Claviceps species are types of fungi that may infect grasses and cereal grains (eg, rye, wheat, barley, and oats) during flowering by invading the plant ovary to produce sclerotia, or ergots, that replace the seed. The sclerotium is a life stage of the fungus that may produce various levels of mycotoxins called ergot alkaloids (EA). When the grains are harvested these ergots containing the toxic EA contaminate the final product. The type and quantity of EA produced by sclerotia can vary, but levels of EA are generally proportional to the quantity of sclerotia.
Canadian harvest sampling by the Canadian Grain Commission reported the highest incidence of EA in rye, then wheat, followed by barley and oats.1 These EA act as noradrenaline, dopamine, and serotonin agonists with various toxic effects. In the lactating and periparturient sow, EA inhibit the secretion of prolactin by binding the lactotrophs in the pituitary and activating D2 dopamine receptors. Vasoconstriction is caused by agonist activity that varies by vascular bed where the EA are alpha two receptor agonists that cause constriction, especially in peripheral arterioles.2
While ergotism is the earliest documented mycotoxicosis as a common cause of gangrene in people in the Middle Ages,3 its relevance to animal agriculture may be increasing. Some data indicate that the incidence of ergot in grains in western Canada is on the rise. For example, the detected incidence of EA in durum wheat samples from 1995 to 2009 was 2.9% compared to 13.1% from 2010 to 2022. However, the levels of EA detected in the samples remained similar across the two periods.1 Levels of ergot may be influenced by farming methods and changing weather patterns associated with climate change, especially given that growing conditions for Claviceps species are favorable during flowering periods with extended moisture.4 Organic farming, while not studied specifically, may predispose cereal grains to ergot contamination. Given that current varieties tend to generate lower yields under organic conditions, they may also have an altered response to fungal growth under such conditions.5
Most research on the impact of EA on sows is in an experimental setting and is constrained in duration and timing of feeding by ethical guidelines because of the predicted impact on piglet mortality due to starvation. There are few cases reported in the literature, with the documented cases occurring in different geographies where the fungal species and EA profile could vary compared to US herds.6,7 Hence, documentation of cases is needed to generate more evidence for the impact on lactating sows. Because the evidence for the impact of differing EA levels in sows is limited, agencies like the European Food Safety Authority (EFSA) Panel on Contaminants in the Food Chain generated the 2022 reference point for EA levels in pig feed based on data primarily derived from growing swine. More information is needed, especially in lactating sows, to determine a no‐observed‐adverse‐effect level (NOAEL).8 Inconsistent recommendations come from the mismatch between controlled toxicology studies and the levels from field exposure reported to have an impact.4 Further understanding of the risks of feeding EA contaminated small grains is needed to prevent toxic levels of exposure.
In this case report, we share the level of EA and duration of exposure found under field conditions in a herd that experienced a high neonatal mortality event consequent to agalactia in the sows. Our aims are to offer practical information for including EA toxicity on the differential list for veterinarians and offer suggestions on practical methods for prevention that should be discussed with feed suppliers.
Case description
Farm description
The affected farm was a 230-sow, farrow-to-feeder herd located in the eastern United States. The farm batch farrowed approximately 20 sows every other week and met the US Department of Agriculture’s organic standards for swine production.9 Every other Friday, typically 3 to 9 days prior to their farrowing date, sows were loaded into two farrowing rooms, each of which contained 10 individual farrowing pens with no farrowing crate. All diets were prepared by a commercial feed mill and developed by a swine nutritionist to meet NRC standards.10 During gestation, the sows were fed an organic gestation diet. Once they entered the farrowing rooms, sows were fed an organic lactation diet. The sows were typically weaned 5 weeks later to meet the organic standard weaning age of at least 28 days (mean [SD] pig age was 34 [2.3] days at weaning). Room temperatures ranged from 12.6°C to 26.2°C during October 2022, the month of the outbreak.
Case presentation
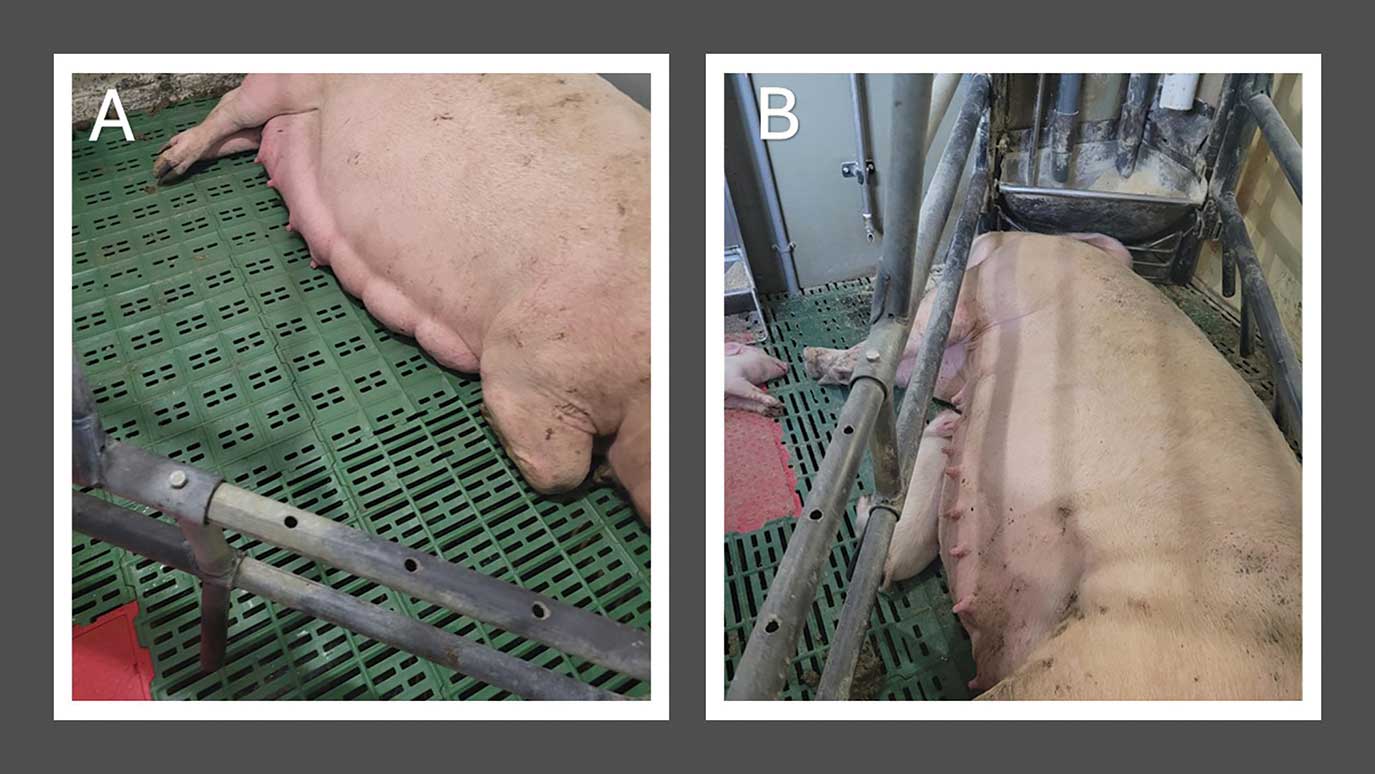
The suspected contaminated feed delivery occurred on October 5, 2022 (Table 1). The first clinical sign reported on October 11, 2022 was loss of udder development in group 4, which were placed in the farrowing rooms on October 7 (Table 2). Udder development also did not occur in those sows after farrowing (Figure 1). In addition, caretakers noted loss of udder development resulting in piglets falling behind in group 3, which had farrowed 2 to 8 days prior to the feed delivery (Table 2). Group 2, which had been loaded into farrowing 26 days prior to feed delivery and had piglets that were 17 to 22 days old, did not show any clinical signs (Table 2). While feed intake was not recorded, farm staff reported that sows had decreased feed intake and increased feed refusal during this period. Piglets appeared agitated and were frequently seen at the udder trying to nurse. There was no report of piglet diarrhea.
| Event date | Day | Event |
|---|---|---|
| Wednesday, October 5, 2022 | 0 | Organic lactation feed delivery |
| Tuesday, October 11, 2022 | 6 | Clinical signs reported |
| Monday, October 17, 2022 | 12 | Diagnostic samples taken |
| Wednesday, October 19, 2022 | 14 | New feed to groups in farrowing |
| Monday, October 31, 2022 | 26 | Contaminated feed removed |
| Tuesday, November 1, 2022 | 27 | Feed analysis results back |
| Monday, November 7, 2022 | 33 | Re-order organic feed |
| Farrowing group | No. of sows | Rooms | Loading date | First farrowing | Last farrowing | Wean date | Max exposure*, d | Pig age at feed delivery, d | Pig age at feed replacement†, d | Mortality, median (IQR), % |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 15 | 1, 6 | 8/26/22 | 8/31/22 | 9/6/22 | 10/6/22 | 1 | 29 to 35 | Weaned | 23.1 (17) |
| 2 | 19 | 2, 3 | 9/9/22 | 9/13/22 | 9/18/22 | 10/20/22 | 14 | 17 to 22 | 31 to 36 | 17.6 (14) |
| 3 | 20 | 4, 5 | 9/23/22 | 9/27/22 | 10/3/22 | 11/3/22 | 14 | 2 to 8 | 16 to 22 | 60.0 (37) |
| 4 | 19 | 1, 6 | 10/7/22 | 10/11/22 | 10/17/22 | 11/17/22 | 12 | -4 to -10 | 2 to 5 | 100.0 (11) |
| 5 | 19 | 2, 3 | 10/21/22 | 10/23/22 | 10/31/22 | 12/1/22 | 0 | Not in farrowing | Not in farrowing | 13.3 (32) |
| 6 | 19 | 4, 5 | 11/3/22 | 11/6/22 | 11/15/22 | 12/14/22 | 0 | Not in farrowing | Not in farrowing | 24.3 (26) |
* The maximum exposure is the maximum number of days the sows could have consumed the feed delivered on October 5th which tested positive for ergot alkaloids.
† The pig age when the feed was replaced represents the range of ages for the piglets when the sows were fed a new feed that was not from the contaminated batch.
The feed was switched to an alternate feed on October 19, 2022. This resulted in an exposure window of 12 to 14 days based on the feed delivery date, the onset of symptoms, and the replacement of the feed to the affected groups (Tables 1 and 2). Subsequently, farrowing groups 3 and 4 that were exposed to the contaminated feed either prior to farrowing or within 8 days of farrowing, experienced increased piglet mortality (Tables 3 and 4). No sows were fed the contaminated feed after the 14 days of exposure (Table 1).
Differential diagnoses
Given that primary agalactia was the clinical diagnosis, ergotism would be on the differential list. However, agalactia in sows could be related to other estrogenic factors such as zearalenone, bacterial infections of the mammary gland, or mastitis-metritis-agalactia syndrome.11 Differentials for anorexia in sows would include other mycotoxins, such as Deoxynivalenol,11 as well as other causes of systemic illness.
Production Effects
Production records were gathered for 6 farrowing groups: group 1 was weaned the day after delivery of the suspected contaminated feed; group 2 farrowed at least 17 days before delivery of the contaminated feed; group 3 farrowed 2 to 8 days before delivery of the contaminated feed; and group 4 consumed the contaminated feed for up to 10 days prior to farrowing and 5 days after farrowing (Table 2). While group 2 consumed the contaminated feed for up to 14 days, there was no impact on mortality for that group (Table 2), so it was combined with groups 1, 5, and 6 to represent groups that had not been affected. None of the production variables were normally distributed and data were reported as medians and interquartile ranges (IQR). To look for differences between medians, a Mann Whitney U-test was done and P < .05 was treated as significant and P < .10 was considered a trend. The mortality data compared the percentages of each cause of death in groups 1, 2, 5, and 6 to affected litters in groups 3 and 4. The mortality for each litter was categorized on the farm as total mortality, low viability, laid on, starved, and euthanized. There was no difference in the percentage of piglets laid on (P > .05). However, there was a significant difference in the percentage of total mortality, as well as the percentages of low viability, starvation, and euthanasia in the exposed litters (P < .001; Table 3). The production data gathered on the litters included the number of liveborn, stillborn, and mummies, litter birth weight, and number weaned. There was no difference in the number of liveborn, stillborn, or mummies per litter (P > .05). There was a trend for lower birth weight in the exposed litters (P = .06) and a significant difference in the number of pigs weaned in the exposed litters (P < .001; Table 4).
| Groups 3 and 4* | Groups 1, 2, 5, and 6† | P | |
|---|---|---|---|
| No. of litters | 39 | 75 | NA |
| Total mortality, median (IQR), % | 79 (50) | 20 (20) | < .001 |
| Low viability, median (IQR), % | 0 (21) | 0 (0) | < .001 |
| Laid on, median (IQR), % | 7.7 (0.2) | 8.3 (0.2) | .76 |
| Starved, median (IQR), % | 17.6 (29.4) | 0 (0) | < .001 |
| Euthanized, median (IQR), % | 26.3 (21.0) | 5.9 (13.3) | < .001 |
* Group 3 farrowed 2-8 days before delivery of the contaminated feed and group 4 consumed the feed for up to 10 days prior to farrowing and 5 days after farrowing.
† Group 1 was weaned the day after delivery of the suspected contaminated feed, group 2 farrowed at least 17 days before delivery of the contaminated feed, and groups 5 and 6 were never fed the contaminated feed.
| Groups 3 and 4* | Groups 1, 2, 5, and 6† | P | |
| Liveborn, median (IQR), No. | 15.0 (5.0) | 16.0 (4.0) | .39 |
| Stillborn, median (IQR), No. | 1 (1.0) | 0 (1.0) | .17 |
| Mummies, median (IQR), No. | 0 (0) | 0 (1.0) | .35 |
| Birth weight, median (IQR), kg | 18.4 (5.9) | 19.8 (6.2) | .06 |
| Weaned, median (IQR), No. | 3 (8) | 12 (3) | < .001 |
* Group 3 farrowed 2-8 days before delivery of the contaminated feed and group 4 consumed the feed for up to 10 days prior to farrowing and 5 days after farrowing.
† Group 1 was weaned the day after delivery of the suspected contaminated feed, group 2 farrowed at least 17 days before delivery of the contaminated feed, and groups 5 and 6 were never fed the contaminated feed.
The data collected on sow outcomes included the date of the next detected estrus, whether the next breeding was successful (ie, resulted in farrowing), removal from the herd before the next breeding, and the size of the next litter. None of the variables were normally distributed and were reported as median and IQR. Differences in medians were calculated using the Mann Whitney U-test. To look for a relationship between the categorical variables of exposure to ergot and farrowing or removal, the Fisher’s Exact test was used. The wean-to-estrus interval was significantly shorter in groups 1, 2, 5, and 6 (5 [1] days) compared to the affected sows in groups 3 and 4 (8.5 [25.0] days; P = .02). The odds of successful breeding were not different in the affected sows (74.1% farrowed) compared to the unaffected sows (84.1% farrowed; P = .38). The odds of removal before next breeding were not different between affected sows (30.8% removed) and unaffected sows (17.3% removed; P = .15). The size of the next litter weaned was no different between the affected sows (12 [2.5]) and the unaffected sows (11 [3]; P = .28).
Diagnostic test results
Fresh and formalin-fixed tissues from four 3-day-old (group 3) and four 2-week-old (group 4) piglets were submitted to the Iowa State University Veterinary Diagnostic Laboratory (ISU VDL; Ames, Iowa) on October 17, 2022. Tissues submitted included lung, heart, liver, kidney, spleen, small intestine, and colon. Oral fluids were collected from individual sows in groups 3 and 4 and pooled by group. Grossly, piglets of both ages were severely underweight and their stomachs empty, although there was some digesta in the cecum and spiral colon. Histopathology on the lung, heart, liver, kidney, spleen, intestine, and colon was unremarkable. Bacterial cultures on the 2-week-old pigs revealed moderate numbers of smooth Clostridium perfringens and low numbers of smooth mucoid Escherichia coli in the colon, and moderate numbers of smooth mucoid E coli in the intestine. Two pools of piglet feces, one for the 3-day-old pigs and one for the 2-week-old pigs tested negative by polymerase chain reaction (PCR) for rotavirus groups A and B and Sapovirus (cycle threshold [Ct] ≥ 37). The pool of feces from the 3-day-old piglets tested positive for rotavirus group C (Ct = 35.5). Oral fluids from both groups of sows tested negative by PCR for both North American and European Union porcine reproductive and respiratory syndrome virus strains (Ct ≥ 37) and for influenza A virus (Ct ≥ 38).
Feed analysis
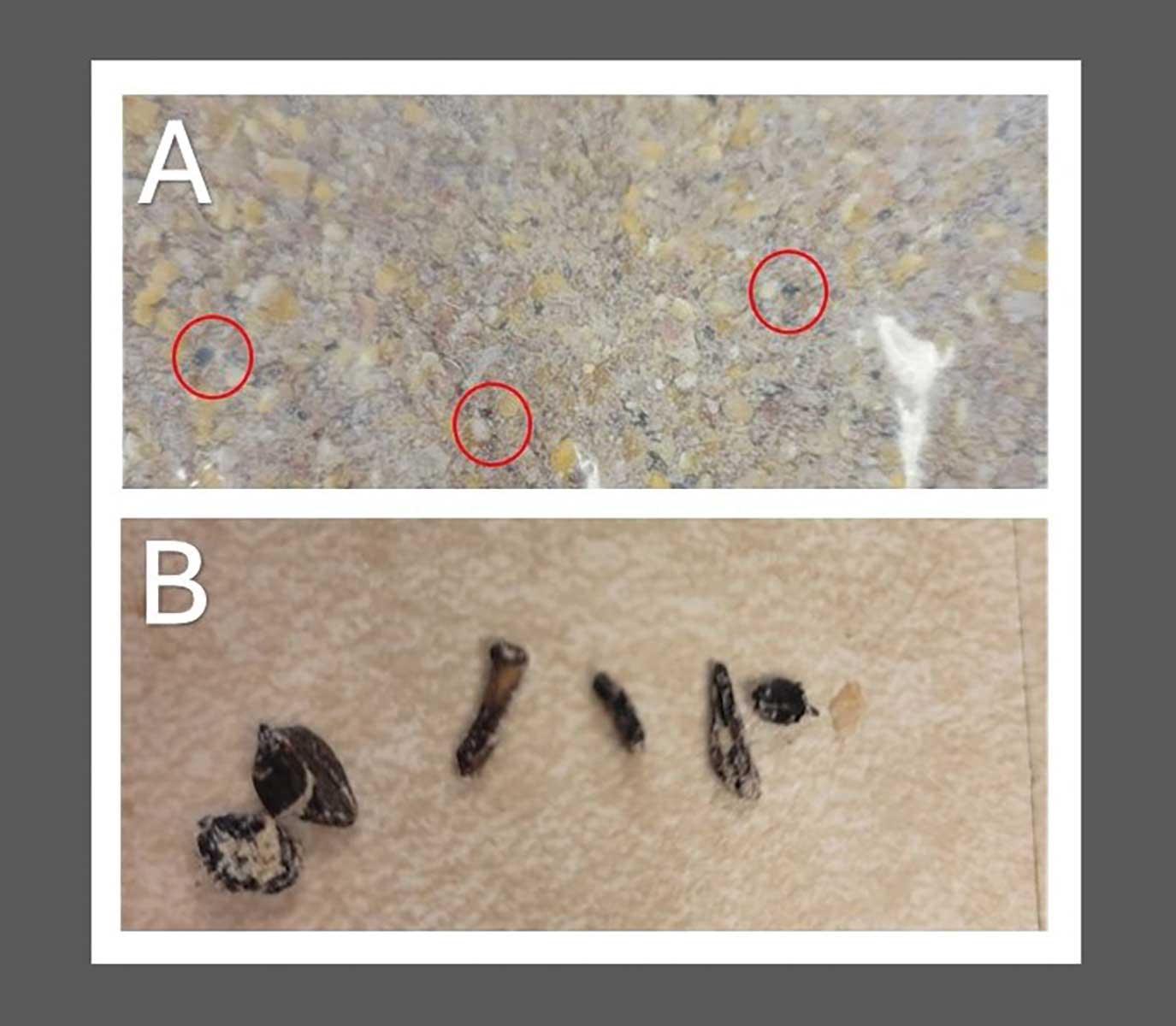
Visual inspection of the lactation diet revealed black material consistent with, but not definitive for, the presence of fragmented ergot sclerotia (Figure 2). Complete feeds taken from the feed carts for the gestation and lactation diet were submitted to the ISU VDL analytical chemistry service for mycotoxin screening by liquid chromatography and tandem mass spectroscopy and an ergopeptine panel using high performance liquid chromatography (HPLC). The HPLC included the six main EA defined by EFSA: ergovaline, ergosine, ergotamine, ergocornine, ergocryptine, and ergocristine.12 Ergovaline was 100 ppb and ergotamine was 230 ppb, for a total of 330 ppb, or 0.33 mg EA per kg of feed, in the lactation diet. However, EA was undetectable in the gestation diet (Table 5). Retained samples from the feed mill of the lactation diet mixed for this farm were tested at Trilogy Analytical Laboratory (Washington, Missouri) for five of the six EA, and none were detectable by HPLC (< 25 ppb; Table 5). It is unknown whether this retained sample was representative of the feed fed to the exposed groups at the farm. In discussions with the feed mill, it was revealed that the lactation feed did contain wheat despite its absence on the feed ticket ingredient list. The wheat was never tested for EA or visually examined or screened for the presence of ergot sclerotia. Deoxynivalenol was not found at levels consistent with anorexia in swine, 2 to 8 ppm (Table 5).11 Neither Zearlenone nor α-Zearalenol were detectable in the samples (Table 5).
| Analyte | Gestation feed* | Lactation feed* | Retained lactation feed† | Retained gestation feed† |
|---|---|---|---|---|
| Ergovaline | NDA < 50 | 100 ppb | Not included | Not included |
| Ergosine | NDA < 50 | NDA < 50 | NDA < 25 | NDA < 25 |
| Ergotamine | NDA < 50 | 230 ppb | NDA < 25 | NDA < 25 |
| Ergocornine | NDA < 50 | NDA < 50 | NDA < 25 | NDA < 25 |
| Ergocryptine | NDA < 50 | NDA < 50 | NDA < 25 | NDA < 25 |
| Ergocristine | NDA < 50 | NDA < 50 | NDA < 25 | NDA < 25 |
| Aflatoxin B1 | < 10 ppb | < 10 ppb | ||
| Aflatoxin B2 | < 10 ppb | < 10 ppb | ||
| Aflatoxin G1 | < 10 ppb | < 10 ppb | ||
| Aflatoxin G2 | < 10 ppb | < 10 ppb | ||
| Deoxynivalenol | 0.2 ppm | 0.4 ppm | ||
| 3-Acetyl Deoxynivalenol | < 10 ppb | < 10 ppb | ||
| Fumonisin B1 | 0.3 ppm | 0.9 ppm | ||
| Fumonisin B2 | < 0.2 ppm | < 0.2 ppm | ||
| Ochratoxin A | < 10 ppb | < 10 ppb | ||
| T-2 | < 10 ppb | < 10 ppb | ||
| HT-2 | < 10 ppb | < 10 ppb | ||
| Zearalenone | < 0.2 ppm | < 0.2 ppm | ||
| α-Zearalenol | < 200 ppb | < 200 ppb |
* Feed samples submitted by the farm were tested at the Iowa State University Analytical Chemistry Laboratory, Ames Iowa.
† Retained feed samples submitted by the feed mill were tested at the Trilogy Analytical Laboratory, Washington Missouri.
NDA = Nondetectable amount.
Diagnosis and treatment
Diagnosis of primary agalactia due to ergotism was based on findings of EA in the diet (Table 5) and absence of other pathogens or toxins to explain the acute rise in agalactia and piglet mortality. Removal of the contaminated feed also supported the diagnosis as groups 5 and 6 did not show an increase in mortality when the feed was changed (Table 2). However, changing the feed did not induce early lactating sows in the exposed groups to resume or commence milk production. Twenty-eight of thirty-nine litters in the exposed group were treated with First Formula (IMPRO products) at a dose of 1mL/pig. This oral liquid supplement containing whey solubles is approved for use as a nutritional supplement in organic herds and is intended to improve gut health but is not a substitution for caloric intake.
Discussion
Different species of Claviceps may have different clinical effects depending on the profile of EA production for the species. A controlled trial by Abdelrahim et al13 found that Claviceps africana in sorghum fed to sows at 0, 11.75, and 23.5 mg of EA per kg of feed at two ill-defined periods showed no influence on lactation performance. This is in contradiction to all other studies. Kopinski et al4 fed C africana contaminated sorghum at 16 mg EA per kg of feed to sows from 14 to 28 days post farrowing and saw decreased feed intake, even with the inclusion of flavoring, decreased serum prolactin in the sows, and decreased piglet weight gain. When C africana contaminated sorghum was fed from 1.4 to 7 mg EA per kg of feed to sows before farrowing, they found an impact on udder development and prolactin levels in all groups, especially parity one animals.15 Though our sows were likely exposed to Claviceps purpurea, our results are consistent with those studies that showed a production impact of EAs from C africana.
Studies on C purpurea are rarer. The EFSA suggests feeding 0.6 mg EA per kg of feed or less is acceptable based on the available evidence, mainly in growing pigs, citing only four studies available in sows, including three that were published in 1945, 1972, and 1986 when EA quantification was done on a dry matter basis.16-18 Our results with feeding EA at 0.33 mg EA per kg of feed, if representative of the concentration in the rest of the feed, were lower than those reported in the literature to cause agalactia for either C purpurea or C africana.
Though the levels of EA found in this case were lower than those reported previously, the clinical presentation was consistent with the literature in both field studies as well as controlled feeding trials. A 160-sow, farrow-to-finish herd in France was exposed to wheat containing C purpurea sclerotia resulting in 3.49 mg of EA per kg of feed for 10 to 15 days at the end of gestation and 8.05 mg of EA per kg of feed in lactation. This resulted in piglet mortality ranging from 23% to 100% of the litter in 13 of 20 sows fed the contaminated feed.7 Kopinski et al14 had 87% piglet mortality when sows were exposed to C africana. Blaney et al6 documented multiple farms exposed to C africana and saw feed refusal and agalactia in sows resulting in piglet losses from a portion of the litter to the whole litter on all the farms examined. In this case, the group of sows fed contaminated feed after their piglets were at least 17 days of age (group 2), saw no difference in mortality from typical groups on this farm. Since 18 to 25 days of age is a typical weaning age, it may be that these sows did experience agalactia, but the piglets were able to cope with the decreased milk intake at that age.
These different species of Claviceps may contain different EA and therefore these comparisons should be interpreted accordingly. We found piglet mortality comparable to previous studies, although none of the other studies reported piglet mortality reasons.6,7,14 When we analyzed the causes of piglet mortality, we saw a difference in piglet mortality reasons (low viability, starvation, and euthanasia) in groups where piglets were less than 8 days of age when exposure started, consistent sequelae to agalactia in the sows.
There were no clinical signs indicative of gangrene in any of the sow exposures documented in the literature, nor were there any in this case. Such signs may develop after long term exposure and are generally associated with exposure to EA for up to 3 months.4 Conditions, such as cold temperatures that favor vasoconstriction,2 would increase the likelihood of gangrene but would be unlikely to occur in traditional farrowing rooms in indoor housed sows. Such temperatures were not found in this study, room temperatures during the outbreak were within to slightly above the thermoneutral zone for sows (10°C-25°C).19
Evidence is mixed for the impact of EA on conception rate and has only been examined in a controlled setting where animals were exposed to C africana. Kopinski et al14 saw decreased litter size in subsequent litters in sows exposed during lactation. In contrast, there was no difference in the number of corpus lutea or embryos in the gilts exposed to EA contaminated sorghum (0, 5, 10 mg/kg of feed) during the growing phase.20 Consistent with that finding, we did not see an impact on the size of the subsequent litter or the likelihood of the next breeding resulting in a successful farrowing. We did find a prolonged wean-to-estrus interval. This longer interval could be due to the sows cycling while in the farrowing room because they were not nursing, and then were out of sync with the rest of the sow group. This could be exacerbated by batch farrowing systems where groups are not weaned weekly, as was done on this farm.
The only known treatment for EA toxicity is removal of the contaminated feed and replacement with a diet with EA below the NOAEL. In this case, the feed was replaced and symptoms did not occur in the following groups, but those sows with severe agalactia did not resume milk production and their litters suffered high mortality. There is little evidence in the literature for other treatments. Kopinski et al14 attempted to cross foster piglets and use milk replacer in affected litters but piglet mortality was still 87%.14 We treated piglets with a product containing whey solubles but this product is not meant to substitute caloric intake, thus we still saw 79% mortality. In Kopinski et al15 they switched some sows that had received contaminated feed prefarrowing to the uncontaminated diet at farrowing and saw no detrimental impacts of EA on those litters, demonstrating a quick response to toxin removal prefarrowing. In this case, there were no groups that ate the contaminated feed and then had it removed prior to parturition. However, one study concluded that sows returned to normal milk production in 3 to 7 days after toxin removal, though this finding was not specific to the stage of lactation at which the sow was exposed.11 This was inconsistent with the return to milk production observed in this case where sows exposed to EAs between 0 to 10 days prior to parturition and 2 to 22 days after farrowing showed no return to milk production. This could be due to the stage of lactation at which the sows were exposed, preventing udder development or redevelopment, or the lack of viable piglets remaining to stimulate a return to milk production.
Given the severity of EA impact, especially on periparturient and lactating sows, special consideration should be given to preventing EA contamination of diets fed to these animals. If cereal grains, such as rye, wheat, sorghum, barley, or oats, are included in a diet fed to sows before and during lactation, visual screening could be a low-cost way to prevent EA contamination. Coufal-Majewski et al4 recommended counting and weighing the sclerotia after screening high-risk ingredients and that more than 5 sclerotia per liter of grain or 0.1% to 0.3% of grain on a dry matter basis is enough contamination that it should not be fed to pregnant or lactating animals. Based on the low levels found in this case, finding sclerotia or suspected sclerotia would warrant further testing using HPLC to test the EA concentration in the ingredient before its inclusion in a sow lactation diet.
If such ingredients are going to be fed to lactating sows, screening or physical removal can be effective but may be challenging if sclerotia are broken and therefore of similar size to the grains.10 This is more likely if grain byproducts are being used, which could result in more broken sclerotia. Chemical binders exist for Deoxynivalenol and other mycotoxins, but more testing is needed to determine their effectiveness for EA.4 Likewise, Mainka et al21 indicated that steam treatment reduced total EA in the feed, but this has not been tested in the field to determine how to implement this technique at varying levels of contamination. Current information would suggest that the safest response to EA contamination of a feedstuff is to avoid feeding it to periparturient or lactating sows and to feed with caution to growing swine using the EFSA guidelines or other science-based recommendations.
More research is needed to determine the safe level of EA that lactating sows can tolerate as this case report is one of 2 in the literature that uses modern testing methodologies, documents field exposure to C purpurea in wheat, and reports the duration of exposure and the concentration of six common EA. Research into how organic production practices influence the likelihood of cereal grain inclusion in the diet and whether organic crop farming increases the chances for EA contamination of such grains is needed so there is a clear understanding of when the EA contamination risk is elevated. Though this farm is small and uses organic production practices, this case should be considered by practitioners when extreme agalactia resulting in high piglet losses is noted in any sow farm where the diet includes cereal grains. The feed mill should be queried about the inclusion of such ingredients as it may not be listed on the feed label. If EA contamination is suspected, multiple feed samples should be gathered from the farm, inspected visually for sclerotia, and sent for EA testing and quantification.
Implications
Under the conditions of this study:
- Severe agalactia resulted from feeding EA at .33mg/kg for 12 to 14 days.
- Lactating sow diets containing wheat should be screened for sclerotia.
- Determination of the NOAEL for EA in sows is needed.
Acknowledgments
The authors acknowledge the cooperation of the farm and the information shared from the feed mill.
Conflict of interest
None reported.
Disclaimer
Scientific manuscripts published in the Journal of Swine Health and Production are peer reviewed. However, information on medications, feed, and management techniques may be specific to the research or commercial situation presented in the manuscript. It is the responsibility of the reader to use information responsibly and in accordance with the rules and regulations governing research or the practice of veterinary medicine in their country or region.
References
1. Walkowiak S, Taylor D, Fu BX, Drul D, Pleskach K, Tittlemier SA. Ergot in Canadian cereals – relevance, occurrence, and current status. Can J Plant Pathol. 2022;44(6):793-805. https://doi.org/10.1080/07060661.2022.2077451
2. Gupta RC, Evans TJ, Nicholson SS. Ergot and Fescue Toxicoses. In: Gupta RC ed. Veterinary Toxicology. 3rd ed. Academic Press; 2018:995-1001. Accessed April 12, 2024. https://doi.org/10.1016/B978-0-12-811410-0.00070-2
3. Council for Agricultural Science and Technology. Task Force Report No. 139 Mycotoxins: Risks in plant, animal, and human systems. 2003. Accessed April 12, 2024. https://www.cast-science.org/publication/mycotoxins-risks-in-plant-animal-and-human-systems
4. Coufal-Majewski S, Stanford K, McAllister T, Blakley B, McKinnon J, Chaves AV, Wang Y. Impacts of cereal ergot in food animal production. Front Vet Sci. 2016;25:3-15. https://doi.org/10.3389/fvets.2016.00015
5. Rempelos L, Wang J, Sufar EK, Almuayrifi MSB, Knutt D, Leifert H, Leifert A, Wilkinson A, Shotton P, Hasanaliyeva G, Bilsborrow P, Wilcockson S, Volakakis N, Markellou E, Zhao B, Jones S, Iverson PO, Leifert C. Breeding bread-making wheat varieties for organic farming systems: The need to target productivity, robustness, resource use efficiency and grain quality traits. Foods. 2023;12(6):1209. https://doi.org/10.3390/foods12061209
6. Blaney BJ, McKenzie RA, Walters JR, Taylor LF, Bewg WS, Ryley MJ, Maryam R. Sorghum ergot (Claviceps africana) associated with agalactia and feed refusal in pigs and dairy cattle. Aust Vet J. 2000;78(2):102-107. https://doi.org/10.1111/j.1751-0813.2000.tb10535.x
7. Waret-Szkuta A, Larraillet L, Oswald IP, Legrand X, Guerre P, Martineau G-P. Unusual acute neonatal mortality and sow agalactia linked with ergot alkaloid contamination of feed. Porcine Health Manag. 2019;5:1-5. https://doi.org/10.1186/s40813-019-0131-z
8. EFSA Panel on Contaminants in the Food Chain. Risks for animal health related to the presence of ergot alkaloids in feed. EFSA J. 2024;22(1):e8496. https://doi.org/10.2903/j.efsa.2024.8496
9. United States Department of Agriculture Agricultural Marketing Service National Organic Program. Organic Livestock and Poultry Standards. 2023. Accessed April 12, 2024. https://www.federalregister.gov/documents/2023/11/02/2023-23726/national-organic-program-nop-organic-livestock-and-poultry-standards
10. Southern LL, Adeola O, De Lange CFM, Hill GM, Kerr BJ, Lindeman MD, Miller PS, Odle J, Stein HH, Trottier NL eds. Nutrient Requirements of Swine. 11th ed. National Research Council. 2012. Accessed April 12, 2024. https://nap.nationalacademies.org/read/13298/chapter/1
11. Ensley SM, Radke SL. Mycotoxins in Grains and Feeds. In: Zimmerman JJ, Karriker LA, Ramirez A, Schwartz KJ, Stevenson GW, Zhang J, eds. Diseases of Swine. 11th ed. John Wiley & Sons, Inc; 2019:1055-1071. https://doi.org/10.1002/9781119350927.ch69
12. Krska R, Crews C. Significance, chemistry and determination of ergot alkaloids: A review. Food Addit Contam A Chem Anal Control Expo Risk Assess. 2008;25(6):722-731. https://doi.org/10.1080/02652030701765756
13. Abdelrahim GM, Richardson RC, Gueye A. Impact of ergot infested sorghum on the reproductive performance of sows. J Anim Res Technol. 2012;1(1):1-6. https://doi.org/10.5147/jart.v1i1.102
14. Kopinski JS, Blaney BJ, Murray S-A, Downing JA. Effect of feeding sorghum ergot (Claviceps africana) to sows during mid‐lactation on plasma prolactin and litter performance. J Anim Physiol Anim Nutr (Berl). 2008;92(5):554-561. https://doi.org/10.1111/j.1439-0396.2007.00747.x
15. Kopinski JS, Blaney BJ, Downing JA, McVeigh JF, Murray S-A. Feeding sorghum ergot (Claviceps africana) to sows before farrowing inhibits milk production. Aust Vet J. 2007;85(5):169-176. https://doi.org/10.1111/j.1751-0813.2007.00139.x
16. Nordskog AW, Clark RT. Ergotism in pregnant sows, female rats and guinea pigs. Am J Vet Res. 1945;8:107-116.
17. Campbell CW, Burfening PJ. Effects of ergot on reproductive performance in mice and gilts. Can J Anim Sci. 1972;52(3):567-569. https://doi.org/10.4141/cjas72-068
18. Dignean MA, Schiefer HB, Blair R. Effects of feeding ergot‐contaminated grain to pregnant and nursing sows. Zentralbl Veterinarmed A. 1986;33(10):757-766. https://doi.org/10.1111/j.1439-0442.1986.tb00588.x
19. Hill G, Lay Jr DC, Richert B. Swine. In: Tucker CB, MacNeil MD, Webster AB, eds. Guide for the Care and Use of Agricultural Animals in Research and Teaching. 4th ed. Federation of Animal Science Societies; 2020:291.
20. Kopinski JS, Blaney BJ, Downing JA. Tolerance of pigs to sorghum ergot (Claviceps africana) during growth and finishing, and effect on conception of replacement gilts. Aust J Exp Agric. 2008;48(5):672-679. https://doi.org/10.1071/EA07326
21. Mainka S, Dänicke S, Ueberschär K-H, V Reichenbach HG. Effect of a hydrothermal treatment on ergot alkaloid content in ergot contaminated rye. Mycotoxin Res. 2005;21:116-119. https://doi.org/10.1007/BF02954433
 PDF version
PDF version RIS citation
RIS citation