Kelly JA. 2001;5:207-213 Analysis of Lightning and BioClean test
Original research
Peer reviewed
Analysis of Lightning and BioClean tests for assessment of sanitation levels in pork production facilities
Jason A. Kelly; Sandra F. Amass, DVM, PhD, Dipl ABVP; Darryl Ragland, DVM, PhD; Pat M. Spicer PhD; Roberta M. Alvarez, DVM
JAK, SFA, DR, RMA: Department of Veterinary Clinical Sciences, Purdue University School of Veterinary Medicine, 1248 Lynn Hall, West Lafayette, Indiana 47907-1248; PMS: Department of Natural Resources and Environment, Box 3100 Bendigo Delivery Centre, Bendigo, Victoria, Australia, 3554
Kelly JA, Amass SF, Ragland D, et al. Analysis of Lightning and BioClean tests for assessment of sanitation levels in pork production facilities. J Swine Health Prod. 2001;9(5):207-213. Also available as a PDF
Summary
Objectives: To determine the test sensitivity and specificity of Lightning (BioControl Systems, Inc, Bellevue, Washington) and BioClean (BioVet, St Anthony, Minnesota) testing systems in determining whether various surfaces in pork production facilities have met the standards for disinfection; and to identify factors in pork production units which may interfere with the test sensitivity and specificity of Lightning or BioClean test results.
Methods: Swab samples were collected from feeders, flooring, and walls in a wean-finish room and a nursery room of a 1600-sow, farrow-to-finish commercial operation. In both facilities, three adjacent swab samples of a 6.16 cm2 (0.955 in2) area were collected at each sampling site and analyzed using Lightning, BioClean, and cultural examination for bacteria. The test sensitivity and specificity of Lightning and BioClean tests were calculated for each surface using cultural examination as the "gold standard" for classifying a sample as clean or contaminated. Factors such as feed, manure, and disinfectant residues were tested to determine if they interfered with the test sensitivity and specificity of Lightning or BioClean tests.
Results: Lightning tests were generally highly sensitive but had low specificity. BioClean tests were generally highly specific but had low sensitivity.
Implications: Lightning and BioClean testing systems are not recommended for use in evaluating sanitation levels on swine farms without prior, independent, on-farm validation. Caution should be exercised when transferring technologies from other industries to pork production.
Keywords:  swine, Lightning,
BioClean, sanitation, disinfectants
swine, Lightning,
BioClean, sanitation, disinfectants
Received: October 31, 2000
Accepted: May 9, 2001
All in-all out management of swine confinement units is strongly recommended to maintain herd health and increase profitability. An essential step for implementation of all in-all out pig flow is the cleaning and disinfection of rooms between pig groups to prevent exposureto pathogens shed by the previous groups.1,2,3,4 Recommendations include pressure washing the room and its equipment with hot water and a detergent to remove organic matter, and then disinfecting with a suitable product.5,67 Consequently, in most cases, an outbreak of neonatal scours or other costly disease must occur before producers and veterinarians reevaluate cleaning methods and disinfectants used. Currently, producers do not have an objective, rapid, proactive method to evaluatethe final cleanliness of the room or the disinfectant’s efficacy in inactivating pathogens enzootic to the farm.
Rapid tests to evaluate sanitation levels have not been developed specifically for use on farms. However, two testing systems, Lightning (BioControl Systems, Inc, Bellevue, Washington) and BioClean (BioVet, St Anthony, Minnesota) were developedfor monitoring food safety in processing plants.8,9 The Lightning system has recently been used without validation for assessing cleanliness of swine transport vehicles.10,11
The Lightning test method quantifies residualadenosine triphosphate (ATP) on surfaces using a luminometer and a swab sample.12 Adenosine triphosphate is found in most food residues and bacteria. Lightning theoretically correlates increased levels of ATP with increased contamination of surfaces. The Lightning test displays a score 11 seconds after the surface sample is obtained. Each test costs approximately US $2.78 after the initial capital outlay for the luminometer.
BioClean is a swab test that uses a color- changing reaction to quantitate protein levels on surfaces as an indicator of food residue. Increasing amounts of protein yield a more intense color change.8 Theoretically, surfaces with a high level of bacterial contamination have a greater protein content. The BioClean test can be prepared in approximately 1 minute and results are obtained within 20 minutes after sampling. Each BioClean test costs approximately US $2.16.
The overall objective of the study was to determine if Lightning or BioClean tests were valid, rapid test methods to assess the efficacy of disinfection in pork production facilities. The specific objectives were to evaluate the test sensitivity and specificity of Lightning and BioClean testing systems in determining whether various surfaces in pork production facilities have met the standards for disinfection,1 and to identify factors in pork production units which may interfere with the test sensitivity and specificity of Lightning or BioClean test results.
Our working hypothesis was that the test sensitivity and specificity of these tests would vary according to the sampled surface.
Materials and methods
Experiment One: Determination of sensitivity and specficity
of Lightning and BioClean tests on surfaces in
swine facilities
Study Design
Swab samples were collected from feeders, flooring, and walls in a wean-finish room and a nursery room of a 1600-sow, farrow-to-finish commercial operation. A 26-pen wean-finish room was emptied of pigs, cleaned, disinfected with a phenolic disinfectant(Triphenol-R/256; ID Russell Company Laboratories, Longmont, Colorado), and allowed to dry for 1 day. Each pen in the facility was sampled on three surfaces: stainless steel feeder trough (Farmweld, Teutopolis, Illinois), concrete slat, and concrete wall approximately 30 cm up from the floor. Pigs were also emptiedfrom a 16-pen nursery room. The room was cleaned and disinfected with Triphenol-R/256 and allowed to dry for 2 days. Samples were taken in each pen from the stainless steel feeder trough (Farmweld, Teutopolis, Illinois), plastic mesh flooring (MIK flooring, MIK Heinrich Michel, Marienhausen, Germany), and PVC plastic-covered wall (AP Livestock, Assumption, Illinois) approximately 30 cm up from the floor. A sterile metal washer with an internal surface area of 6.16-cm2 was used to standardize the sampled surface area. In both facilities, three adjacent swab samples of a 6.16-cm2 area were collected at each sampling site and analyzed using Lightning, BioClean, and cultural examination for aerobic bacteria.
Background contamination control samples
Sterile washers were exposed to facility airspacefor approximately 5 seconds at five different locations in each room to quantitate background aerosol contamination of washers that might occur during sampling. The inner surface of each washer was swabbed and analyzed using Lightning, BioClean, and cultural examination for bacteria as described below. Additionally, metal washers autoclaved in the same batch as those used for on-farm sampling were culturally examined under aseptic conditions to confirm sterility.
Bacterial plating analysis
Each surface was sampled with sterile, cotton-tipped swabs which were placed into 1-mLaliquots of 0.9% saline stored on ice packs. Original samples and serial dilutions were plated onto trypticase soy agar with 5% sheep blood (BBL Stacker Plate, Beckton Dickenson Microbiology Systems, Cockeysville, Maryland) within 5 hours of collection and incubated aerobically at 37 degrees C for 18 hours. Colonies were counted on each plate and converted to colony forming units (CFUs) per cm2 of surface sampled. The average bacterial count for the five background contamination control samples for the room was subtracted from each sample swab count. A surface was classified as either clean (<=1 CFU per cm2) or contaminated (>1 CFU per cm2).1 Plates indicating more than 487 CFUs per cm2 surface area were considered "too numerousto count" (TNTC) and classified as contaminated. Samples that detected less than the mean background contamination level were classified as sterile (0 CFUs per cm2).
Lightning analysis
The Lightning system was used according to test instructions. Briefly, each surface was sampled with a Lightning swab that consists of three basic parts. A swab, attachedto an ampule containing buffer solutionat the top, is enclosed in a sheath containing a luciferin-luciferase pellet. Buffer contained in the ampule is used to rinse the sample from the swab and mix with the luciferin-luciferase pellet to activatethe reaction. Residual ATP present on surfaces reacts with the luciferin-luciferase pellet to yield light. The Lightning luminometer measures the light output and calculates a score. Each sample was classified by the standard Lightning cut-off as either clean (score <=2.5) or contaminated (score >2.5). Alternative cut-off values (3.0, 3.5, 4.0) were applied to determine their effects on test sensitivity and specificity.
BioClean analysis
Sample tubes were prepared according to test instructions by adding 1 drop of reagent B to the tube containing reagent A. Each surface was sampled with a sterile cotton swab provided with the BioClean kit. Protein contaminants react with copper ions to form a complex with the biuret reagent that causes a color-changing reaction. The sample swab was allowed to react for 20 minutes in the tube containing the reagent mixture. The surface was classified as clean (no color change after 20 minutes) or contaminated (any color change after 20 minutes).
Data analysis
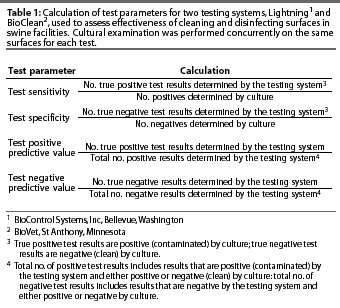 The sensitivity, specificity, positive
predictive value (PPV), and negative predictive value (NPV) of
Lightning and BioClean tests were calculated (Table 1) for each
surface, using cultural examination as the "gold standard"
for classifying a sample as truly clean or contaminated. Sensitivity
was defined as the ability of the test to detect a contaminated
surface when the surface was truly contaminated. Specificity was
defined as the ability of the test to detect a clean surface when
the surface was truly clean. The PPV was defined as the probability
of a contaminated test result corresponding to a truly contaminated
surface. The NPV was defined as the probability of a clean test
result corresponding to a truly clean surface.
The sensitivity, specificity, positive
predictive value (PPV), and negative predictive value (NPV) of
Lightning and BioClean tests were calculated (Table 1) for each
surface, using cultural examination as the "gold standard"
for classifying a sample as truly clean or contaminated. Sensitivity
was defined as the ability of the test to detect a contaminated
surface when the surface was truly contaminated. Specificity was
defined as the ability of the test to detect a clean surface when
the surface was truly clean. The PPV was defined as the probability
of a contaminated test result corresponding to a truly contaminated
surface. The NPV was defined as the probability of a clean test
result corresponding to a truly clean surface.
Experiment Two: Factors in pork production units that may interfere with sensitivity and specificity of Lightning or BioClean tests
Effect of manure residue on test results
Five manure samples were collected from a growing-finishing facility housing pigs weighing approximately 45 kg. Manure samples were diluted with sterile 0.22-µm-filtered water to a final dilution of 0.001 g manure per mL water. Each sample was divided into two equal aliquots. One aliquot was autoclaved for 20 minutes at 121 degrees C and the other remained nonautoclaved. A negative control consistedof a sterile, 0.22-µm-filtered watersample. The nonautoclaved manure samples, the autoclaved manure samples, and a negative control were analyzed using cultural examination for bacteria, Lightning, and BioClean methods.
Effect of feed residue on test results
Five grow-finish feed samples were collected. Feed samples were diluted with sterile0.22-µm-filtered water to a final dilution of 0.083 g feed per mL water. Each sample was divided into two equal aliquots. One aliquot was autoclaved for 20 minutes at 121 degrees C and the other remained nonautoclaved. A negative control consisted of a sterile, 0.22-µm-filtered water sample. The nonautoclaved feed samples, the autoclaved feed samples, and a negative control were analyzed using cultural examination for bacteria, Lightning, and BioClean tests.
Effect of disinfectant residue on test results
Disinfectant sampling was performed undercontrolled laboratory conditions to determine if disinfectant residue affected Lightning or BioClean test results. Representatives of six classes of disinfectants were used (Table 2). Clean glass slides (Esco 3"x 1" microscope slides, Erie Scientific Company, Portsmouth, New Hampshire) were submerged in disinfectant solution prepared according to label instructions in sterile, 0.22-µm-filtered water. Slides were dried in a sterile, HEPA-filtered biosafety cabinet. Dry slides were sampled using each of the three testing methods. The top one third of the slide was culturally examined for bacteria, the middle one third was sampled for the Lightning test, and the bottom one third was sampled for the BioClean test. Five replicates were performed for each disinfectant. Five control slides were submerged in sterile, 0.22-µm-filtered water and tested as above.

Effects of disinfected manure and feed on test results
Five manure samples were collected from a continuous-flow finishing facility housing pigs weighing approximately 68 kg. Manure samples were diluted in sterile, 0.22-µm-filtered water to a concentration of 0.01 g manure per mL water. Five samples of an SEW diet containing carbadox were collected and diluted in sterile, 0.22-µm-filtered water to a concentration of 0.01 g feed per mL water. To simulate contamination with organic matter and bacteria, seven slides were submerged in each sample of manure and seven slides were submerged in each sample of feed. All slides were allowedto dry in a sterile, HEPA-filtered biosafety cabinet. Then, for both the manure-coated slides and the feed-coated slides, six of the seven inoculated slides were each submerged in one of the six disinfectants (Table 2) for 5 seconds and dried a second time. The seventh slide was used as a positive control that was dried but not disinfected. Five replicates were performed for each disinfectant and positive control. A negative control slide was submerged in sterile 0.22-µm-filtered water and allowed to dry in the biosafety cabinet to confirm sterility of the water and the biosafety cabinet. All slides were evaluated using cultural examination for bacteria, Lightning, and BioClean tests. The top one third of the slide was culturally examined for bacteria, the middle one third was sampled for the Lightning test, and the bottom one third was sampled for the BioClean test.
Results
Experiment One: Determination of sensitivity and specificity of Lightning and BioClean tests on surfaces in swine facilities
Variation existed among cultural examination for bacteria, Lightning, and BioClean tests with respect to classification of surfaces as clean or contaminated (Table 3). Lightning was highly sensitive but had low specificity on stainless steel wean-finish feeders, stainless steel nursery feeders, concrete slats, plastic flooring, and concrete walls. However, sensitivity was low and specificity was high on PVC plastic-covered walls using the standard cut-off score of 2.5 to distinguish between clean and contaminated surfaces (Table 4). Incrementally increasingthe Lightning cut-off score did not improve the accuracy of test results on surfaces (Table 5). BioClean had high specificity and low sensitivity on stainless steel wean-finish feeders, stainless steel nursery feeders, concrete slats, concrete walls, and PVC plastic-covered walls. BioClean sensitivity on plastic flooring was low but exceeded specificity (Table 4).
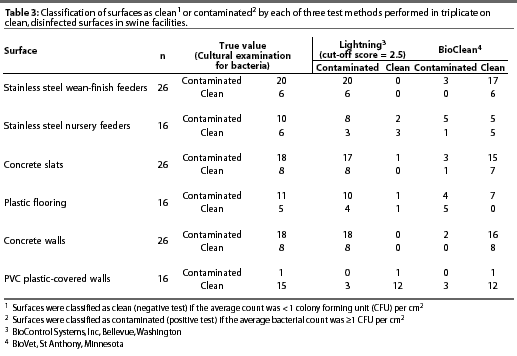
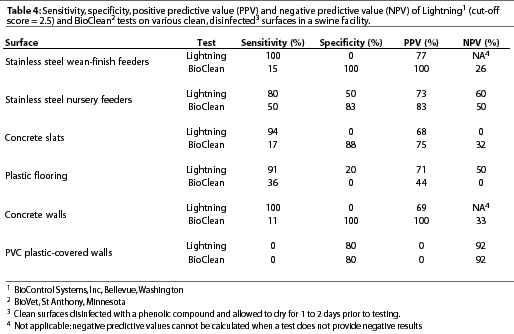
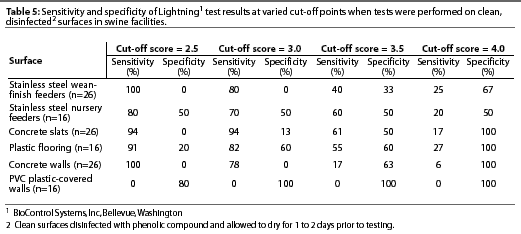
Experiment Two: Factors in pork production units that may interfere with sensitivity and specificity of Lightning or BioClean tests
Effect of manure residue on test results
Negative control: A sterile water negative control was classified as clean using all three tests.
Nonautoclaved manure: All nonautoclaved manure samples grew bacteria in numbers TNTC on cultural examination and were classified as contaminated. Lightning (cut-off score 2.5) correctly classified four of five samples (80%) as contaminated and incorrectly classified one of five samples (20%) as clean. BioClean incorrectly classified five of five samples (100%) as clean.
Autoclaved manure: All autoclaved manure samples were sterile on cultural examination and classified as clean. Lightning (cut-off score 2.5) correctly classified three of five samples (60%) as clean and incorrectly classified two of five samples (40%) as contaminated. BioClean correctly classified all autoclaved manure samples as clean.
Effect of feed residue on test results
Negative control: A sterile water negative control was classified as clean using all three tests.
Nonautoclaved feed: All nonautoclaved feed samples grew bacteria in numbers TNTC on cultural examination and were classified as contaminated. Both Lightning (cut-off score 2.5) and BioClean tests correctly classified all samples as contaminated.
Autoclaved feed: All autoclaved feed samples were sterile on cultural examination and classified as clean. Both Lightning (cut-off score 2.5) and BioClean tests incorrectlyclassified five of five autoclaved feed samples (100%) as contaminated.
Effect of disinfectant residue on test results
Thirty-five of 35 slides (100%) were sterile on cultural examination. Lightning (cut-off score 2.5) correctly classified all disinfected slides and control slides as clean. BioClean correctly classified all control slides and all slides coated with Clorox Bleach, Nolvasan solution, 1-Stroke Environ, Roccal D-Plus, and Virkon S as clean. However, BioClean incorrectly classified all slides coated with Cidex Activated Dialdehyde solution as contaminated.
Effect of disinfected manure on test results
Negative control: A negative control slide coated with sterile water was classified as clean using all three tests.
Disinfected manure: Twenty-five of 25 disinfected manure samples (100%) were sterileon cultural examination and classified as clean. Lightning (cut-off score 2.5) correctly classified as clean three of five (60%) manure-coated slides disinfected with Cidex Activated Dialdehyde solution and incorrectly classified two of five (40%) as contaminated. Lightning (cut-off score 2.5) correctly classified one of five (20%) manure-coated slides disinfected with Clorox Bleach as clean and incorrectly classified four of five (80%) as contaminated. Lightning (cut-off score 2.5) correctly classified four of five (80%) manure-coated slides disinfected with Nolvasan solution as clean and incorrectly classified one of five (20%) as contaminated. Lightning (cut-off score 2.5) correctly classified one of five (20%) manure-coated slides disinfected with 1-Stroke Environ as clean and incorrectly classified four of five (80%) as contaminated. Lightning (cut-off score 2.5) correctly classified two of five (40%) manure-coated slides disinfected with Roccal D-Plus as clean and incorrectly classified three of five (60%) as contaminated. BioClean correctly classified all disinfectedmanure samples as clean.
Effect of disinfected feed on test results
Negative controls: A control slide coated with sterile water was classified as clean using all three tests.
Disinfected feed: Twenty-five of 25 disinfected feed samples (100%) were sterile on cultural examination and classified as clean. Lightning (cut-off score 2.5) correctly classified all feed-coated slides disinfected with Cidex Activated Dialdehyde solution or Clorox Bleach as clean. Lightning (cut-off score 2.5) correctly classified two of five (40%) feed-coated slides disinfected with Nolvasan solution as clean and incorrectly classified three of five (60%) as contaminated. Lightning (cut-off score 2.5) correctly classified three of five (60%) of feed-coated slides disinfected with 1-Stroke Environ or Roccal D-Plus as clean and incorrectlyclassified two of five (40%) as contaminated. BioClean correctly classified all disinfected feed samples as clean.
Discussion
An ideal test for assessing sanitation on pork production facilities would be inexpensive, rapid, 100% sensitive, and 100% specific. Lightning and BioClean testing systems were not designed for use in pork production facilities. Lightning and BioClean technology were transferred to swine production because of the need by the industry to quickly and objectively evaluate sanitation protocols. Unfortunately, Lightning tests were recommended and implemented without prior validation for on-farm use.10,11
To compensate for the greater contamination of swine facilities compared to food processing plants, some have recommended using a higher Lightning cut-off score for classifying a surface as clean. In our study, incremental increases in cut-off scores improvedspecificity, but decreased sensitivity of Lightning for all surfaces except PVC plastic-covered walls. Therefore, altering cut-off scores did not improve overall test accuracy.
Further evaluation of Lightning under experimental conditions demonstrated that sterile organic material such as autoclaved or disinfected feed and manure residues were sometimes classified as contaminated according to the Lightning tests. Feed is composed primarily of plant products. Living cells present in the ground seed coats may contain sufficient ATP to cause false- positive Lightning results. Sterile manure residue could cause false-positive results because of ATP in fibrous seed coats, which pass through the digestive tract of the pig with little degradation. An additional source of ATP in manure could be epithelial cells sloughed from the digestive tract. Pure disinfectant residues did not affect Lightning results.
BioClean tests were recently made available for commercial use and have not been widely implemented in the pork industry. Under the conditions of this study, BioClean tests were generally highly specific for all surfaces except plastic flooring, but sensitivity was low. Both sensitivity and specificity were low for plastic flooring. Low overall BioClean sensitivity might result from inability of reagents in the kit to adequately detect small quantities of protein. An alternative explanation is that residual protein is not a good indicator of bacterial contamination. A second disadvantage of the BioClean test is cross-reaction with Cidex Activated Dialdehyde solution,an aldehyde disinfectant, resulting in the classification of sterile solutions of pure disinfectant as contaminated. However, false classification did not occur when Cidex Activated Dialdehyde solution was used to disinfect feed or manure samples. One explanation is that the organic material bound to or reacted with the residue in Cidex that caused false-positive reactions. Other classes of disinfectants tested did not affect BioClean test results.
In conclusion, the low test specificity of Lightning and the low test sensitivity of BioClean tests resulted in an inability to accurately assess the efficacy of disinfection in swine production facilities under the conditions of this study. This experiment took place in two rooms of a single commercial pork production facility. The walls, equipment, and flooring of this facility may not represent those on other swine farms. The investigators encourage practitioners and producers to validate Lightning and BioClean testing systems in their own facilities before implementing biosecurity programs utilizing these testing methods. Future research should focus on validating additional rapid testing systems used by other industries to determine whether they are applicable to the pork industry. Development of rapid testing systems designed for use in the pork industry should be considered if technology transfer from other industries is not possible.
Implications
- Lightning and BioClean testing systems are not recommended for use in evaluating sanitation on swine farms unless prior, independent, on-farmvalidation is performed.
- Caution should be exercised when transferring technologies from other industries to pork production.
- When evaluated on three different surface types in a wean-finish building and a nursery, under the conditions of this study, Lightning tests were generally highly sensitive but specificity was low when used on stainless steel feeders, concrete slats, and plastic flooring, and Lightning tests were highly specific but had low sensitivity when used on PVC plastic-covered walls.
- When evaluated on three different surface types in a wean-finish building and a nursery, under the conditions of this study, BioClean tests were highly specific, but sensitivity was low.
- Under the conditions of this study, Lightning tests classified sterile organic material such as autoclaved or disinfected feed and manure residues as contaminated, and BioClean tests cross-reacted with an aldehyde disinfectant resulting in false classification of sterile disinfectant solutions as contaminated.
Acknowledgements
Support for this research was provided by the National Pork Producers Council.
References — refereed
1. Tamasi G. Testing disinfectants for efficacy. Rev Sci Tech. 1995;14:75-79.
2. Quinn PJ. Disinfection and disease prevention in veterinary medicine. In: Block, SS. Disinfection, sterilization, and preservation. 4th ed. Philadelphia: Lea and Febiger; 1991:846-868.
3. Fotheringham VJC. Disinfection of livestock production premises. Rev Sci Tech. 1995;14:191-205.
4. Owen JM. Disinfection of farrowing pens. Rev Sci Tech. 1995;14:381-391.
5. Bruins G, Dyer JA. Environmental considerations of disinfectants used in agriculture. Rev Sci Tech. 1995;14:81-94.
7. Kennedy MA, Mellon VS, Caldwell G, Potgieter LND. Virucidal efficacy of the newer quaternary ammonium compounds. JAVMA. 1995;31:254-257.
12. Ford SR, Chenault KD, Hall MS, Pangburn SJ, Leach FR. Effect of periodate-oxidized ATP and
other nucleotides on firefly luciferase. Arch Biochem Phys. 1994;314:261-267.
References — non refereed
6. Thompson, R. Transportation cleaning and disinfection. Swine Health Fact Sheet. Jan. 2000; 2(2).
8. BioClean qualitative control of surface cleanliness: Your surfaces are clean or Bioclean? (Biovet, St Anthony, Minnesota)
9. Lightning Luminometer Operator’s Manual. Westbrook, Maine: IDEXX Laboratories, Inc; 35.
10. Faust, C. The determination of surface levels of ATP as a biosecurity measure. Proc AASP. Quebec City, Quebec. 1997;301-311.
11. Dee, SA. An overview of methods for measuring the impact of sanitation procedures for swine transport vehicles. Swine Health Fact Sheet. November 1999;1(2).