| Case report | Peer reviewed |
Cite as: Lugar DW, Ragland D, Stewart KR. Influenza outbreak causes reduction in semen quality of boars. J Swine Health Prod. 2017;25(6):303–307. https://doi.org/10.54846/jshap/1018
Also available as a PDF.
SummaryAn influenza outbreak occurred at Purdue University’s swine barn, resulting in infection of 28 boars with influenza A virus (H3N2) and causing the death of two boars. The 28 boars, approximately 35 weeks of age, were enrolled in a study at the time of the outbreak and the case report herein describes the effects of the unintended influenza outbreak on sperm production. Semen was collected from the boars once a week and evaluated for total sperm production and concentration, semen volume, and relative motility. Compared to previous collections, total sperm production was substantially decreased (26% reduction) approximately 4 weeks after the first observed clinical signs and remained low for 6 subsequent weeks. Semen production then returned to pre-outbreak levels and was maintained for the duration of the observation period. Sperm motility and percent normal sperm production were also slightly reduced 2 weeks after infection. | ResumenUn brote de influenza sucedió en una sala de la granja porcina de la Universidad de Purdue, resultando en la infección de 28 machos con el virus de la influenza tipo A (H3N2 por sus siglas en inglés), y causando la muerte de dos machos. Los 28 machos de aproximadamente 35 semanas de edad, se incluyeron en un estudio al momento del brote, y el reporte descrito en este caso, así como los efectos de un brote de influenza no intencionado sobre la producción de semen. Una vez a la semana, se recolectó semen de los machos, y se evaluó la concentración y la producción total, el volumen, y la motilidad relativa del semen. Comparándolo con las colecciones previas, la producción total de semen este disminuyó sustancialmente (26% de reducción) aproximadamente 4 semanas después del los primeros signos clínicos observados, este permaneció bajo por las siguientes 6 semanas. La producción de semen regresó a los niveles previos al brote, y se mantuvo durante del periodo de observación. La motilidad del semen y el porcentaje de producción normal también disminuyeron ligeramente 2 semanas después de la infección. | ResuméUne épidémie d’influenza est survenue à la porcherie de l’université Purdue, entrainant l’infection de 28 verrats avec le virus de l’influenza A (H3N2) et causant la mort de deux verrats. Les 28 verrats, âgés d’environ 35 semaines, faisaient partie d’une étude au moment de l’épidémie et le présent rapport de cas décrit les effets de cette épidémie inattendue d’influenza sur la production de sperme. De la semence était prélevée des verrats une fois par semaine et évaluée pour la production totale de sperme et la concentration, le volume de semence, et la mobilité relative. Comparativement aux collectes antérieures, la production totale de sperme était diminuée de manière substantielle (26% de réduction) environ 4 semaines après l’observation des premiers signes cliniques et est demeurée faible pour les 6 semaines subséquentes. La production de semence retourna ensuite aux niveaux pré-épidémie et fut maintenue pour la durée de la période d’observation. La mobilité du sperme et le pourcentage de production de sperme normal étaient également réduits 2 semaines après l’infection. |
Keywords: swine, influenza A virus, boar, semen quality
Search the AASV web site
for pages with similar keywords.
Received: December 20, 2016
Accepted: April 25, 2017
Intensive farming strategies create opportunities for pathogens to enter and rapidly spread throughout herds, causing reduced growth rates, overall performance, and health compared to unchallenged herds. These reductions in growth and performance also have a consequential economic impact on producers. In addition to nutrition-related measures of performance, reproductive performance and efficiency are also keys to producer profitability and are susceptible to reductions caused by physiological stressors like heat stress and diseases. However, there is a lack of literature on the effects of disease on semen quality in boars. Porcine reproductive and respiratory syndrome virus is thought to impair motility and morphology of sperm without a major change in sperm production, though most research is focused on its transmission through semen.1,2 The Torque teno virus3 and porcine circovirus type 24 are also transmitted through the semen, but do not appear to alter semen quality in boars.
Influenza A virus is a pathogen that induces clinical signs, resulting in fever, which leads to elevated core body temperature, but there is a lack of published information on the effects of this disease on semen quality in boars. Choi et al5 reported that 22.8% of pigs in the United States test positive for swine influenza virus via hemagglutination inhibition and real time-polymerase chain reaction.5 Globally, influenza is one of the most widespread respiratory viruses affecting the swine industry and is considered endemic throughout most of the world.6 One of the main issues with influenza infections in swine is that pigs are susceptible to swine, avian, and human strains of influenza virus.6 Influenza-infected animals generally display clinical signs shortly after infection, which include, but are not limited to fever, diarrhea, and sneezing and (or) coughing.7 Influenza is primarily spread via nose-to-nose contact between pigs and via aerosolized, infectious respiratory excretions.7
To the authors’ knowledge, this is the first report on the effects of influenza A virus infection on the subsequent semen quality of boars. The purpose of this case report is to elucidate the effects of influenza A virus infections on sperm production and semen quality in boars.
Influenza outbreak and treatment timeline
Twenty-eight 35-week-old boars were being evaluated for semen quality parameters at the Purdue University Animal Sciences Research and Education Center when an influenza outbreak occurred. The first observation of mild clinical signs of illness (intermediate coughing and lethargy) in three boars occurred April 22, 2016, three weeks into the evaluation of semen quality. On the morning of April 24, 2016 (start of week 4), one boar was found dead and mild clinical signs were observed in eight to 10 boars. By April 25, 2016, moderate clinical signs (persistent coughing and lethargy) were observed in over 75% of the boars present. At this point, all of the boars were treated with a single injection of tulathromycin at a dosage of 2.5 mg per kg to minimize the potential for secondary infections, and with a single intramuscular injection of flunixin meglumine at a dosage of 2.2 mg per kg to manage pyrexia. On April 26, 2016, a second boar was found dead in the early afternoon and moderate clinical signs were observed in all of the remaining boars. These moderate clinical signs persisted for 2 to 3 days post treatment in a majority of the boars, and mild clinical signs lasted up to 10 days in some boars. By day 11 post treatment, 2 weeks after initial clinical signs were observed (end of week 5), no clinical signs of illness were observed.
The boars that died on April 24 and April 26 were necropsied by a licensed veterinarian and lung samples were collected and submitted for diagnostic testing. The necropsies revealed consolidation of the cranial and ventral lung lobes and accessory lobes, with some fibrinous exudate in the pleural cavity. The lungs had varying degrees of pulmonary edema and congestion and there appeared to be increased fluid in the pericardial sac of the affected boars. Lung tissue was submitted to the Indiana Animal Disease Diagnostic Laboratory (ADDL; West Lafayette, Indiana) and the cause of the influenza outbreak was confirmed. Testing of the diseased lung tissue via polymerase chain reaction revealed the presence of influenza type A nucleic acids belonging to the H3N2 strain of influenza virus. Blood was collected and serum was harvested from all of the boars during week 5 and samples were submitted to the Indiana ADDL. The serum was tested for influenza type A virus antibodies via enzyme-linked immunosorbent assay (ELISA), and the results showed that all boars tested positive for influenza A virus antibodies.
Effects on reproductive performance
The influenza outbreak had a major impact on the semen quality parameters measured on freshly collected semen in this incident. Prior to the influenza outbreak, the boars were housed in individual stalls and semen was routinely collected one time per week via the double-gloved hand technique and an artificial sow. Semen was evaluated on site immediately after each collection for sperm concentration, semen volume, relative motility, and total sperm production (concentration × volume). Semen was evaluated on site starting at week 1 (April 4, 2016) through week 17 (July 29, 2016). At weeks 5 through 11, semen was diluted with a commercial semen extender on site and also evaluated by computer-assisted sperm assessment (CASA; CEROS II, IMV Technologies USA; Maple Grove, Minnesota) for sperm total motility and progressive motility, as well as by microscopic examination for percent normal sperm morphology, once weekly for each boar. Total motility refers to any movement of the sperm, whereas progressive motility, a subset of total motility, refers to sperm movement in a mostly straight manner. To determine percent normal morphology, a phase contrast microscope was used to count a minimum of 200 sperm.
Statistical analysis was performed using the PROC MIXED function of SAS (version 9.4; SAS Institute Inc, Cary, North Carolina). Statistical analysis of semen parameters utilized repeated measures criteria of boar by week. Different covariance structures (compound symmetry, heterogeneous autoregressive, and unstructured) were tested in order to minimize Akaike information criterion. Where appropriate, collection and laboratory technician were utilized as potential random effects. A P value < .05 was considered statistically significant and a P value < .10 was considered a tendency.
The influenza outbreak occurred at the end of week 3 of the study and lasted through the end of week 5. Normal semen production was seen in weeks 1 through 6 (70.74, 65.14, 70.89, 65.31, 63.98, and 71.53 ± 3.72 × 109 sperm per ejaculate, respectively). Sperm production was significantly reduced during weeks 7 through 12 (54.22, 49.45, 49.45, 48.43, 53.41, and 48.94 ± 3.72 × 109 sperm per ejaculate, respectively), returning to normal during weeks 13 through 17 (69.23, 67.45, 72.82, 66.79, and 65.95 ± 3.72 × 109 sperm per ejaculate). For this reason, total sperm production, total motile sperm production, semen volume, and semen concentration were analyzed in three phases: Phase 1 (weeks 1 to 6), Phase 2 (weeks 7 to 12), and Phase 3 (weeks 13 to 17). Total sperm production data are summarized in Table 1. Total sperm production did not differ in phases 1 and 3 (P > .05). Total sperm production was greater in phases 1 and 3 compared to Phase 2 (67.92 and 68.33 versus 50.52 ± 2.73 × 109 sperm; P < .001). Average total sperm production in phases 1 and 3 was 68.13 × 109 sperm compared with 50.52 × 109 sperm in Phase 2, a difference of 17.61 × 109 sperm representing a 26% reduction in sperm production. Total motile sperm production data are summarized in Table 1. Total motile sperm production was lower in Phase 2 than in phases 1 and 3 (P < .001), and did not differ in phases 1 and 3 (P > .05). Semen volume was greater in Phase 3 than in phases 1 and 2 (P < .001 and P < .01, respectively). Semen volume was greater in Phase 2 than in Phase 1 (P < .05). Sperm concentration was lower in Phase 3 than in Phase 1 (P < .001), and lower in Phase 2 than in phases 1 and 3 (P < .001).
Table 1: Sperm production data by phase from 28 boars unintentionally infected with influenza A virus at a university research farm, with a pre-influenza phase (Phase 1), an influenza-affected phase (Phase 2), and a post-influenza phase (Phase 3)*
| Sperm production | Phase† | SE | P | ||
|---|---|---|---|---|---|
| 1 | 2 | 3 | |||
| Total sperm production × 109 | 67.92a | 50.52b | 68.33a | 2.730 | < .01 |
| Relative motility (%) | 89.82a | 83.30b | 82.80b | 0.005 | < .01 |
| Total motile sperm × 109‡ | 58.25a | 40.89b | 56.03a | 2.430 | < .01 |
* Clinical signs observed during the influenza outbreak included coughing and lethargy. The outbreak resulted in the deaths of two boars during the observation period. Lung samples from the dead boars were submitted to a diagnostic laboratory, which confirmed by polymerase chain reaction the presence of type A influenza virus (H3N2). Following the laboratory diagnosis, blood was collected and serum harvested and submitted for diagnosis, confirming type A influenza by an enzyme-linked immunosorbent assay.
† Phase 1 (weeks 1-6) where first clinical signs were observed at week 3 and were no longer observed by week 5; Phase 2 (weeks 7-12); Phase 3 (weeks 13-17). Phases were based on the weekly analysis of the data that showed a substantial reduction in total sperm production in weeks 7-12.
‡ Total motile sperm = total sperm production (total sperm production = semen volume × sperm concentration) × relative motility (as assessed by phase contrast microscopy).
ab Within a row, values with different superscripts are different (P < .05; ANOVA).
SE = standard error.
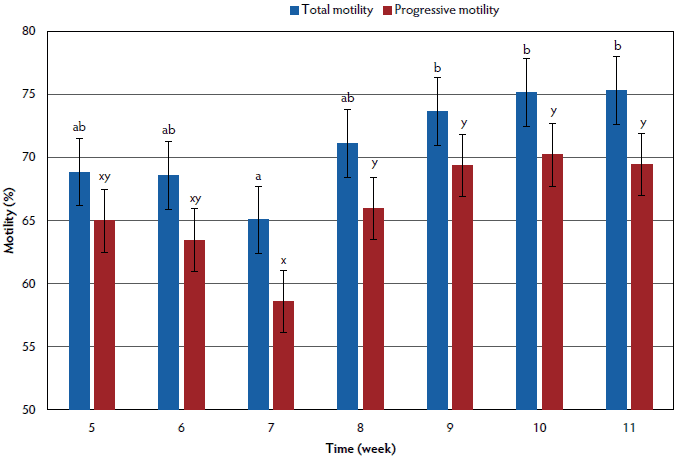
Sperm motility and progressive motility data are summarized in Figure 1. Sperm motility was lower in week 7 than in weeks 9 through 11 (P < .05), weeks 5, 6, and 8 through 11 did not differ (P > .05), and weeks 5 through 8 did not differ (P > .05). Progressive motility did not differ for week 5 compared to other all weeks (P > .05). Progressive motility tended to be lower for week 6 than for week 10 (P < .10). Week 7 progressive motility was lower than in weeks 8 through 11 (P < .05, P < .01, P < .01, P < .01; respectively), and within weeks 8 to 11 progressive motility did not differ (P > .05).
Figure 1: An unintentional outbreak of influenza A (H3N2) occurred at a university research farm, affecting total sperm motility and progressive motility of 28 boars. This outbreak caused clinical signs consisting of coughing and lethargy, as well as the deaths of two boars. Laboratory diagnosis confirmed the presence of this virus in lung tissue samples, as well as in serum samples from the boars. Clinical signs were observed in weeks 3-5 and caused latent effects on semen production and quality. The figure shows the effect of influenza on sperm total motility and progressive motility as assessed by computer assisted sperm assessment weeks 5-11. Blue bars represent total motility where bars with differing letters (a, b) are statistically different (P < .05). Red bars represent progressive motility where bars with differing letters (x, y) are statistically different (P < .05). Error bars for both total motility and progressive motility represent the standard error. This graph shows that both total motility and progressive motility of sperm were decreased at week 7 and then returned to normal beginning at week 8. Sperm motility parameters were not measured prior to week 5, so the total extent of the effects of influenza on sperm motility parameters cannot be estimated. Statistical analyses on total and progressive motility were performed using repeated measures in the MIXED procedure of SAS (version 9.4) with collection and laboratory technician as random effects. Significant differences were determined at a P value < .05 and a tendency at a P value < .10.
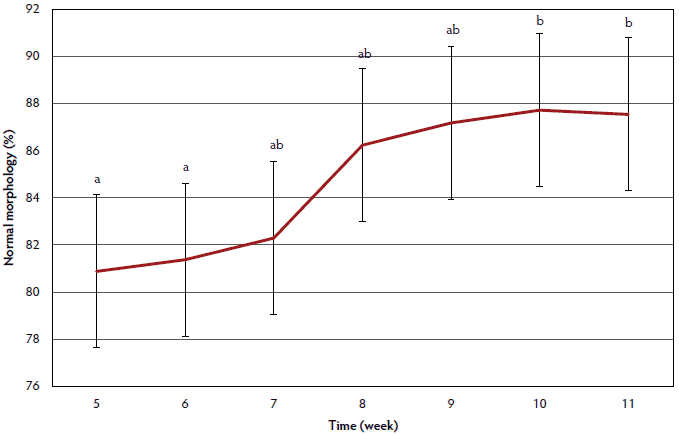
Percent normal morphology data are summarized in Figure 2. Percent normal morphology was lower for weeks 5 and 6 than for weeks 10 and 11 (P < .05). Week 5 percent normal morphology did not differ from that in weeks 6 to 9 (P > .05), and percent normal morphology in week 6 did not differ from that in weeks 5, 7, and 8 (P > .05). Week 6 percent normal morphology tended to be lower than week 9 percent normal morphology (P < .10).
Figure 2: An unintentional outbreak of influenza occurred at a university research farm, affecting the percentage of morphologically normal sperm of 28 boars. This outbreak caused clinical signs which consisted of coughing and lethargy, as well as the deaths of two boars. Laboratory diagnosis confirmed the presence of a type A influenza virus, specifically H3N2, in lung tissue samples from the dead boars, as well as in serum samples from the remaining boars. Clinical signs of the outbreak were observed weeks 3-5 and caused latent effects on semen production and quality. The figure illustrates the effect of influenza virus on the percentage of morphologically normal sperm. Sperm morphology was assessed weeks 5-11 using phase contrast microscopy, where a minimum of 200 sperm were counted and categorized as normal or abnormal. Weeks with differing letters (a, b) are different (P < .05) and error bars are represented by the standard error. This figure reveals that the percentage of morphologically normal sperm was low at weeks 5 and 6, and then increased over time. It is likely that percent normal morphology during weeks 1-3 would have been similar to weeks 9-11, where a plateau was observed. Since morphological assessment was not conducted weeks 1-4, the full effect of influenza A virus on sperm morphology cannot be completely estimated. Statistical analysis on sperm morphology was performed using repeated measures in the MIXED procedure of SAS (version 9.4) with collection and laboratory technician as random effects. Significant differences were determined at a P value < .05 and a tendency at a P value < .10.
Discussion
The results of this report indicate that influenza A infection had a significant impact on the semen quality of boars, including total sperm production, motility, and morphology. To the authors’ knowledge, this is the first reported case of influenza A infection affecting semen quality in boars, although there are reports of influenza’s impact on semen quality in humans and mice.8,9 Research suggests that other diseases can impact semen quality of boars; however, most research on disease in boars is primarily focused on the transmission of pathogens through semen. This report suggests that 4 weeks after the onset of clinical signs of infection, total sperm production is reduced in the boar. This reduction resulted from reduced sperm production within the testes and was confirmed by a reduction in sperm concentration. Semen volume increased during the observation period, as is normally seen in growing, maturing boars. These data suggest that infection with influenza virus causes a physiological response to the extent that there is a reduction in the production of sperm, likely caused by fever and elevated core body temperature. This reduced sperm production has been seen in men infected with febrile (fever causing) diseases.10,11 It is unlikely that influenza A virus directly impacted semen production in the testes due to its localization in lung tissue; instead, influenza A virus likely had an indirect effect by initiating a fever response which could cause a reduction in sperm production. Further research should investigate the effects of a febrile infection on semen quality parameters in boars to determine the direct cause of the reduced sperm production.
During the 17-week observation period, sperm motility and morphology were analyzed for weeks 5 through 11. Data suggest sperm motility and progressive motility may decline at a minimum of 2 to 4 weeks after the onset of clinical signs of fever or infection with influenza virus and return to normal after 5 to 6 weeks. The percentage of normal spermatozoa was reduced at a minimum of 2 to 3 weeks after the initial onset of clinical signs and steadily returned to normal by 7 to 8 weeks. This case report did not analyze data on CASA parameters and percent normal sperm morphology during the first 4 weeks of the observation period and thus the entire impact on motility and morphology parameters cannot be estimated. However, the results of analysis suggest that these parameters are temporarily decreased a few weeks after influenza A infection, which agrees with results in men infected with febrile diseases.12
The semen analysis results presented in this report are common in boars that have undergone a stressful event able to increase core body temperature, such as heat stress. Boars that are heat stressed, for example, typically have reduced sperm motility and increased sperm abnormalities within 2 weeks of the heat stress event.13 The length of time the stressor impacts the boars also plays a role, as motility and morphology parameters do not return to normal until 4 to 6 weeks after the stressor is removed.13 Similar results were seen in this case, where there was a clear delay in affected sperm reaching the caudal epididymis and having an effect on spermatogenesis. Influenza and (or) the consequential physiological responses to influenza A virus infection appear to affect spermatogenesis to the extent that an entire spermatogenic cycle (approximately 41 days) is required for sperm production to return to normal.14 To the authors’ knowledge, this is the first reported case of impaired semen quality due to an influenza outbreak in boars. While it is unlikely that influenza virus directly impairs sperm production, the residual effects of viral infections may be seen in the reproductive function of boars.
Implications
- Influenza and its effects on the body can negatively impact normal sperm production in boars.
- The consequences of influenza on total sperm production are delayed due to the nature of spermatogenesis.
Conflict of interest
None reported.
Disclaimer
Scientific manuscripts published in the Journal of Swine Health and Production are peer reviewed. However, information on medications, feed, and management techniques may be specific to the research or commercial situation presented in the manuscript. It is the responsibility of the reader to use information responsibly and in accordance with the rules and regulations governing research or the practice of veterinary medicine in their country or region.
References
1. Prieto C, Suarez P, Bautista JM, Sanchez R, Rillo SM, Simarro I, Solana A,Castro JM. Semen changes in boars after experimental infection with porcine reproductive and respiratory syndrome (PRRS) virus. Theriogenology. 1996;45:383–395.
2. Prieto C, Castro JM. Porcine reproductive and respiratory syndrome virus infection in the boar: a review. Theriogenology. 2005;63:1–16.
3. Kekarainen T, Lopez-Soria S, Segales J. Detection of swine Torque teno virus genogroups 1 and 2 in boar sera and semen. Theriogenology. 2007;68:966–971.
4. Rose N, Opriessnig T, Grasland B, Jestin A. Epidemiology and transmission of porcine circovirus type 2 (PCV2). Virus Res. 2012;164:78–89.
5. Choi YK, Goyal SM, Joo HS. Prevalence of swine influenza virus subtypes on swine farms in the United States. Arch Virol. 2002;147:1209–1220.
6. Brown IH. The epidemiology and evolution of influenza viruses in pigs. Vet Microbiol. 2000;74:29–46.
7. Lange E, Kalthoff D, Blohm U, Teifke J, Breithaupt A, Maresch C, Starick E, Fereidouni S, Hoffmann B, Mettenleiter T, Beer M, Vahlenkamp T. Pathogenesis and transmission of the novel swine-origin influenza virus A/H1N1 after experimental infection of pigs. J Gen Virol. 2009;90:2119–2123.
8. Evenson DP, Jost LK, Corzett M, Balhorn R. Characteristics of human sperm chromatin structure following an episode of influenza and high fever: a case study. J Androl. 2000;21:739–746.
9. Sharma G, Polasa H. Cytogenetic effects of influenza virus infection on male germ cells of mice. Hum Genet. 1978;45:179–187.
10. MacLeod, J. Effect of chickenpox and of pneumonia on semen quality. Fertil Steril. 1951;6:523–533.
11. Soehadi K. Azoospermia caused by typhoid fever A: case report. Andrologia. 1982;14:31–34.
12. Carlsen E, Andersson AM, Petersen JH, Skakkebaek NE. History of febrile illness and variation in semen quality. Hum Reprod. 2003;10:2089–2092.
13. Wettemann RP, Wells ME, Johnson RK. Reproductive characteristics of boars during and after exposure to increased ambient temperature. J Anim Sci. 1979;49:1501–1505.
14. Almeida FFL, Leal MC, Franca LR. Testis morphometry, duration of spermatogenesis, and spermatogenic efficiency in the wild boar (Sus scrofa scrofa). Biol Reprod. 2006;75:792–799.