Brief communication |
Peer reviewed |
Impact of sample collection location and grain fraction when assessing corn for aflatoxin contamination
Impacto de la ubicación de la toma de muestras y del grano quebrado cuando se analiza el maíz en busca de contaminación con aflatoxinas
Influence du site d'échantillonnage et de la portion du grain analysé lors de l'évaluation de la contamination du maïs par l'aflatoxine
Allen F. Harper, PhD; Junmei Zhao, MS, PhD; J. Blair Meldrum, DVM, PhD; Mark J. Estienne, PhD
AFH, JZ, MJE: Virginia Tech Department of Animal and Poultry Sciences, Blacksburg, Virginia. JBM: Virginia-Maryland Regional College of Veterinary Medicine, Blacksburg, Virginia. Corresponding author: Dr Allen F. Harper, Virginia Tech Tidewater AREC, 6321 Holland Road, Suffolk, VA 23437; Tel: 757-657-6450, ext 106; Fax: 757-657-9333; E-mail: alharper@vt.edu.
Cite as: Harper AF, Zhao J, Meldrum JB, et al. Impact of sample collection location and grain fraction when assessing corn for aflatoxin contamination. J Swine Health Prod. 2006;14(3):149-152.
Also available as a PDF.
SummaryIn corn containing 57 ng aflatoxin per g, toxin concentration was greater at a sampling depth of 1 meter than in deeper regions of the bin, and significantly lower in whole kernels than in fines. These data illustrate the importance of proper sampling when testing feedstuffs to diagnose mycotoxin. | ResumenEn maíz con un contenido de 57 ng de aflatoxinas por gramo, la concentración de la toxina fue mayor en una muestra tomada a un metro de profundidad que en partes más profundas del silo, y significativamente más baja en granos enteros que en pedazos pequeños de grano. Esta información ilustra la importancia de un muestreo apropiado cuando se analizan los componentes del alimento en el diagnóstico de micotoxinas. | ResuméDans du maïs contenant 57 ng d'aflatoxine par gramme, la concentration de toxine était plus élevée à une profondeur d'échantillonnage de 1 mètre que plus profondément dans la trémie, et significa-tivement plus faible dans des grains entiers que dans les moutures. Ces données démontrent l'importance d'un échantil-lonnage approprié lors de l'analyse d'aliments pour détecter la présence de mycotoxines. |
Keywords: swine, corn,
aflatoxin, sampling, screening
Search the AASV web site
for pages with similar keywords.
Received: July
5, 2005
Accepted: August
19, 2005
Aflatoxins are toxic by-products produced by the fungi Aspergillus flavus and Aspergillus parasiticus. These toxins can infect a variety of grains and oilseeds, but most often occur in corn, the predominant feed grain in North America. Four principle metabolites have been characterized and identified as aflatoxins B1, G1, B2, and G2, but the B1 metabolite occurs most frequently and is considered the most toxic.1 Aflatoxin M1 and M2 are related metabolites expressed in the milk of animals consuming aflatoxins. Swine are particularly susceptible to negative effects associated with consumption of aflatoxins: it has been reported that among food-producing and companion animals, only ducklings, trout, and cats are more susceptible to aflatoxicosis than swine.2
Clinical effects of aflatoxicosis include reduced growth rate and feed consumption, liver damage, internal hemorrhage, and impaired immunity, with younger pigs being more susceptible than older ones.3,4 Reduced growth rate in young pigs has been reported when feed was contaminated with aflatoxin at levels of 125 to 140 ng per g, with more pronounced effects at 260 ng per g.5,6 Levels of aflatoxins in food and feedstuffs at which the US Food and Drug Administration (FDA) initiates regulatory enforcement action are summarized in Table 1. Maximum levels indicated are 20, 100, and 200 ng per g for immature pigs, breeder swine, and finisher swine, respectively.4
Table 1: US Food and Drug Administration regulatory enforcement action levels for aflatoxins in food and feedstuffs*
* Adapted from Council for Agricultural Science and Technology Task Force Report No. 139, 2003.4 † Aflatoxin M1 is a metabolite expressed in the milk of animals consuming aflatoxins. |
When confirming or ruling out aflatoxin as a potential swine herd health problem, sampling and assaying feed or feed grains is typically employed. Thin-layer chromatography and high-performance liquid chromatography have been used to accurately detect and quantify aflatoxin in grain and feed samples. More recently, commercial laboratories have developed rapid test kits based on enzyme-linked immunosorbent assays (ELISAs) that are used in field testing of grain and feed samples for aflatoxins.
The goal of any aflatoxin sampling and analysis procedure is to determine as precisely as possible the concentration of toxin in feedstuffs in order to assess the potential for negative effects on animal health and performance. The objective of this experiment was to determine if depth within an on-farm storage bin and mechanical screening of the sample would have effects on aflatoxin concentration in corn previously determined to be contaminated with 57 ng aflatoxin per g.
Materials and methods
In October of 2002, approximately 73,600 kg of shelled corn was purchased from a commercial grain elevator in Suffolk, Virginia. The corn was transported in multiple truck loads (approximately 7360 kg each load) and placed in a steel grain bin (5.5 m diameter x 5.2 m height) at the Virginia Tech Tidewater Agricultural Research and Extension Center Swine Unit, Suffolk, Virginia, for routine use in swine feed formulation. The corn was grown locally in the 2002 season, a year in which regional drought conditions were considered potentially conducive to aflatoxin problems. As a precaution before using the corn in feed formulation, a representative sample of the total quantity of corn placed in the bin was tested for aflatoxins. The sample was obtained by collecting periodic subsamples using a plastic beaker (0.5 L per subsample) from the flowing grain being discharged from each truck load (four subsamples per load) and placing these subsamples in a large plastic pail. Thus, approximately 40 subsamples collected from the flowing grain stream were pooled to represent the total lot of corn placed in the bin. After the pooled subsamples were thoroughly mixed, a single representative sample (0.5 kg) was submitted for analysis at the Virginia-Maryland Regional College of Veterinary Medicine (VMRCVM) toxicology laboratory. Results indicated a contamination level for the bin of 57 ng aflatoxin per g.
Prior to delivery, the corn had been mechanically dried for proper storage (< 13.5% moisture) and was at ambient temperature when placed in the bin. Furthermore, it was not deemed necessary to apply forced aeration during the 12 -week storage period (October, November, and December) preceding the experiment.
In this experiment, four replicate samples (1100 g per sample) were collected from each of three depth regions in the bin. A probe-type grain sampler (Nasco Agricultural Sciences, Fort Atkinson, Wisconsin) was used to extract the samples 1 meter from the bin sidewall at depths of 1, 3, and 5 meters from the top surface of the corn. Each sample was then mechanically shaken over a stainless steel screen with round openings 6.75 mm in diameter. Using this process, the samples were separated into a fine-particles fraction (fines) and a fraction consisting of intact corn kernels. Each sample fraction was weighed and placed in a separate labeled container. For the 12 experimental samples treated in this manner, the weight of the fines fractions ranged from 7.9% to 9.2% of the total sample weight. For each sample fraction, dry matter content was determined using a still-air drying oven, and bulk density (weight per unit volume) was determined by weighing a fixed sample volume. The sample fractions were ground in a laboratory grist mill and transported to the VMRCVM toxicology laboratory for determination of aflatoxin concentration.
Aflatoxin concentration was determined in duplicate for each sample using the Veratox ELISA test kit (Neogen Corporation, Lansing, Michigan). Briefly, after thorough mixing of each ground-corn sample, a 5-g aliquot was extracted using 25 mL of 70% methanol and vigorous mechanical shaking for 3 minutes. The extract was filtered and 100-mL aliquots were placed in each of two antibody-coated wells. Reagents were added and incubated and the resultant color development was read on a microplate reader (Spectramax Plus; Molecular Devices Corporation, Sunnyvale, California) according to manu-facturer's specifications. Each Veratox kit includes standards and a predetermined standard curve. The limit of detection for the assay is 5 ng per g.
Statistical analysis
The data were subjected to analysis of variance (ANOVA) using the Statistical Analysis System (SAS Inc, Cary, North Carolina). The statistical model included effects of sampling depth (1 m, 3 m, or 5 m), grain fraction (whole kernels or fines), and the interaction of sampling depth and grain fraction on the following dependent variables: dry matter, bulk density, and aflatoxin concentration. When appropriate, individual means were compared by multiple t tests using the PDIFF option of the GLM procedure of SAS. Mean differences were considered statistically significant at P < .05.
Results
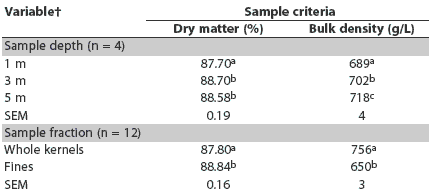
Overall sample dry matter ranged from 87.7% to 88.7% across all sampling depths and was slightly but significantly lower at the shallow depth (87.7%). Sample depth and sample fraction had modest but statistically significant effects on dry matter and bulk density (Table 2). Sample dry matter content at the 1-m depth was lower than at the 3-m and 5-m depths (P < .05). Bulk density of samples was lowest at 1-m depth, intermediate at 3 m, and greatest at 5 m (P < .05). Whole kernels were slightly lower in dry matter than the separated fines (P < .05) and also had greater bulk density (P < .05). The fines fraction, which made up 7.9% to 9.2% of the total material sampled, appeared to consist largely of small pieces of broken kernel and light-weight chaff material, such as small bits of cob, weed seeds, and other foreign material.
Table 2: Main effect means for dry matter and bulk density in samples of corn grain from a bin sampled using a probe-type grain sampler at three depths from the surface*
* The bin contained approximately 73,600 kg of corn grain previously determined to be contaminated with 57 ng/g aflatoxin. Corn in the bin was tested for aflatoxin using a modified ELISA test kit (Veratox; Neogen Corporation, Lansing, Michigan). Each sampling depth was measured from the corn surface 1 m from the bin sidewall. Four replicate samples were collected from each depth using a sampling probe (Nasco Agricultural Sciences, Fort Atkinson, Wisconsin). Sample fractions (whole kernels or fines) were separated by mechanical screening with a 6.75-mm screen. The interaction of sample depth and sample fraction was not significant for dry matter (P = .14) or bulk density (P = .83). abc Within a variable (sample depth or fraction), means in a column with no common superscript differ (P < .05; t test) |
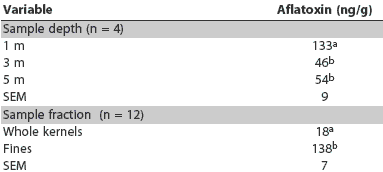
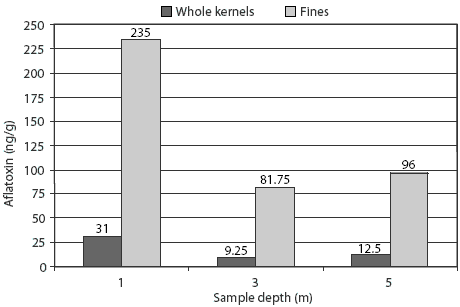
Main effects of sampling depth and sample fraction on aflatoxin concentration are presented in Table 3. Aflatoxin concentration was markedly greater at the 1-m sampling depth than at depths of 3 m and 5 m (P < .001). Sample fraction also had a major impact on mean aflatoxin concentration, which was 18 ng per g in whole kernels and 138 ng per g in separated fines (P < .001). The difference in aflatoxin concentration between whole kernels and fines was approximately 2.4 times greater at the 1-m sampling depth than at the 3-m and 5-m depths (Figure 1), resulting in a significant interaction of sampling depth and sample fraction (P < .001).
Table 3: Main effect means for aflatoxin concentration in samples of corn grain from a bin sampled using a probe-type grain sampler at three depths from the surface*
* Corn and sampling and testing techniques described in Table 2. ab Within a variable (sample depth or fraction), means in a column with no common superscript differ (P < .001; t test). |
Figure 1: Interaction of sampling depth and sample fraction (whole kernels or fines) on aflatoxin concentration in a bin containing 73,600 kg of corn. A single representative sample from the bin, tested using a modified ELISA test kit (Veratox; Neogen Corporation, Lansing, Michigan), had an aflatoxin concentration of 57 ng/g. Sample fractions (whole kernels and fines) were then separated by mechanical screening with a 6.75-mm screen and tested using the same ELISA. Each mean represents four replicate samples with an SEM of 13. The interaction of sample depth and sample fraction was significant (P < .001).
|
Discussion
In-field contamination of corn with aflatoxin is more common in years characterized by drought stress and above normal temperatures.7 The bin of corn used in this experiment was produced in southeast Virginia during the 2002 season, a year noted for drought stress and below-average crop yields for the region. The composite sample representing the entire bin (73,600 kg) was analyzed within a week of filling the bin. The subsequent sampling experiment verified that this level of contamination was generally representative of the total bin. However, absolute concentration of aflatoxin varied considerably, with the highest concentration in the most shallow sampling zone (1-m depth).
The bin was filled with consecutively delivered farm truckloads of approximately 7300 kg each, and it is possible that the final loads delivered were higher in aflatoxin than the earlier loads. Another possibility is that aflatoxin production during storage occurred to a greater degree near the grain surface than in deeper regions of the bin. Bulk density of the grain was lower in the shallow sampling zone, suggesting more fines in this region. The interaction between sample fraction and sampling depth indicated that the fines component contributed to overall aflatoxin concentration in greater magnitude in the shallow bin region than in deeper regions. Stored grain dry matter levels above 87% (ie, < 13% moisture) are considered adequate for long-term storage, and aflatoxin production under field conditions is minimal at moisture levels below 15%.8 In this study, overall sample dry matter exceeded 87% at all sampling depths and was slightly but significantly lower at the shallow depth. However, as no grain cooling or aeration procedures were applied during the 12-week storage period, the combined factors of slightly greater moisture content and greater percentage of fine material near the surface may have promoted aflatoxin production in this zone.
These sampling-depth data show that concentration of aflatoxin (and presumably other mycotoxins) may vary substantially at different locations within masses of stored feed grains. It is well known that "hot spots," ie, areas of high mycotoxin concentration, develop in stored grain. When grain for swine feed is purchased or assembled at mills or farms, it should be recognized that different lots or sources of grain have unique potentials for mycotoxin contamination. Sampling and testing programs for quality control and preventive health maintenance should be set up to account for this variation. Furthermore, when grains or feeds are tested to confirm or rule out a potential mycotoxin-related swine health problem, a single representative sample from a large mass of stored grain or feed appears to be inadequate. For more precise diagnosis of mycotoxicosis problems, samples for testing should be collected from the grain and compounded feed that the affected animals are eating.
Negative effects of aflatoxin-contaminated corn on swine health and performance may be moderated by techniques that include post-harvest cleaning to remove moldy or damaged kernels, treatment of infected corn with ammonia gas, and addition of certain clay-based products to swine feeds.9 The latter approach has received substantial research interest and practical use in the industry. Harvey and co-workers10 demonstrated that addition of a hydrated sodium calcium aluminosilicate product to a pig diet that had been artificially contaminated with aflatoxin was effective in preventing abnormal liver enzymes, prothrombin times, and growth by binding aflatoxins in the gastrointestinal tract and preventing absorption. Similar amelioration has been demonstrated in growing pigs when sodium bentonite, calcium bentonite, or other clay-based feed additives are added to corn-based diets naturally contaminated with aflatoxin.11,12 Many of these products are labeled for use in swine feeds as anti-caking agents or pelleting aids, and do not hold specific label claims to prevent aflatoxin absorption in pigs.
The sample-screening component of this experiment demonstrates another technique for substantially reducing aflatoxin contamination in corn. In this study, Aspergillus growth and aflatoxin production might have been greater in the fines, as aflatoxin concentration was seven-fold greater in the fines fraction. Under these conditions, grain screening brought the overall aflatoxin concentration to < 20 ng per g, below FDA enforcement action levels for all classes of swine and other livestock. Physical separation has been described as a means to reduce mycotoxin levels in foodstuffs,9,13 and technology to accomplish physical separation in large grain handling and feed mill operations has been described.14
Implications
- Under the conditions of this experiment, screening to remove fine particles from intact kernels is highly effective in reducing aflatoxin concentration in moderately contaminated corn grain.
- As aflatoxin is not distributed homogenously in a large mass of stored grain, a single representative grain sample is inadequate for diagnostic aflatoxin testing.
- For precise diagnosis of mycotoxicosis problems, composite sampling should be directed to the grain and compounded feed that the affected animals are consuming.
Acknowledgements
The research reported herein was conducted as a component of Project VA-135586, Virginia Agricultural Experiment Station and US Department of Agriculture cooperating. Provision of aflatoxin assay kits by the Neogen Corporation, Lansing, Michigan, is gratefully acknowledged. Appreciation is also expressed to Ms Barbara Wise for technical assistance and performing the aflatoxin analysis.
References
1. Smith JE. Aflatoxins. In: D'Mello JPF, ed. Handbook of Plant and Fungal Toxicants. Boca Raton, Florida: CRC Press; 1997.
2. Pier AC. Mycotoxins and animal health. Adv Vet Sci Comp Med. 1981;25:185.
3. Harvey RB, Kubena LF, Huff WE, Corrier DE, Rottinghaus GE, Phillips TD. Effects of treatment of growing swine with aflatoxin and T-2 toxin. Am J Vet Res. 1990;51:1688-1693.
*4. Council for Agricultural Science and Technology (CAST). Mycotoxins - Risks in Plant, Animal and Human Systems. Task Force Report No. 139. Ames, Iowa; 2003.
5. Coffey MT, Hagler WM Jr, Cullen JM. Influence of dietary protein, fat or amino acids on the response of weanling pigs to aflatoxin B1. J Anim Sci. 1989;67:465-472.
6. van Heugten E, Spears JW, Coffey MT, Kegley EB, Qureshi MA. The effect of methionine and aflatoxin on immune function in weanling pigs. J Anim Sci. 1994;72:658-664.
7. Widstrom NW. The aflatoxin problem in corn grain. In: Sparks D, ed. Advances in Agonomy. New York, New York: Academic Press; 1996.
8. Payne GA, Hagler HW Jr, Adkins CR. Aflatoxin accumulation in inoculated ears of field-grown maize. Plant Dis. 1988;72:422-424.
9. Park DL. Effect of processing on aflatoxin. Adv Exp Med Biol. 2002;504:173-179.
10. Harvey RB, Kubena LF, Phillips TD, Huff WE, Corrier DE. Prevention of aflatoxicosis by addition of hydrated sodium calcium aluminosilicate to the diets of growing barrows. Am J Vet Res. 1989;50:416-420.
11. Lindemann MD, Blodgett DJ, Kornegay ET, Schurig GG. Potential ameliorators of aflatoxicosis in weanling/growing swine. J Anim Sci. 1993;71:171-178.
12. Schell TC, Lindemann MD, Kornegay ET, Blodgett DJ, Doerr JA. Effectiveness of different types of clay for reducing the detrimental effects of aflatoxin-contaminated diets on performance and serum profiles of weanling pigs. J Anim Sci. 1993;71:1226-1231.
13. Scott PM. Effects of food processing on myco-toxins. J Food Protection. 1984;47:489-499.
*14. Paulsen RB. Productivity, efficiency key to grain cleaner selection. Feedstuffs. December 30, 2002.
* Non-refereed references.