| Original research | Peer reviewed |
Cite as: Wiedmeyer CE, Fangman TJ, Schwartz K, et al. Fine-needle aspiration and cytology as an antemortem method for evaluating injection-site lesions. J Swine Health Prod. 2014;22(4):244–247.
Also available as a PDF.
SummaryObjectives: To apply a fine-needle aspirate (FNA) technique to evaluate grossly visible injection-site reactions by cytologic examination and determine agreement with gross and histopathological findings. Materials and methods: Two trials were conducted. In both, pigs were vaccinated with porcine circovirus type 2 vaccine at weaning and 17 days later. Seven days after the second vaccination, pigs with grossly visible injection-site lesions were selected (Trial 1, n = 40; Trial 2, n = 12). In Trial 1, pigs were manually restrained for the FNA procedure. In Trial 2, pigs were sedated and the FNA procedure was conducted using two different-sized hypodermic needles (18-gauge and 22-gauge). After the FNA procedure, pigs were euthanized and the injection-site lesions and lymph nodes dissected and submitted for histopathologic interpretation. All cytologic preparations were examined by a board-certified veterinary clinical pathologist. Results: In Trial 1, the cytologic interpretation of the samples was mild lymphocytic to mixed inflammation. Lesions were suggested to be the result of an immunologic response to the vaccine, not hemorrhage or abscess. In Trial 2, no differences were detected between preparations made with an 18-gauge or 22-gauge needle. Cytologic and histological findings agreed, reporting low to moderate numbers of lymphocytes and macrophages, with low numbers of neutrophils, foreign material, and bacteria. Implications: The FNA procedure described is a potential technique practitioners can utilize to characterize tissue-reaction lesions without the need for euthanasia or surgical biopsy. | Resumen
Objetivos: Utilizar la técnica de aspiración de aguja fina (FNA por sus siglas en inglés) para evaluar lesiones visibles por reacciones en el sitio de inyección por medio de la evaluación histológica y determinar la concordancia entre los hallazgos macro e histopatológicos. Materiales y métodos: Se realizaron dos pruebas. En ambas, los cerdos fueron vacunados con una vacuna de coronavirus porcino tipo 2 al destete y 17 días después. Siete días después de la segunda vacunación, se seleccionaron los cerdos con lesiones visibles en el sitio de inyección (Prueba 1, n = 40; Prueba 2, n = 12). En la Prueba 1, se contuvo manualmente a los cerdos para el procedimiento FNA. En la Prueba 2, se sedó a los cerdos y se realizó el procedimiento FNA utilizando dos agujas hipodérmicas de diferentes tamaños (calibre 18 y calibre 22). Después del procedimiento FNA, los cerdos fueron sacrificados y las lesiones del sitio de la inyección y los nódulos linfáticos fueron disecados y enviados para la interpretación histopatológica. Todas las preparaciones histológicas fueron examinadas por un patólogo clínico veterinario certificado. Resultados: En la Prueba 1, la interpretación histológica de las muestras fue desde inflamación linfocítica ligera hasta mixta. Se sugirió que las lesiones eran resultado de una respuesta inmunológica a la vacuna, no había hemorragia ni absceso. En la Prueba 2, no se detectaron diferencias entre las preparaciones hechas con aguja calibre 18 o calibre 22. Los hallazgos histológicos y citológicos concordaron, reportando bajos números a moderados de linfocitos y macrófagos, con números bajos de neutrófilos, material extraño, y bacterias. Implicaciones: El procedimiento FNA descrito es una potencial técnica que los médicos pueden utilizar para caracterizar las lesiones de reacción del tejido sin la necesidad de hacer una eutanasia o biopsia quirúrgica. | ResuméObjectifs: Utiliser une technique d’aspiration à l’aiguille fine (FNA) pour évaluer par examen cytologique les réactions aux sites d’injection visibles à l’œil nu et déterminer l’accord avec les trouvailles des examens macroscopiques et histopathologiques. Matériels et méthodes: Deux essais ont été réalisés. Dans les deux, des porcs furent vaccinés avec le vaccin contre le coronavirus de type 2 au sevrage et 17 jours plus tard. Sept jours après la deuxième administration, les porcs avec des lésions visibles au site d’injection ont été sélectionnés (Essai 1, n = 40; Essai 2, n = 12). Dans l’Essai 1, les porcs étaient contentionnés manuellement pour la procédure de FNA. Dans l’Essai 2, les porcs étaient mis sous sédation et la procédure de FNA effectuée en utilisant des aiguilles hypodermiques de deux tailles différentes (18-gauge et 22-gauge). Suite à la procédure de FNA, les porcs étaient euthanasiés et les lésions aux sites d’injection ainsi que les nœuds lymphatiques furent disséqués et soumis pour examen histopathologique. Toutes les préparations cytologiques furent examinées par un pathologiste clinique vétérinaire certifié. Résultats: Dans l’Essai 1, l’interprétation cytologique des échantillons était une légère inflammation lymphocytaire à mixte. Les lésions semblaient être le résultat d’une réponse immunologique au vaccin, et non une hémorragie ou un abcès. Dans l’Essai 2, aucune différence ne fut détectée entre la préparation faite avec l’aiguille 18-gauge ou l’aiguille 22-gauge. Les trouvailles cytologiques et histologiques concordaient, rapportant des quantités faibles ou modérées de lymphocytes et de macrophages, avec de faibles quantités de neutrophiles, de matériel étranger, et de bactéries. Implications: La procédure de FNA décrite est une technique potentielle que les praticiens peuvent utiliser pour caractériser des lésions associées à des réactions tissulaires sans avoir à euthanasier l’animal ou prélever chirurgicalement une biopsie. |
Keywords: swine, antemortem, injection sites, fine-needle aspirate, cytology
Search the AASV web site
for pages with similar keywords.
Received: August 13, 2013
Accepted: February 11, 2014
Pigs in commercial production units are routinely vaccinated to aid in prevention of a variety of diseases. Most vaccines used today are safe and efficacious and result in a very low incidence of complications. Occasionally, vaccination can cause adverse systemic reactions that may result in poor production or death. Additionally, local reactions can cause permanent tissue damage, resulting in undesirable carcass quality and economic losses.1 Local tissue reactions that can occur are granulomatous or lymphocytic inflammation, hemorrhage (ie, hematoma), fibrosis, abscessation (sterile or septic), or a combination of these.1 Each type of reaction has a characteristic cell and tissue architecture that may be discernible by cytologic examination. Defining the type of reaction is useful in order to institute prevention or control. Currently, there are no routinely utilized, simple techniques to characterize the type of local tissue reaction present without pig sacrifice. This study describes the use of fine-needle aspirate and cytologic examination as an antemortem technique to characterize local vaccine reactions. The purpose of this study was to apply a fine-needle aspirate (FNA) technique to evaluate grossly visible injection-site reactions by cytologic examination and determine its agreement with gross and histopathologic findings. Additionally, the optimal needle size used to perform the aspirate was evaluated. By using cytology to characterize lesions, decisions regarding future vaccination management or protocols can be developed.
Materials and methods
The studies were approved by the institutional animal care and use committees of Boehringer Ingelheim Vetmedica, Inc (Trial 1) and the Iowa State University (Trial 2).
Two trials were conducted. The first trial utilized 139 pigs housed in a commercial production unit. Pigs had received an intramuscular (IM) porcine circovirus type 2 vaccine administered at weaning (21 days of age) and a second dose 17 days later (38 days of age). Both injections were administered in the same anatomical location on the pig, the left cervical region. Seven days after the second dose, 69 pigs with grossly visible swelling at the injection site were identified. Of these, 40 pigs were conveniently selected and manually restrained for the FNA procedure. The lesion was manually isolated and punctured by inserting the needle into the lesion several times at different angles in order to obtain tissue within the needle and its hub (“woodpecker method”) using a 1.5-inch, 22-gauge hypodermic needle (no syringe was attached). The cellular contents in the needle and hub were expelled onto a clean glass microscope slide by pushing 5 mL of air retained in a syringe through the hypodermic needle. To spread the cellular material onto the slide, another clean glass slide was placed on top of the material and the slides slowly slid apart as for a standard cytologic preparation. Both cytologic preparations were air dried and sent for examination by a board-certified veterinary clinical pathologist (CEW). After FNA acquisition, the pigs were returned to their pens.
Trial 2 was conducted to compare the cytologic findings obtained by FNA with gross and histopathologic findings. Additionally, the effect of needle size on cytologic findings was evaluated. In Trial 2, a population of 1495 pigs within a different commercial production unit was utilized. Pigs had been subjected to the identical vaccination protocol using the same vaccine as in Trial 1. Forty percent of the pigs receiving this injection protocol demonstrated injection-site swellings. Twelve pigs with grossly visible lesions at the site of the vaccine (Figure 1) were selected by reaching into an affected pen and removing one pig from each of 12 pens. The selected pigs were then sedated with xylazine (2.0 mg per kg IM) and ketamine (20.0 mg per kg IM). Two separate cytologic samples were obtained per pig following the same FNA technique used in the first trial, but using two different-sized hypodermic needles (18-gauge and 22-gauge). The only difference between the FNA techniques utilized during Trial 2 versus Trial 1 was the addition of a sanitization step. The site chosen for FNA and approximately 1cm around the site was wiped with an alcohol-soaked gauze several times to remove surface fecal matter. The cytologic preparations were air-dried and sent for interpretation to a board-certified clinical pathologist. Following the FNA procedure, pigs were humanely euthanized. The injection-site lesion and regional lymph node were dissected from the neck area and submitted to Iowa State University Veterinary Diagnostic Laboratory for histopathologic interpretation by a diagnostic pathologist.
Figure 1: Pigs received an intramuscular porcine circovirus type 2 vaccine administered at weaning (21 days of age) and a second dose 17 days later (38 days of age). Both injections were administered in the left cervical region. Seven days after the second dose, pigs with a grossly visible swelling at the injection site were identified, as illustrated (Trial 2).

All cytology slides from both groups of animals were stained with a Wright-Giemsa stain and characterized for cellularity, cell populations present and their relative percentages, and the presence of organisms or foreign material. A final interpretation was determined, based on the following cytologic criteria. Granulomatous inflammation was characterized by a predominance of macrophages, lymphocytic inflammation by a predominance of lymphocytes. Hemorrhagic inflammation or hematoma was determined by the presence of macrophages containing hemosiderin or displaying erythrophagocytosis, and abscesses were defined by an infiltrate of degenerate or non-degenerate neutrophils with or without bacteria.2 Fibrosis cannot be reliably identified by cytology, but a cytologic preparation with low cellularity may suggest fibrosis. Mixed inflammation is best characterized by an overlap of cell populations from each cytologic category. The sensitivity and specificity of cytologic compared to histopathologic findings was determined using a 2 × 2 table.
Results
In Trial 1, at least one of the two cytologic samples taken per pig contained adequate material for interpretation. Nearly all of the samples were of low cellularity and displayed mostly small mature lymphocytes with lesser numbers of nondegenerate neutrophils and red blood cells. In the background, a mixed population of bacteria was noted. The cytologic interpretation of the samples with these findings was mild lymphocytic to mixed inflammation. The bacteria present were believed to be environmental contaminates, most likely from fecal material on the skin of the pig. On the basis of the cytologic findings, it was suggested that the vaccine reactions were a result of an immunologic response to the vaccine and not hemorrhage or abscess. Due to the low cellularity, fibrosis could not be completely ruled out. From this portion of the study, it was concluded that the FNA technique was adequate for cytologic characterization of the lesions (Figure 2). However, it was recommended that the site be lightly cleaned prior to the FNA procedure to avoid fecal contamination of the sample.
Figure 2: Fine-needle aspirate cytological preparation of an injection-site lesion (500× magnification). This cytological slide reveals adequate cellularity with a mixture of non-degenerate neutrophils, small lymphocytes, and macrophages set within a background of red blood cells. The interpretation is mild mixed inflammation. Vaccination and sampling methods described in Figure 1.
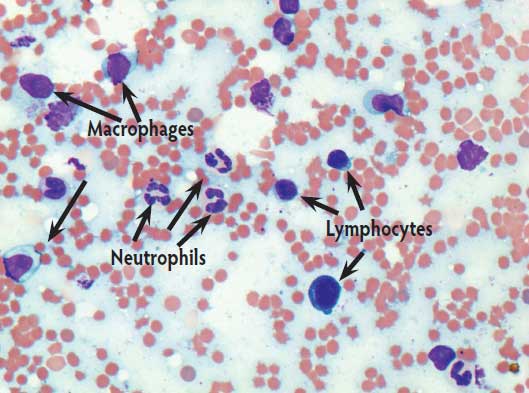
In Trial 2, no differences in cytologic quality were noted between the preparations made with an 18-gauge or 22-gauge needle. Cytologic findings from this group varied from granulomatous to mixed inflammatory response characterized by macrophages, small lymphocytes, and very low numbers of nondegenerate neutrophils. Histopathologic examination revealed subacute to severe mononuclear cell infiltrations, primarily macrophages with lesser numbers of lymphocytes, within muscle and connective tissue. Histopathologic examination also revealed that neutrophils, foreign material, or bacteria were not a prominent feature in any of the lesions examined. On the basis of these results, it was determined that the cytologic and histopathologic methods agreed. The 2 × 2 table analysis revealed a sensitivity of 100%; specificity could not be determined due to the lack of negative findings (ie, no cells observed on cytologic preparations or tissue for histopathologic examination) on both cytology and histopathology, respectively.
Discussion
This study demonstrates that the FNA technique described in this paper, and cytologic examination of the samples, can be utilized to obtain cellular components from a raised injection-site lesion. This technique can provide useful information regarding the cytologic characteristics of a vaccine-reaction lesion. While cytologic characterization can provide information about the type of tissue reaction, the exact cause of the reaction cannot be discerned by this technique. Additionally, cytologic examination cannot provide an entirely comprehensive architectural characterization of the lesion as can histopathologic examination, but it is able to provide relative proportions of the cells present. We believe this technique will be most useful for discriminating between rather benign vaccine reactions (ie, granulomatous or lymphocytic) and abscesses. A finding of mostly neutrophils, with or without bacteria, indicates the presence of an abscess, which may be the result of an unsanitary vaccination. If FNA and cytologic examination reveal the presence of an abscess, management control measures can be employed for future prevention. If FNA and cytologic examination routinely reveal other cellular processes, management protocols may be directed at the type of vaccine used rather than the vaccine procedure.
Implications
• These studies provide a description of how a practitioner can collect needle-aspirate samples and evaluate the cause of injection-site lesions.
• To ensure adequate cytologic preparation for examination, clean the site to be sampled of debris and feces, spread the sample on clean glass slides, and either ship air dried or stain the cytologic preparations in house with hematology-cytology stains.
• Either a 22-gauge or 18-gauge needle with proper technique can provide adequate cytologic preparations for interpretation.
• This technique can characterize tissue-reaction lesions without the need for pig euthanasia or surgical biopsy.
• Use of the cytologic examination of samples collected by fine-needle aspiration allows practitioners to evaluate the causes of injection-site lesions and alter vaccination protocols if necessary.
Acknowledgments
The authors would like to acknowledge the owner of the production sites and facilities that were used for this study, the producer who provided the pigs, and the animal-care staff who provided daily care and vaccination assistance over the course of the study, for which we are very grateful.
Conflict of interest
None reported.
References
1. Deville S, Ascarateil S, De Potter A, Gaucheron J, Dupuis L, Belloc C, Laval A. Control of pig vaccine safety trought adjuvant design and vaccination protocol: example of a divalent Pasteurella multocida toxin and Bordetella bronchiseptica vaccine. Revue de Médecine Vétérinaire. 2009;60:514–519.
2. Raskin R, Meyer D. General categories of cytologic interpretation. In: Canine and Feline Cytology. 2nd ed. St Louis, Missouri: Saunders Elsevier; 2010:15–25.
