Pittman JS. Enteritis in grower-finisher pigs caused by F18-positive Escherichia coli. J Swine Health Prod. 2010;18(2):81–86
| Case report | Peer reviewed |
Cite as: Pittman JS. Enteritis in grower-finisher pigs caused by F18-positive Escherichia coli. J Swine Health Prod. 2010;18(2):81–86.
Also available as a PDF.
SummaryEscherichia coli is a common cause of postweaning diarrhea in swine, but usually does not affect grower-finisher pigs. Eleven-week-old grower pigs presented with an acute and severe watery diarrhea, initially considered to be transmissible gastroenteritis. Diagnostic submissions showed the causative agent to be an F18-positive E coli producing shiga-like toxin. No pigs developed neurological signs or other conditions usually associated with edema disease. Affected pigs responded to treatment with neomycin. No significant difference was observed in mortality or growth performance between clinically affected and unaffected groups. This case report describes an unusual presentation of F18-positive E coli infection in 11-week-old pigs. | ResumenLa Escherichia coli es una causa común de diarrea post destete en cerdos, sin embargo comúnmente no afecta a los cerdos en crecimiento-finalización. Cerdos de crecimiento de 11 semanas de edad presentaron una diarrea acuosa severa, inicialmente considerada como gastroenteritis transmisible. Los resultados de las muestras enviadas a diagnóstico mostraron que el agente causante era una E coli F18-positiva que producía una toxina shiga-semejante. Ningún cerdo desarrolló signos neurológicos u otro problema comúnmente asociados a la enfermedad del edema. Los cerdos afectados respondieron al tratamiento con neomicina. No se observó una diferencia significativa en mortalidad ó desempeño del crecimiento entre grupos clínicamente afectados y no afectados. Este reporte de caso describe una presentación inusual de la E coli F18-positiva en cerdos de 11 semanas de edad. | ResuméEscherichia coli est une cause fréquente de diarrhée post-sevrage chez le porc, mais n’affecte pas habituellement les porcs en période de croissance-finition. Des porcs en croissance âgés de 11 semaines ont été présentés avec une diarrhée aqueuse aiguë et sévère, suspectée initialement comme étant de la gastro-entérite transmissible. Les résultats des analyses diagnostiques ont permis de montrer que l’agent causal était E coli F18-positif producteur de shiga-toxine apparentée. Aucun porc ne développa de signe neurologique ou d’autres conditions associées habituellement avec la maladie de l’Å“dème. Les porcs atteints ont bien répondu à un traitement avec de la néomycine. Aucune différence significative n’a été observée entre les groupes affectés et les groupes non-affectés quant à la mortalité ou les performances de croissance. Ce rapport de cas décrit une présentation inhabituelle d’infection par un isolat d’E coli F18-positif chez des porcs âgés de 11 semaines. |
Keywords: swine, diarrhea, Escherichia coli, F18,
shiga-like toxin
Search the AASV web site
for pages with similar keywords.
Received: August 29, 2009
Accepted: November 12, 2009
Esscherichia coli is a common cause of disease in pigs, other livestock species, and humans. In pigs, the main syndromes associated with E coli infection are enteritis, edema disease, and septicemia.1 Enterotoxigenic E coli strains associated with enteritis in swine are commonly characterized by their pilus type (F4 [K88], F5 [K99], F6 [987P], F41, and F18) and the presence or absence of gene combination encoding two heat stable (STa and STb) or one heat labile (LT) enterotoxin. Enteritis caused by E coli is seen in pigs of varying ages, and age of onset is usually related to the pilus type due to the presence or absence of pilus-specific receptors on the enterocytes. Escherichia coli strains positive for pilus types F5, F6, and F41 affect pigs at a younger age (< 3 weeks of age), while F4-positive E coli can cause enteritis in the preweaning and postweaning periods.1 For E coli strains positive for the F18 pilus to cause disease, age-dependent F18 receptors must be present on the enterocytes, and pigs are affected post weaning, usually at > 3 weeks of age.2 Both F4-positive and F18-positive E coli cause postweaning diarrhea, also characterized by dehydration, lethargy, and wasting, often resulting in a high mortality rate. In addition, F18-positive E coli strains can be classified as shiga toxin-producing E coli (STEC) if they contain a gene that encodes for a shiga-like endotoxin (Stx2e). The Stx2e toxin is responsible for vascular damage and permeability, resulting in edema disease in pigs, characterized by generalized edema in several tissues (eg, brain, forehead, eyelids, stomach), ataxia, lateral recumbency, dyspnea, and acute mortality.1
Enteritis associated with E coli is less common in older pigs (> 10 wk), and when it does occur in grower pigs, it is usually associated with other diseases (eg, rotavirus, porcine reproductive and respiratory syndrome [PRRS], porcine circovirus associated disease [PCVAD]). This case describes an uncharacteristic presentation of F18-positive E coli enteritis in 11-week-old pigs.
Case description
Housing
The case farm had been recently converted from a 675-sow, one-site farrow-to-finish operation to a 5600-head finishing facility. It was composed of six original finishing barns (Barns 1 to 6), one converted breeding and gestation barn (Barn 7), and a combined farrowing and nursery building that was no longer being utilized. Each of the six finishing barns was divided into three rooms, each separated by a full wall and door, but with a common pull-plug pit and attic air space. All rooms were fully slatted and housed a total of 250 pigs in 10 equally sized pens. The converted breeding and gestation barn was partially slatted with 50% solid floors and contained 1400 finishing spaces divided among 44 equally sized pens. The site was completely empty of pigs and cleaned prior to receiving feeder pigs from a different farm.
Pig flow
At the time of clinical disease, 10-week-old feeder pigs (average weight, 22.7 kg) were being placed from multiple nursery sites, but originating from a single sow farm. Approximately 950 pigs were placed each week for 6 consecutive weeks until the finishing site was filled. Pigs were placed in a barn to capacity and remaining pigs were placed in the next sequential barn. Each barn was emptied, washed, and disinfected prior to placement of new feeder pigs. The site was not completely emptied of older animals (near market weight) before the subsequent groups of feeder pigs were placed. At the time of clinical disease, the farm was receiving its second group of feeder pigs since its conversion from a farrow-to-finish farm.
One trailer load (Load C, approximately 475 pigs) of the placement group (that developed clinical signs) experienced a stress event on the day of placement (Day 0). The trailer hauling the pigs became stuck in mud at the finishing site while backing up to the loading chute prior to unloading. The pigs spent 2 hours in the trailer while it sat idle. Weather conditions during that time were 7°C, 79% average humidity, overcast, and no wind. These pigs, along with another trailer load (Load D, an additional 475 pigs) were placed in Barns 2 and 3. Approximately 750 pigs in Barn 1 and 200 pigs in Barn 2 had been placed 1 week previous (Loads A and B, Day -7). Barns 4, 5, and 6 were empty and clean, while 140 market-weight pigs remained in Barn 7.
Clinical description
Six days after feeder pigs from Loads C and D were placed in Barns 2 and 3, acute watery diarrhea was seen in > 10% of the group. The following day (Day 7), this increased to > 50%, and diarrhea was seen in pigs from Loads A and B placed in Barn 2 the previous week (Day -7), but not in pigs from these loads placed in Barn 1. On Day 7, Barns 3 and 4 received a new shipment of feeder pigs (Loads E and F) that were clinically unaffected. A company ser-viceperson was present at the farm on Day 7 to receive feeder pig Loads E and F. Phone conversations between the serviceperson and the author indicated that watery diarrhea was affecting a large number of pigs, and several pigs were seen vomiting.
Diagnostic testing and results
Preliminary diagnosis
Immediately, a tentative and precautionary diagnosis of transmissible gastroenteritis (TGE) was made. Swabs (BBL CultureSwabs with Stuart’s medium; Becton, Dickinson and Company, Sparks, Maryland) were used by the serviceperson to collect pooled samples from three to five fresh diarrhea puddles throughout Barns 2 and 3. A total of 10 swabs were collected. Swabs were placed on ice and submitted overnight to the Iowa State University Veterinary Diagnostic Laboratory (ISU-VDL) for testing by polymerase chain reaction (PCR) for TGE and porcine respiratory coronavirus (PRCV). Communications were made to key personnel in the production system to limit the exposure of this site to other farms, pigs, trucks, and equipment. Unnecessary visits to the farm were prohibited until a diagnosis could be determined.
Preliminary diagnostic testing
Each of the 10 fecal swabs was PCR-negative for TGE-PRCV. It was then determined that a herd visit by the veterinarian would be immediately conducted to inspect the clinical presentation of the disease, collect further information about the course of disease, and collect additional fecal and tissue samples. On the basis of the clinical signs, TGE was still suspected as the cause, and this raised concerns about the quality, handling, or testing of samples.
Herd visit
The farm was visited by the veterinarian 3 days after the onset of diarrhea (Day 9). Pigs in Barns 2 and 3 still had severe watery diarrhea. By this time, > 50% of the pigs from the first placement (Loads A and B, Day -7) of Barn 2 were affected. There was still no evidence of diarrhea in pigs in Barn 1 or in pigs placed in Barns 3 or 4 (Loads E and F, Day 7). The 140 remaining market animals in Barn 7 had been sold that morning prior to the veterinarian’s arrival; however, no clinical signs were noted in these pigs by the farm manager or serviceperson. There were no other animals on-site.
Clinical signs were characterized by severe, watery, uncontrolled, and projectile diarrhea. Diarrheic feces were present throughout each pen, along the walls, on the side curtains, and in the aisles. Diarrhea from some pigs was seen to project as far as 1 meter and across the aisles into adjacent pens. Individual diarrhea varied in color from dark green-brown, tan, and yellow, to watery-clear appearance (Figure 1). Nondigested feed particles were present in the stools. Diarrhea was also apparent on many pigs in the perineal region and between the hind legs. During the visit, there was evidence of at least five animals vomiting, either observed directly or by finding fresh vomitus in separate pens. There was no evidence of blood, mucous, or parasites in the stools. Approximately 30% to 40% of the pigs appeared dehydrated and chilled, although room temperatures were 18°C to 21°C. Only about 5% of the animals had a noticeable cough, rectal temperatures were within normal range, and no other major clinical signs were observed. There was no evidence of moldy feed in the feeders or feed bins. Feed in all barns was from the same delivery. On the basis of clinical presentation, TGE was still the primary differential, while other considerations were salmonellosis, rotavirus diarrhea, spirochetal colitis (Brachyspira pilisicoli), and swine dysentery (Brachyspira hyodysenteriae).
Figure 1: Eleven-week-old grower-finisher pig with severe watery diarrhea caused by a strain of Escherichia coli positive for the gene responsible for the F18ac pilus and for genes coding for heat-labile enterotoxin (LT), heat-stable enterotoxins STb and EAST-1, and shiga-like endotoxin (Stx2e).  |
Further diagnostic procedures
Sample collection
A swab set, comprising one each of three types of swabs, was used to sample composite diarrhea puddles: a Universal Viral Transport Sterile Swab Applicator (Becton, Dickinson and Company, Franklin Lakes, New Jersey) placed in a sterile 7.0-mL serum Vacutainer (Becton, Dickinson and Company) containing 4.0 mL of sterile 1× phosphate buffered saline (1× PBS) (Fisher Scientific, Pittsburgh, Pennsylvania); a BBL CultureSwab with Stuart’s media; and a Viral Culturette collection and transport system (Becton, Dickinson and Company). Each swab in a set was used to sample the same five diarrhea puddles. This process was repeated three more times in different areas of the barn, for a total of 20 diarrhea puddles sampled and four of each type of swab (12 total fecal swabs). In addition, one swab set was collected from a pool of three fresh vomitus samples.
Four untreated pigs showing signs of active profuse watery diarrhea were selected and euthanized (two pigs from each affected barn). Postmortem examinations of all four animals revealed large volumes of watery ingesta and fecal material throughout the small intestine, cecum, and colon. All animals had full stomachs. Spleen, liver, and mesenteric lymph nodes were of normal size and without gross pathology. There were no gross lesions suggestive of ulcerative colitis. Small areas of patchy consolidated pneumonia in one pig affected < 5% of the lung. All other pigs and organs were unremarkable. Tissue samples, focused on intestinal tract organs, were collected from each pig and pooled by barn. Multiple sections of stomach, duodenum, jejunum, ileum, cecum, colon, and lymph nodes (mesenteric and colonic) were collected fresh and fixed in 10% formalin. Tissues and swabs were placed on ice and shipped overnight to the ISU-VDL for testing.
Diagnostic testing
Tissue samples were submitted for histopathology and for immunohistochemistry (IHC) testing for TGE virus, rotavirus, PRRS virus (PRRSV) and porcine circovirus type 2 (PCV2). Fecal contents were submitted for bacterial culture and sensitivity. The four fecal swabs and one vomitus swab submitted in 1× PBS were pooled at the laboratory and tested for TGE-PRCV by PCR and for type A rotavirus by antigen-capture enzyme-linked immunosorbent assay (ELISA). All other swabs were held in case additional testing was warranted.
Diagnostic results
Histopathological examination of the small intestines of all pigs revealed long slender villi with mats of short, rod-shaped bacteria adhered to the surface of the enterocytes (Figure 2). Small intestinal mucosa was diffusely infiltrated by lymphocytes and lesser numbers of plasma cells. Colonic lesions were described as having multifocally eroded and flattened enterocytes with subjacent neutrophils. Glands were infrequently dilated and contained necrotic cellular debris. The mucosa was expanded by lymphocytes, plasma cells, and lesser numbers of neutrophils. No lesions suggestive of viral enteritis (ie, villous atrophy) were seen. Histological diagnoses of acute diffuse enteritis and acute multifocal erosive colitis were made. Immunohistochemistry testing on intestinal sections was negative for TGE virus, Type A rotavirus, PRRSV, and PCV2. The fecal-vomitus swab pool was negative by PCR for TGE-PRCV and by antigen-capture ELISA for type A rotavirus. Culture of fecal contents revealed high numbers of hemolytic E coli. Further characterization of the E coli isolate was carried out. Salmonella enrichment and Brachyspira species cultures on intestinal contents were negative.
Figure 2: Mats of small, rod-shaped bacteria (arrows) adhered to enterocytes of an 11-week-old pig with severe acute watery diarrhea (H&E stain; magnification ×200). Culture of fecal contents revealed high numbers of hemolytic Escherichia coli, and molecular genotyping identified E coli positive for genes coding for the F18ac pilus and for heat-labile enterotoxin (LT), heat-stable enterotoxins STb and EAST-1, and shiga-like endotoxin (Stx2e). Salmonella enrichment and Brachyspira species cultures of intestinal contents were negative. 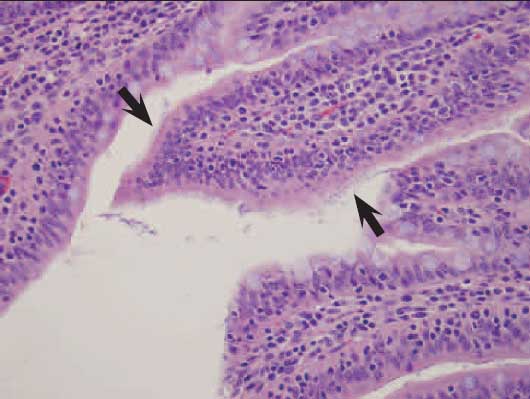 |
Escherichia coli isolate characterization
The E coli isolate was sensitive to ampicillin, ceftiofur, enrofloxacin, neomycin, and trimethoprim-sulphamethoxazole and was resistant to all other antimicrobials tested. Molecular genotyping of the E coli isolate was positive for genes encoding the F18 pilus and toxins STb, LT, and Stx2e.
The E coli isolate was forwarded to the University of Minnesota Veterinary Diagnostic Laboratory, St Paul, Minnesota, for E coli virulence-factor PCR (genotyping), specifically to evaluate the presence of genes encoding for adhesion involved in diffuse adherence (AIDA-1), attaching and effacing factor (eae), and porcine attaching and effacing-associated factor. The isolate was confirmed to have STb, LT, and Stx2e toxin genes, and also a gene encoding for heat-stable enterotoxin 1 (EAST-1). The isolate was negative for genes encoding AIDA-1, eae, and porcine attaching and effacing-associated factor.
The isolate was also forwarded to the E coli Reference Center at the Pennsylvania State University, University Park, Pennsylvania, for further characterization. Testing confirmed the above characteristics and further classified the E coli isolate as F18ac-positive.
Diagnosis
Histological lesions, culture, and isolate genotyping were consistent with enteric colibacillosis caused by E coli positive for F18ac, LT, STb, EAST-1, and Stx2e.
Treatment
At the time of sample collection (Day 9), treatment of the affected animals was initiated with neomycin sulfate (NeoMed 325; Bimeda, Inc, Le Sueur, Minnesota) in the drinking water at a rate of 22 mg per kg of body weight for 5 days, followed by 5 days of electrolytes (Ag ProVision electrolyte pack; Animal Science Products, Inc, Nacogdoches, Texas) also metered through the water. The course of treatment was completed and no other antibiotics were given when sensitivity testing and clinical response to treatment confirmed a sensitivity of the E coli isolate to neomycin. Treatment of disease was considered successful by the cessation of clinical diarrhea near the end of the neomycin treatment period, as witnessed by the producer and company serviceperson.
Serological testing
Twenty-one days after clinical signs had first been noticed, blood samples were collected from 10 pigs in each of three barns (Barn 1, unaffected; Barn 2, severely affected; and Barn 3, moderately affected) to attempt to better understand the disease process. Samples were submitted overnight to the ISU-VDL for testing for antibodies to TGE and PRCV, PRRSV, Salmonella, and Lawsonia intracellularis. All samples were seronegative for TGE virus and seropositive for PRCV (Svanovir TGEV-PRCV-Antibody ELISA; Svanova Veterinary Diagnostics, Uppsala, Sweden; positive test, > 60% inhibition). All pigs tested in Barns 1, 2, and 3 were seropositive for PRRSV (HerdChek PRRS 2XR ELISA; Idexx Laboratories, Inc, Westbrook, Maine; positive test, sample:positive [S:P] ratio ≥ 0.40), with average S:P ratios of 2.44 (SD, 0.92), 2.29 (SD, 0.81), and 2.33 (SD, 0.73), respectively. Ten samples of 10, 8 of 10, and 7 of 10 were seropositive for Salmonella in Barns 1, 2, and 3, respectively (HerdChek Swine Salmonella Antibody ELISA; Idexx Laboratories, Inc; positive test, S:P ≥ 0.25). Two of 10 samples from each of the three barns were seropositive for Lawsonia intracellularis (bioScreen Ileitis Antibody ELISA; Synbiotics Europe SAS, Lyon, France; positive test, S:P ≥ 0.40).
Finishing performance
Finishing performance was evaluated for affected lots and compared to an equal number of lots not clinically affected. Only group summaries were available for comparison and not individual pig performance; therefore, weights, average daily gain, and feed conversion could not be compared. Chi-square analysis of percent mortality, percent culls, and both combined demonstrated no statistical difference between clinically affected and unaffected groups (Table 1). The impact of subclinical disease or other diseases on the clinically unaffected barns is not known.
Table 1: Comparison of finishing performance between groups of 11-week-old pigs clinically affected with severe watery diarrhea (Barns 2 and 3) and unaffected (Barns 1 and 4)*
* Percent mortality, culls, and mortality and culls combined were not statistically different between groups as tested by chi-square at P < .05. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Discussion
The mechanism by which E coli causes diarrhea is primarily hypersecretory. Production of diarrhea is mediated first by attachment of the E coli via fimbria (pili) to receptors on enterocyte microvilli, then initiation of production of one or more enterotoxins released into the intestinal lumen. These enterotoxins enhance chloride ion secretion (LT), inhibit sodium ion absorption (LT, STa, and EAST-1), cause fluid secretion (STa and EAST-1), and possibly activate enteric nerves (STb), leading to severe fluid loss into the intestinal lumen, thus resulting in diarrhea and dehydration.3 It has been shown that E coli strains with the capability to produce multiple enterotoxins produce more severe diarrhea, likely through additive effects of the enterotoxins.4,5 Shiga-like toxin, also known as a verotoxin, is considered an endotoxin.6 The Stx2e toxin enters the bloodstream via the intestinal wall and causes destruction of vascular endothelium, resulting in inflammation, permeability, and edema. In addition to being an endotoxin, the Stx2e toxin experimentally causes diarrhea, sometimes hemorrhagic, due to the toxin’s ability to cause ischemic necrosis and hemorrhage of the intestinal epithelium.6,7 Diarrhea in cases of edema disease is usually related to the STEC’s ability to co-produce endotoxins and enterotoxins. Multiple combinations of these toxins can be found in various E coli isolates and pilus types; however, the genes associated with Stx2e production are found most commonly in F18-positive E coli isolates that produce one or more enterotoxins.8-10 Two fimbrial variants are currently classified within F18-positive E coli: F18ab and F18ac.11 Most F18-positive isolates from cases of edema disease have been characterized as F18ab-positive; however, this is not exclusive and some have been classified as F18ac-positive, as in this case.11-13 The characteristics, classifications, and pathogenesis of E coli have been reviewed in further detail elsewhere.14,15
In the case described above, it is clear that the severe diarrhea was caused by the enterotoxins (LT, STb, and EAST-1) produced by the F18-positive E coli. Clinical disease was limited to two barns where one shipment of feeder pigs were placed, indicating a difference in the epidemiology of the disease in this subpopulation. The fact that one shipment of pigs had a stress event prior to unloading likely predisposed this group of pigs to develop disease. Colibacillosis in grower-finisher pigs is uncommon and is usually attributed to a stressful event, immunocompromised hosts, or concurrent disease (eg, PRRS, PCVAD).1,16-19 Stressful events, such as weaning, cold stress, and mixing, can enhance E coli infections, disrupt the intestinal mucosa, and increase fecal shedding of E coli in swine.20-22 In addition to the stressful event, these pigs had been exposed to PRRS, Salmonella serovars, and L intracellularis, as demonstrated by ELISA serological testing; however, clinically unaffected pigs in other barns had similar levels of exposure to these diseases. No concurrent infections were diagnosed in the samples submitted to the ISU-VDL. It is unclear whether secondary disease predisposed these pigs to develop such severe diarrhea.
Interestingly, there was a lack of typical neurological signs usually associated with edema disease and no evidence of edema, which would be expected with an F18-positive, Stx2e-positive E coli infection. There are several possibilities as to why edema disease did not manifest in this case.
The E coli was positive for the Stx2e gene by PCR; however, this suggests only that the isolate has the ability to express the gene and does not relate to expression in vivo. Clinical development of edema disease is dose-dependent on the number of STEC colony-forming units, likely relating to the total number of STEC organisms present in the gut, and also possibly delayed until adequate levels of toxin are present.23,24 It is possible that either numbers of STEC or amount of Stx2e were not at high enough concentrations to cause edema disease in these relatively older, larger pigs.
Incidence of edema disease in pigs is age-dependent due to increased expression of F18 receptors on enterocytes as the pig increases in age.2 Receptors are expressed starting at approximately 3 weeks of age and persist until at least 23 weeks of age,25 which can result in edema disease in older pigs.19 Grange et al26 showed age-related reduction in expression of the LT receptor GM1. It may be speculated that similar age-related reduced expression of the Stx2e receptor, Gb4, may explain the lower incidence of edema disease in older pigs. In this case, the severity of the diarrhea suggests that the dose of STEC and amount of toxin production were sufficient to cause edema disease. Therefore, it is more likely that either a higher tolerance to the Stx2e endotoxin or fewer Gb4 receptors in these older pigs was responsible for the lack of edema disease.
This case demonstrates an unusual presentation of F18-positive, Stx2e-positive E coli infection in 11-week-old grower-finisher pigs resulting in severe watery diarrhea but without neurological signs of edema disease.
Implications
- Strains of E coli positive for the F18 pilus, enterotoxins, and Stx2e endotoxin should be added to the list of differential diseases that cause severe diarrhea and vomiting in grower-finisher pigs.
- Episodes of stress or concurrent disease may predispose grower-finisher pigs to clinically express diseases not commonly observed in those age groups.
Acknowledgments
The author would like to thank Dr Darin Madson, ISU-VDL; Dr Kurt Rossow, University of Minnesota Veterinary Diagnostic Laboratory; and Dr Chobi DebRoy, Pennsylvania State University E coli Reference Center, for providing assistance with diagnostic testing, pathogenesis, and the included histopathology image.
References
1. Fairbrother JM, Gyles CL. Postweaning Escherichia coli diarrhea and edema disease. In: Straw BE, Zimmerman JJ, D’Allaire S, Taylor DJ, eds. Diseases of Swine. 9th ed. Ames, Iowa: Blackwell Publishing; 2006:649–662.
2. Nagy B, Casey TA, Whipp SC, Moon HW. Susceptibility of porcine intestine to pilus-mediated adhesion by some isolates of piliated enterotoxigenic Escherichia coli increases with age. Infect Immun. 1992;60:1285–1294.
3. Moeser AJ, Blikslager AT. Mechanisms of porcine diarrheal disease. JAVMA. 2007;231:56–67.
4. Zhang W, Berberov EM, Freeling J, He D, Moxley RA, Francis DH. Significance of heat-stable and heat-labile enterotoxins in porcine colibacillosis in an additive model for pathogenicity studies. Infect Immun. 2006;74:3107–3114.
5. Berberov EM, Zhou Y, Francis DH, Scott MA, Kachman SD, Moxley RA. Relative importance of heat-labile enterotoxin in the causation of severe diarrheal disease in the gnotobiotic piglet model by a strain of enterotoxigenic Escherichia coli that produces multiple enterotoxins. Infect Immun. 2004;72:3914–3924.
6. Gannon VP, Gyles CL, Wilcock BP. Effects of Escherichia coli shiga-like toxins (verotoxins) in pigs. Can J Vet Res. 1989;53:306–312.
7. Dykstra SA, Moxley RA, Janke BH, Nelson EA, Francis DH. Clinical signs and lesions in gnotobiotic pigs inoculated with Shiga-like toxin I from Escherichia coli. Vet Pathol. 1993;30:410–417.
8. Moon HW, Hoffman LJ, Cornick NA, Booher SL, Bosworth BT. Prevalences of some virulence genes among Escherichia coli isolates from swine presented to a diagnostic laboratory in Iowa. J Vet Diagn Invest. 1999;11:557–560.
9. Post KW, Bosworth BT, Knoth JL. Frequency of virulence factors in Escherichia coli isolated from pigs in postweaning diarrhea and edema disease in North Carolina. Swine Health Prod. 2000;8:119–120.
10. Zhang W, Zhao M, Ruesch L, Omot A, Francis D. Prevalence of virulence genes in Escherichia coli strains recently isolated from young pigs with diarrhea in the US. Vet Microbiol. 2007;123:145–152.
11. Rippinger P, Bertschinger HU, Imberechts H, Nagy B, Sorg I, Stamm M, Wild P, Wittig W. Designations F18ab and F18ac for the related fimbrial types F107, 2134P and 8813 of Escherichia coli isolated from porcine postweaning diarrhoea and from oedema disease. Vet Microbiol. 1995;45:281–295.
12. Bosworth BT, Dean-Nystrom EA, Casey TA, Neibergs HL. Differentiation of F18ab+ from F18ac+ Escherichia coli by single-stranded conformational polymorphism analysis of the major fimbrial subunit gene (fedA). Clin Diag Lab Immun. 1998;5:299–302.
13. DebRoy C, Roberts E, Scheuchenzuber W, Kariyawasam S, Jayarao BM. Comparison of genotypes of Escherichia coli strains carrying F18ab and F18ac fimbriae from pigs. J Vet Diagn Invest. 2009;21:359–364.
14. Nataro JP, Kaper JB. Diarrheagenic Escherichia coli. Clin Microbiol Rev. 1998;11:142–201.
15. Fairbrother JM, Nadeau E, Gyles CL. Escherichia coli in postweaning diarrhea in pigs: an update on bacterial types, pathogenesis, and prevention strategies. Anim Health Res Rev. 2005;6:17–39.
16. Amezcua R, Friendship RM, Dewey CE, Gyles C, Fairbrother JM. Presentation of postweaning Escherichia coli diarrhea in southern Ontario, prevalence of hemolytic E coli serogroups involved, and their antimicrobial resistance patterns. Can J Vet Res. 2002;66:73–78.
17. Merialdi G, Bonilauri P, Granelli F, Luppi A, Dottori M. Bacterial pathogens on field cases of clinical colitis in growing and finishing pigs in Italy. Vet Rec. 2003;153:529–530.
*18. Leiting R. All finishing diarrhea is not ileitis. Nat Hog Farm. 2004;1:22.
*19. Rademacher CJ. Experiences with edema disease in 10-week-old feeder pigs. Proc Iowa State Univ Swine Dis Conf. Ames, Iowa. 2004;27–28.
20. Jones PH, Roe JM, Miller BG. Effects of stressors on immune parameters and on the faecal shedding of enterotoxigenic Escherichia coli in piglets following experimental inoculation. Res Vet Sci. 2001;70:9–17.
21. Dowd SE, Callaway TR, Morrow-Tesch J. Handling may cause increased shedding of Escherichia coli and total coliforms in pigs. Foodborne Pathog Dis. 2007;4:99–102.
22. Moeser AJ, Ryan KA, Nighot PK, Blikslager AT. Gastrointestinal dysfunction induced by early weaning is attenuated by delayed weaning and mast cell blockade in pigs. Am J Physiol Gastrointest Liver Physiol. 2007;293:G413–G421.
23. Cornick NA, Matise I, Samuel JE, Bosworth BT, Moon HW. Shiga toxin-producing Escherichia coli infection: temporal and quantitative relationships among colonization, toxin production and systemic disease. J Infect Dis. 2000;181:242–251.
24. Tsukahara T, Nakanishi N, Nakayama K, Matsubara N, Ushida K. Experimental infection of enterotoxigenic Escherichia coli associated with porcine edema disease and its pathological characteristics in the intestine. J Vet Med Sci. 2005;67:1167–1171.
25. Coddens A, Verdonck F, Tiels P, Rasschaert K, Goddeeris BM, Cox E. The age-dependent expression of the F18+ E coli receptor on porcine gut epithelial cells is positively correlated with the presence of histo-blood group antigens. Vet Micro. 2007;122:332–341.
26. Grange PA, Parrish LA, Erickson AK. Expression of putative Escherichia coli heat-labile enterotoxin (LT) receptors on intestinal brush borders from pigs of different ages. Vet Res Commun. 2006;30:57–71.
*Non-refereed references.