaminated complete feed as a vehicle for porcine epidemic diarrhea virus infection of naive pigs following consumption via natural feeding behavior: Proof of concept. BMC Vet Res. 2014;10:176. https://doi.org/10.1186/s12917-014-0176-9
12. Greiner LL. Evaluation of the likelihood of detection of porcine epidemic diarrhea virus or porcine delta coronavirus ribonucleic acid in areas within feed mills. J Swine Health Prod. 2016;24(4):198-204.
* Non-refereed reference.
