Case study |
Peer reviewed |
The effect of porcine proliferative enteropathy on the introduction of gilts into recipient herds
El efecto de la enteropatía proliferativa del cerdo en la introducción de hembras en granjas receptoras
Effet de l'entéropathie proliférative porcin sur l'introduction des cochettes dans les troupeaux récepteurs
Robert M. Friendship, DVM, MSc, Diplomate ABVP; Cesar A. Corzo, DVM, MSc; Cate E. Dewey, DVM, MSc, PhD; Tim Blackwell, DVM, MSc, PhD
RMF, CAC, CED: Department of Population Medicine, Ontario Veterinary College, University of Guelph, Guelph, Ontario; TB: Ontario Ministry of Agriculture and Food (OMAF), Fergus, Ontario; Corresponding author: Dr Robert Friendship, Department of Population Medicine, University of Guelph, Guelph, Ontario, Canada N1G 2W1; Tel: 519-824-4120, ext 54022; Fax: 519-763-3117; E-mail: rfriends@uoguelph.ca
Cite as: Friendship RM, Corzo CA, Dewey CE, et al. The effect of porcine proliferative enteropathy on the introduction of gilts into recipient herds. J Swine Health Prod. 2005;13(3):139-142.
Also available as a PDF.
SummaryPorcine proliferative enteropathy (PPE) caused by Lawsonia intracellularis (LI) is one of the most important enteric diseases in growing swine. The acute form of this disease, proliferative hemorrhagic enteropathy (PHE), has become a common problem when replacement breeding stock are introduced into a herd. In the cases described, animals of different immune status were moved between supply and recipient herds. An LI-free breeding-stock herd (Herd A) supplied gilts to four herds, one of which was LI-free (Herd B). During a 1-year period, PHE was observed in replacement animals after they entered the LI-infected herds (Herds C, D, and E); however, no problems were reported in Herd B. After an outbreak of PHE occurred in Herd A, replacement animals from this herd no longer developed PHE in the three LI-infected recipient herds. However, an outbreak of PHE occurred in Herd B when LI-infected replacement gilts were introduced. | ResumenLa enteropatía proliferativa (PPE por sus siglas en inglés) causada por el Lawsonia intracellularis (LI por sus siglas en inglés) es una de las enfermedades entéricas mas importantes en los cerdos en crecimiento. La forma aguda de la enfermedad, enteropatía hemorrágica proliferativa (PHE por sus siglas en inglés), se ha convertido en uno de los problemas más comunes al introducir pie de cría de reemplazo a un hato. En los casos descritos en este reporte, animales con diferentes niveles de inmunidad se introdujeron de engordas de producción de pie de cría a granjas receptores. Un hato de pie de cría libre de LI (Hato A) envió hembras a cuatro granjas: B, C, D y E. Durante un periodo de un año, se observó la PHE en los animales de reemplazo después de que se introdujeron a las granjas C, D y E, que estaban infectadas con LI. Sin embargo, no se reportaron problemas en las granjas B, que era libre de LI. Después de un brote de PHE en la granja A, los animales de reemplazo ya no desar-rollaron PHE en las tres granjas receptoras infectadas con LI. Sin embargo, un brote de PHE se presenté en la granja B cuando las hembras de reemplazo infectadas con LI, de la granja A, fueron introducidas. | ResuméL'entéropathie proliférative du porcin (PPE par ses initiales en anglais) causé par le Lawsonia intracellularis (LI par ses initiales en anglais) est une de les maladies entériques plus importantes dans les animaux en croissance. La forme aiguë de cette maladie, l'entéropathie hémorragique proliférative (PHE par ses initiales en anglais), est devenu un problème commun quand les animaux de remplacement sont introduits dans un troupeau. Dans les cas décrits en cet article, les animaux de statut immunitaire différent ont été déplacés des engraissements de production de cochettes aux troupeaux récepteurs. Un troupeau sans LI (Troupeau A) a fourni des cochettes à quatre troupeaux, les troupeaux B, C, D, et E. Pendant une période de 1 année, le PHE a été observé dans les animaux du rem-placement après qu'ils sont entrés dans les troupeaux C, D, et E qui ont été infectés avec LI. Cependant, aucuns problèmes n'ont été rapportés dans le troupeau B qui était sans LI. Après d'une première manifestation du PHE que s'est produite dans le troupeau A, les animaux du remplacement de ce troupeau n'ont plus développé de PHE dans les trois troupeaux récepteurs sans LI. Cependant, une première manifestation du PHE s'est produite dans le troupeau B quand les cochettes du remplacement infectées avec LI ont été introduites du troupeau A. |
Keywords: swine, Lawsonia
intracellularis, porcine proliferative enteropathy, proliferative hemorrhagic
enteropathy
Search the AASV web site
for pages with similar keywords.
Received: August
31, 2004
Accepted: November
30, 2004
Porcine proliferative enteropathy (PPE) has become a concern in swine health and production because of the effect it has on the performance of growing pigs. The causative bacterium, Lawsonia intracellularis (LI), is present in many herds around the world,1 and some details of the epidemiology of this organism are known.2,3 It has been reported that affected pigs shed the organism for approximately 10 to 12 weeks,4 and that it is able to survive in the environment for up to 2 weeks.5 These two facts explain in part why the disease is widespread in the pig population and why it is difficult to maintain a herd free of LI infection.
If the disease enters a herd for the first time, the consequences are likely to be serious. Mature naive pigs that become infected commonly develop porcine hemorrhagic enteropathy (PHE), the acute form of PPE, resulting in high mortality. This case report describes an outbreak of disease caused by LI infection in a previously naive breeding-stock supply herd and the subsequent effects on herds receiving gilts from this source.
Original status of case herds
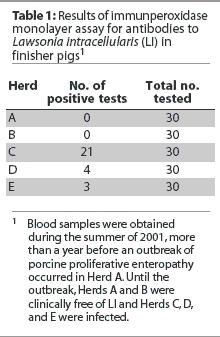 In the summer of 2001, 30 blood samples from finisher pigs in each of five
Ontario farrow-to-finish herds (Herds A, B, C, D, and E) were serologically tested for LI
as part of a general prevalence study. Samples were submitted to the University of
Minnesota for testing by immunoperoxidase monolayer assay
(IPMA).6 Herds were classified as positive if at least one sample
was positive. Only Herds A and B were serologically negative for LI (Table 1).
In the summer of 2001, 30 blood samples from finisher pigs in each of five
Ontario farrow-to-finish herds (Herds A, B, C, D, and E) were serologically tested for LI
as part of a general prevalence study. Samples were submitted to the University of
Minnesota for testing by immunoperoxidase monolayer assay
(IPMA).6 Herds were classified as positive if at least one sample
was positive. Only Herds A and B were serologically negative for LI (Table 1).
Outbreak in Herd A
In November 2002, Herd A, a breeding-stock supplier herd, experienced an outbreak of PHE in the breeding herd. Three gestating sows became pale, developed bloody diarrhea, and died within 24 hours. A fourth sow with similar clinical signs died in the farrowing room soon after becoming ill. Shortly after the initial sow deaths, diarrhea and thin, unthrifty pigs were noted in the grower room. Diagnosis was based on postmortem examination findings and detection of LI in feces and tissue samples by polymerase chain reaction (PCR). Tylan 10 premix (Elanco Animal Health, Guelph, Ontario) was added to sow feed at a rate of 5 kg per tonne of finished feed (110 g of tylosin per tonne of feed), and Lincomycin 44 premix (Bio Agri Mix, Mitchell, Ontario) was also added at 2.5 kg per tonne of finished feed (110 g of lincomycin per tonne of feed). Sows that became clinically affected were treated with injectable tylosin (Tylan 200; Elanco Animal Health) at a dosage of 9 mg per kg body weight for 3 days.
About 1% of all animals in the herd died in the outbreak. Animals responded to treatment and there were no more new cases of PHE in the breeding herd, but problems in the grower room persisted. Although not all pigs appeared to be affected, some exhibited poor growth, moderate diarrhea, "razor-back" appearance, and wasting, and some had red-tinged, very loose diarrhea.
Health histories of case herds
The health history of each herd before and after the outbreak in Herd A was collected either by visit or telephone.
Herd A (original LI status: naive)
Herd A, a one-site operation with 170 sows, was a naive herd, free of LI and other major pathogens for 18 years before the outbreak. Farrowing and nursery rooms were managed all-in all-out by room, and continuous flow management was used in the grower and finisher rooms. The herd was completely closed, using internal replacements only. Biosecurity measures were strictly enforced, including downtime of 48 hours after exposure to other pigs, shower-in and shower-out, and use of farm-specific coveralls and boots. There were no other pig farms within a 5-km radius, and no other animal species were housed in or near the facility. The herd was serologically negative for Mycoplasma hyopneumoniae, Actinobacillus pleuropneu-moniae (serotypes 1 and 5), and porcine reproductive and respiratory syndrome (PRRS) virus.
Herd B (original LI status: naive)
Herd B, a 140-sow, one-site commercial operation with approximately 480 nursery pigs and 1400 grower-finisher pigs, was also a naive herd. All-in all-out management was used in the nurseries, and continuous flow in the grower-finisher barn. At the end of 2001, Herd B had been depopulated and repopulated with stock from Herd A and one other source known to be free of important pathogens. Further groups of 20 to 30 replacement gilts were later introduced from Herd A. No problems were reported during acclimatization of these gilts.
Herds C, D, and E (original LI status: infected)
Herd C was a one-site, one-building operation with 175 sows, 300 nursery pigs, and 1100 grower-finisher pigs. Continuous flow management was used both in the nursery and grower-finisher rooms. As a new manager had been recently hired at the time of the farm visit, no health history prior to the outbreak in Herd A was available for this herd.
Herd D had 615 sows, 2100 nursery pigs, and approximately 1300 grower-finisher pigs that were housed in an off-site barn, all managed all-in all-out by room. The producer reported that previous to the outbreak of PHE in Herd A, one of two clinical syndromes had occurred in Herd A gilts introduced into Herd D. Gilts either had black tarry diarrhea associated with high mortality, or they developed intermittent diarrhea that was difficult to control. Clinical signs appeared within 2 to 4 weeks after replacement gilts entered the herd. Approximately a third of each group of Herd A gilts exhibited black tarry diarrhea. Treatment with injectable lincomycin (Lincomix injectable solution, 100 mg per mL; Pfizer Canada Inc, Kirkland, Quebec) at 10 mg per kg of body weight daily for 3 days was successful in many affected gilts, but others died before treatment began. Eventually, the disease was controlled by including tylosin in the feed (110 g per tonne) on alternate weeks for 4 weeks after gilts entered the facility.
Herd E had 130 sows, 200 nursery pigs, and 700 grower-finisher pigs, with all-in all-out flow in the nursery and continuous flow in the grower-finisher barn. Problems with PPE had occurred in the herd since the 1980s, when poor-doing pigs with thickened intestines had been observed. When Herd A gilts entered this herd, one or two of each group of 15 usually developed tarry stools 7 to 10 days after arrival. Initially, sick pigs had been treated with injectable lincomycin (Lincomix injectable solution; Pfizer Canada Inc) at 10 mg per kg of body weight daily for 3 days with little success. For several years prior to 2001, gilts had been treated with tylosin (Tylan 10 premix; Elanco Animal Health) at 110 g per tonne of feed for the first 2 weeks after arrival, and for a second 2-week period after an interval of 2 weeks on unmedicated feed.
Health histories of recipient herds after the outbreak in Herd A
Herd B
After the introduction of the last group of Herd A gilts in December 2002, twenty sows in the breeding herd developed bloody diarrhea, became pale, and died suddenly. In the grower-finisher herd, a large number of pigs had loose stools, and the number of poor-doing pigs suddenly increased. Antibiotic treatment in the feed and water was initiated to control the outbreak. This consisted of tiamulin (Denagard Liquid Concentrate; Boehringer Ingelheim Canada Ltd, Burlington, Ontario) in the drinking water at a level of 0.0049% continuously for 5 days, and tiamulin (Denagard Medicated Premix; Boehringer Ingelheim Canada Ltd) in the feed at 178 g per tonne. Although treatment did control the outbreak, costs due to mortality and treatment became a concern. More than a year later, chronic cases of PPE were still occurring in the finishing barn. Tylosin (110 g per tonne) was included in the feed and clinically affected animals were treated with injectable tylosin (Tylan 200; Elanco Animal Health) at a dosage of 9 mg per kg body weight intramuscularly for 3 days. After the outbreak, no more purchased replacement gilts were introduced into the herd.
Herd C
Groups of Herd A gilts acclimatized well with regard to PPE: no signs of tarry stools or diarrhea were observed.
Herd D
After the in-feed medication protocol was established for incoming Herd A gilts, problems with the acute form of PPE disappeared. In addition, internal replacement gilts were introduced from the finisher barn, entering the breeding herd with no health problems and without the need for medication.
Herd E
Gilts from Herd A introduced after the fall of 2002 displayed no signs of PHE and acclimatized well. However, tylosin continued to be used prophylactically.
Testing of Herds A and B after the outbreak
In the summer of 2003, animals in Herds A and B were serologically tested for LI to verify the PPE status of these herds after the outbreaks of diarrhea in both herds. Blood samples were obtained from 20 finisher pigs in each herd and tested by the indirect fluorescent antibody test (IFAT) for LI. All Herd B samples were IFAT-negative. Nineteen of the 20 samples from Herd A were IFAT-positive. Two Herd A grower pigs that were in poor physical condition were euthanized and submitted for necropsy at the Animal Health Laboratory, University of Guelph, Guelph, Ontario. Macroscopic and microscopic lesions were consistent with a diagnosis of PPE.7 Ileal specimens and fecal samples from the two Herd A pigs were submitted for further testing by PCR for LI, and both samples from both pigs were PCR-positive.
Discussion
This case reflects the importance of knowing the health status of the herd supplying breeding stock and the recipient herd, and also emphasizes the importance of matching the LI immune status of the source and recipient herds. A serologic test (IPMA) was used in 2001 to classify herds as either LI-naive or LI-infected. On the basis of this test, two herds (A and B) were categorized as naive and the remaining three as infected. Only Herd B shared the LI status of Herd A (the breeding-stock supplier), and only Herd B had no problems acclimatizing Herd A gilts. Conversely, Herds C, D, and E did have problems introducing Herd A gilts, which did not share the LI status of the recipient herds. The LI-naive gilts were challenged with LI for the first time on entering the infected herds, and developed PHE.
The immune system of a naive gilt is easily overwhelmed by a large challenge of Lawsonia organisms, and the acute form of the disease is triggered. Some animals treated early may be saved, but treatment may be costly. Guedes et al8 reported that twice-daily injections of tylosin plus in-feed tylosin and tiamulin in the water stopped clinical signs from appearing and minimized mortality during an outbreak of PHE in a recently repopulated farm.
Administration of high doses of antibiotics may prevent animals from developing an immune response to LI, extending the susceptibility period until after antibiotics are withdrawn. However, researchers found that administering chlortetracycline at 400 ppm in feed for 10 days after infection allowed some degree of immunity.9 The problem with this approach is that it must be known when the pigs are becoming infected and when treatment should be initiated, as well as what dose of medication should be used and for what treatment period, so that active immunity against LI can be developed. Prior to the PHE outbreak in Herd A, almost no antibiotics were used in the grower-finisher barn, and therefore it was assumed, on the basis of clinical and serologic evidence, that Herd A was free of LI.
No Herd A replacement gilts entered Herd B after the outbreak of disease in Herd B in December of 2002. Sow mortality and diarrhea in the Herd B grower-finisher barn started occurring after the introduction of infected Herd A gilts in late 2002, indicating that Herd A gilts were LI-carriers and disseminated the bacteria throughout Herd B. There should be no further difficulty with acute disease if LI-positive replacement gilts are introduced into Herd B. However, problems associated with chronic PPE infection may become evident, including poor average daily gain and feed conversion rate, and an increase in weight variation of grower and finisher pigs. Interestingly, no positive samples were found from the group of Herd B finisher pigs sampled after the outbreak. The results of later postmortem examinations and observation of poor-doing pigs with diarrhea in the grower herd showed that the organism was present in the herd. However, the finisher pig ration was medicated with tylosin at 1 kg per tonne, which might have prevented pigs from developing detectable antibodies.9
A commercial LI vaccine is available that might be helpful when the LI-disease status of a purchasing herd differs from that of the breeding-stock supplier herd. If the receiving herd is LI-positive, but replacement pigs are purchased from a negative herd, the vaccine could be used in incoming replacement gilts during acclimatization or quarantine. Thus, naive gilts will have time to develop immunity and there should be no problem when these vaccinated gilts enter the receiving sow herd. This would have been an option for Herds C, D, and E when Herd A gilts were introduced, if the vaccine had been available in 2001. Instead, protocols were developed in these herds to treat Herd A replacement gilts with in-feed antibiotics at the time of exposure to LI, and this strategy worked well in preventing the gilts from developing PHE. After the outbreak in Herd A, Herds C, D, and E no longer had problems with PHE in Herd A gilts during acclimatization. Since Herd A gilts had already been exposed to the organism in the herd of origin, they had sufficient immunity to resist an LI challenge at the receiving herd. Although the prophylactic program of antibiotics used in these herds was likely no longer of value and could have been discontinued, producers were reluctant to take this risk.
There are still herds in Ontario at risk of LI infection (ie, LI-negative herds) which must take steps to prevent the organism from entering their facilities, eg, in rodents or on fomites. Herd A had good biosecurity and had been a closed herd. It is difficult to explain how this herd had been able to remain free of many pathogens (eg, PRRS virus) over such a long period of time and yet was unable to prevent introduction of LI. The mechanism by which LI gained access to this herd remains unknown. A pathogen such as LI is most readily introduced into a herd by carrier pigs, but in Herd A, this was not the case. Conversely, Herd B probably introduced the disease by purchasing infected Herd A gilts, and, as a consequence, experienced significant losses. It has been reported that the chronic form of PPE produces losses due to slow growth and increased weight variation.10 This case study suggests that acute PPE (PHE) may also result in heavy losses due to the deaths of valuable incoming naive gilts and the need for antibiotic treatment.
To avoid outbreaks of disease, producers introducing new genetic lines of animals into a herd must take into account the LI-status of both the breeding-stock supplier herd and recipient herd. Gilt acclimatization is critical when animals from new sources are brought into the herd, in order to maintain a stable herd immunity. It might be necessary for producers to tolerate LI infection in their herds, minimizing the economic impact PPE has on performance by use of management practices including all-in all-out flow, and judicious use of antibiotics, vaccine, or both.
Implications
- To avoid outbreaks of PPE and the economic consequences of such outbreaks when replacement animals enter a herd, the LI status of both the breeding-stock supplier and recipient herd must be known.
- Serologic testing may be a practical tool to determine the status of the herd supplying replacement gilts.
- Suspicious results of serological tests for LI must be carefully interpreted and the herd retested if necessary.
- If a stable herd immunity to LI is to be maintained, immune status of replacement animals should be similar to that of the recipient herd.
- If LI-positive herds introduce LI-naive gilts, an acclimatization protocol and vaccination program should be established in an off-site facility.
References
1. McOrist S, Barcellos DE, Wilson RJ. Global patterns of porcine proliferative enteropathy. Pig J. 2003;51:26-35.
2. Smith SH, McOrist S, Green LE. Questionnaire survey of proliferative enteropathy on British pig farms. Vet Rec. 1998;142:690-693.
3. Bronsvoort M, Norby B, Bane D, Gardner I. Management factors associated with seropositivity to Lawsonia intracellularis in US swine herds. J Swine Health Prod. 2001;9:285-290.
4. Guedes RM, Gebhart CJ. Onset and duration of fecal shedding, cell-mediated and humoral immune responses in pigs after challenge with a pathogenic isolate or attenuated vaccine strain of Lawsonia intracellularis. Vet Microbiol. 2003;91:135-145.
5. Collins A, Love R, Pozo J, Smith S, McOrist S. Studies on the ex-vivo survival of Lawsonia intracellularis. J Swine Health Prod. 2000;8:211-215.
6. Guedes RM, Gebhart CJ, Deen J, Winkelman NL. Validation of an immunoperoxidase monolayer assay as a serologic test for porcine proliferative enteropathy. J Vet Diagn Invest. 2002;14:528-530.
7. McOrist S, Gebhart CJ. Porcine proliferative enteropathies. In: Straw BE, D'Allaire S, Mengeling WL, Taylor DJ, eds. Diseases of Swine. 8th ed. Ames, Iowa: Iowa State University Press; 1999:521-534.
8. Guedes RM, Gebhart CJ, Armbruster GA, Roggow BD. Serologic follow-up of a repopulated swine herd after an outbreak of proliferative hemorrhagic enteropathy. Can J Vet Res. 2002;66:258-263.
*9. Collins AM, van Dijk N, Vu NQ, Pozo P, Love RJ. Immunity to Lawsonia intracellularis. Proc AD Leman Swine Conf. St Paul, Minnesota. 2001:115-120.
10. McOrist S, Smith SH, Green LE. Estimate of direct financial losses due to porcine proliferative enteropathy. Vet Rec. 1997;140:579-581.
*Non-refereed reference
