What’s your interpretation? September and October 2001
What’s your interpretation?
Non refereed
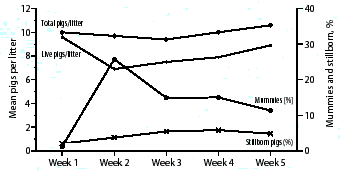
This graph contains data from 503 consecutive farrowings in
one new 2800-sow farrowing
operation. Our first concern was to identify the reason for the
high numbers of mummified and stillborn piglets so that corrective
measures could be instituted. How do you interpret
these findings and what action plan would you propose?
Case report of reproductive problem in a new startup operation
As the data were not helpful in making a diagnosis, a farm visit was made.
The breeding herd was one of two 2800-sow herds that had been established within a few months of each other. Shortly after the first gilts started farrowing in Unit 1, a marked increase in the numbers of mummified piglets was noted. The reproductive problems expanded rapidly to include increased numbers of stillbirths; weak, nonviable neonates; late term abortions; and gilts identified as "not in pig" (NIP). Many of these NIP sows expelled entire litters of mummified fetuses, often at full term. The problem continued for 7 weeks after the first farrowing in both units, but was much more severe in Unit 1 than in Unit 2.
Gilts in both units had originated from the same high-health status nucleus herd and had been vaccinated against PRRS, erysipelas, leptospirosis, and parvovirus at the appropriate times. During the startup phase, 180 gilts, approximately 175 days of age, arrived on each farm weekly. Since there was inadequate isolation space to handle this number of incoming gilts, and all gilts had come from the same source, there was no quarantine period. After a 21-day acclimatization period, gilts were bred and entered the gestation area.
Further investigation revealed that the gilts had actually originated from two different gilt grow-out facilities. One of these herds had been in operation for several years (Facility B), but the other had recently been established and was housed in a new facility (Facility A), which was operated on an all in-all out basis. The gilts were produced in a three-site production system using early weaning. Gilts delivered each week to a farrowing unit all came from the same facility.
 The farm manager soon realized
that there was a marked difference in incidence of reproductive
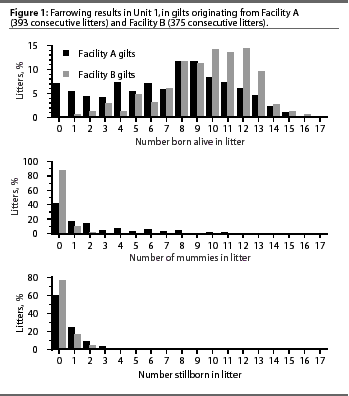
problems between gilts from the two facilities. Figure 1 identifies
the extent of this variation in Unit 1 and compares the numbers
of born alive, mummified, and stillborn pigs in litters of gilts
that originated from Facility A and Facility B. Approximately
70% of the gilts in Unit 1 came from Facility A, and these animals
experienced the most severe problems. The remaining 30% of the
gilts came from Facility B, the older facility, which was run
in a semi-continuous flow manner. Ten percent of the gilts in
Unit 2 came from Facility A, with the remaining 90% from Facility
B.
The farm manager soon realized
that there was a marked difference in incidence of reproductive
problems between gilts from the two facilities. Figure 1 identifies
the extent of this variation in Unit 1 and compares the numbers
of born alive, mummified, and stillborn pigs in litters of gilts
that originated from Facility A and Facility B. Approximately
70% of the gilts in Unit 1 came from Facility A, and these animals
experienced the most severe problems. The remaining 30% of the
gilts came from Facility B, the older facility, which was run
in a semi-continuous flow manner. Ten percent of the gilts in
Unit 2 came from Facility A, with the remaining 90% from Facility
B.
For the initial 7 weeks of farrowings, gilts from Facility A averaged 7.15 born alive, 2.17 mummified and 0.65 stillborn pigs per litter, whereas gilts from Facility B averaged 9.53 born alive, 0.18 mummified, and 0.33 stillborn pigs per litter. During the second week of farrowing, when 25.7% of piglets were mummified, approximately 40% of piglets from Facility A gilts were mummified.
Diagnostics
A full diagnostic workup was ordered. Aborted or mummified fetuses and weak, nonviable neonatal piglets were submitted to a diagnostic laboratory. Parvovirus fluorescent antibody testing (FAT) on lung tissue was negative. Bacterial cultures of various tissues were negative, as were virus isolation attempts. Testing for PRRSV was negative, using FAT, virus isolation, and polymerase chain reaction (PCR) techniques. In addition, sera from affected dams was negative for PRRSV, encephalomyocarditis virus, and Leptospira interrogans serovars pomona, grippotyphosa, and bratislava.
A consistent finding was identification of porcine circovirus type II (PCV-2). Polymerase chain reaction and restriction fragment length polymorphism testing identified PCV-2 in 29 of 34 samples from lung and (or) heart tissue from aborted, mummified, stillborn, and (or) weak neonatal piglets. Diffuse, coalescing, nonsuppurative, and necrotizing myocarditis was identified in stillborn and weak, nonviable neonates. Immunohistochemistry identified moderate to extensive staining for PCV-2 in both myocytes and inflammatory cells in the areas of myocarditis.
Recommendations
There were two areas of concern: treatment of on-farm animals and preventive measures concerning incoming animals. In the short term, deliveries of gilts from Facility A were discontinued. In the meantime, gilts from Facility A were exposed to tissues from affected fetuses and (or) piglets.
Outcome
The problem resolved spontaneously in gilts in Unit 1, even though they had not been exposed to infected tissues on entry. Reproductive performances in gilts that remained in inventory, and that had originated from both sources, reached expected levels.
What happened?
It is our belief that PCV-2 was the agent responsible for these reproductive problems, with the source of infection in the nucleus breeding herd. The segregated early weaning process produced a population of colostrum-protected pigs in Facility A. Until their arrival at the breeding operation, these pigs had not been exposed to PCV-2 and had not developed an active immunity. Gilts from Facility B acted as a source of infection for these naïve animals, which became infected during gestation. It is possible that the problem resolved spontaneously when the PCV-2 began to circulate in the facility A nursery of the gilt grow-out barn.
Why was the problem not as severe in Unit 2?
Only 10% of gilts in Unit 2 were from Facility A, so, although a similar reproductive problem did exist, it was not of the same magnitude. A high percentage of affected gilts were of Facility A origin.
This case highlights the potential for swine pathogens to express themselves in new ways as our production systems change. It is also an example of problems that can occur in newly established herds that receive animals from more than one source.
— Gaylan Josephson, DVM, Dipl Path,
Animal Health Laboratory, University of
Guelph, Guelph, Ontario
— George Charbonneau, DVM,
Stratford, Ontario