Diagnostic Notes |
Non refereed |
How to evaluate herds using swine influenza serology
Gene A. Erickson, DVM; Marie R. Gramer, DVM; Richard J. Webby, PhD
GAE: Rollins Laboratory, North Carolina Veterinary Diagnostic Laboratory System, Raleigh, North Carolina. MRG: Minnesota Veterinary Diagnostic Laboratory, Veterinary Population Medicine, College of Veterinary Medicine, University of Minnesota, St Paul, Minnesota. RJW: Department of Infectious Diseases, St Jude Children’s Research Hospital, Memphis, Tennessee.Corresponding author: Dr Gene A. Erickson, Rollins Laboratory, North Carolina Veterinary Diagnostic Laboratory System, 1031 Mail Service Center, Raleigh, NC 27699-103; E-mail: gene.erickson@ncmail.net
Cite as: Erickson GA, Gramer MR, Webby RJ. How to evaluate herds using swine influenza serology. J Swine Health Prod. 2005;13(4):222-224.
Also available as a PDF.
Serologic monitoring of swine influenza virus (SIV) has become extremely frustrating due to multiple antigenic strains of H1N1 and H3N2 circulating in swine herds today.1-3 Variation of hemagglutination inhibition (HI) test titers between laboratories is just one of the continuing issues to consider. However, a much larger issue for HI tests is the type of viral antigen used by the laboratory.
H1N1 serologic testing
Classical H1N1 SIV strains have been essentially displaced by reassortant strains. Sequence analyses performed to date show that three groups or clusters of H1 hemagglutinin are present in US herds: the reassortant-like, the H1N2-like, and classical-like groups. In samples from midwestern states (Minnesota and Iowa) submitted to the Minnesota Veterinary Diagnostic Laboratory (MVDL) between 2001 and 2004, prevalences for HI groups were 56% reassortant-like, 35% H1N2-like, and 15% classical-like. During the same time period, for H1-positive samples genetically analyzed at MVDL from US swine, regardless of region, prevalences for the H1 groups were 42% reassortant-like, 47% H1N2-like, and 1% classical-like.
Prevalences of the H1 groups are much different in North Carolina than in the Midwest. For 40 recent H1N1 isolates from North Carolina sequenced at St Jude Children’s Research Hospital, prevalences were 27% reassortant-like and 73% H1N2-like. The presence of different variants of H1 SIV in different regions must be considered when H1 SIV serology tests are selected.
Currently, there are two options for H1N1 SIV serological testing: ELISA and HI. The Idexx HerdChek swine influenza virus H1N1 ELISA antibody test kit (Idexx Laboratories, Westbrook, Maine) contains antigen prepared from classical H1N1 SIV, therefore its utility for routine surveillance is limited. The test works extremely well with samples from pigs previously infected with that classical H1N1 SIV and some reassortant-like and H1N2-like strains. Due to the very high prevalence of reassortant-like and H1N2-like SIVs in infected herds, the Idexx H1N1 ELISA is useful only for compliance testing on pigs or sows that are routinely vaccinated with vaccines containing classical H1N1 SIV and one of the two currently licensed vaccines containing reassortant H1N1 SIV. Consult with your diagnostic laboratory to determine if the assay will work for your vaccine program.
Serologic monitoring by the HI test for H1N1 antibodies is far more versatile if multiple test strains are used. The HI test antigens must be complementary to the antibodies made by the pig due to natural infection or vaccination. Due to the displacement of classical H1N1 by reassortant H1N1 or H1N2-like H1N1 SIV, classical H1N1 SIV HI test antigen is useful only for compliance testing of pigs vaccinated or infected with closely-related strains of virus. Two options currently exist for monitoring to determine if naive or vaccinated pigs have been infected by field strains of H1N1 SIV. Routine use of the reassortant H1N1 SIV HI test strain provided by the National Veterinary Services Laboratories (NVSL) at the MVDL has been reliable for midwestern pigs due to the dominance of reassortant H1N1 SIV in those herds. A genetically conserved strain of H1N2-like H1N1 SIV is needed to provide a reliable second test antigen for routine monitoring of US swine. In lieu of such a strain, some laboratories have conducted serologic testing with HI test antigens made from herd isolates.
From a panel of 40 North Carolina isolates sequenced at St Jude Children’s Hospital, 29 were H1N2-like H1N1 SIV strains. In an attempt to find a suitable H1N2-like H1N1 test antigen, those isolates are currently being evaluated to identify two or three that appear to be most representative of the isolates sequenced. They will be evaluated for both homology of protein expression and location of glycosylation sites on the hemagglutinin that may mask antigenic sites on the virus. When there are changes to the antigenic sites, a very different virus is presented to the pig. Selected strains will be used to prepare prototype HI test antigens for evaluation against field sera from North Carolina and midwestern herds to determine which will be most suitable for routine serologic monitoring.
H3N2 serologic testing
H3N2 serology has been just as frustrating for diagnostic laboratories and producers trying to determine if pigs have been recently infected with a field strain of the virus. Antigenic drift among naturally occurring H3N2 SIV strains has been rapid in US swine. Three different genotypes or clusters of the virus have been characterized to date. The first type was dominant in the initial wave of widespread infection of herds in 1999, and is represented by Texas 98 (TX 98) prototype SIV,4 which appeared to be most closely related to H3N2 influenza strains associated with human infection in 1995. During 1999, two other types of H3N2 SIV were detected that were most similar to strains of H3N2 influenza that circulated in humans in 1996 and 1997. The TX 98-like strains were quickly displaced. Currently, the H3N2 SIV strains similar to 1996 human strains are predominant in the US swine industry and are represented by an Illinois prototype characterized by Webby et al,4 A/Sw/IL/21587/99 (IL 99).
The IL 99-like strains have undergone significant antigenic drift, resulting in eight twofold or greater differences in titers between the TX 98 HI test antigen provided by NVSL for diagnostic laboratories and test antigens made from recent field strains. The H3N2 SIV strains similar to 1997 human strains are represented by a 1999 Colorado isolate, A/Sw/CO/23619/99 (CO 99), which is dramatically different from the other two types of H3N2 SIV, and requires a dedicated HI test antigen for diagnostic testing. Due to the major antigenic differences among H3N2 strains (represented by TX 98, Il 99, and CO 99), serologic monitoring for natural H3N2 infection has been very complicated. The best solution has been to use the TX 98 test antigen provided by NVSL and a custom HI test antigen made from strains isolated from individual herds infected with IL 99-like H3N2 SIV strains. However, accurate and timely serologic testing is not always readily available due to the time required to isolate and make new test antigens against a herd isolate.
Fortunately, a new H3N2 ELISA test kit (HerdChek H3N2 ELISA; Idexx Laboratories) has just been licensed by the United States Department of Agricultute (USDA). One concern was whether this ELISA would detect antibodies against antigenically diverse strains of H3N2 SIV. In a cooperative effort, 36 sera (provided by Dr Mary Battrell, Murphy-Brown LLC) from selected gilt pigs infected with a recent antigenic drift variant of IL 99-like H3N2 were evaluated with both the H3N2 ELISA and TX 98 HI test (Figure 1). These pigs had received two doses of a bivalent H1N1 and H3N2 USDA-licensed SIV vaccine at 10 and 12 weeks of age, but broke with clinical influenza 7 to 8 weeks after receiving the second dose. Sera were collected months after the outbreak. Before testing with the ELISA, samples were screened with the TX 98 HI test and sera with the lowest titers were selected to evaluate natural immune response to the outbreak strain. This allowed for critical evaluation of the kit’s ability to detect antigenic-drift variant strains of H3N2 SIV (Figure 2). Sera from two pigs experimentally infected with CO 99 H3N2 SIV were also tested with the ELISA.
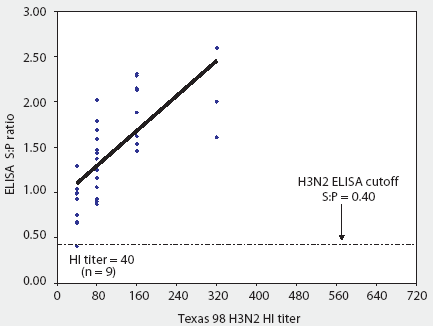
Figure 1: Convalescent sera from 36 pigs naturally infected with a recent antigenic drift variant of IL 99-like H3N2 swine influenza virus (SIV) were tested both by HerdChek H3N2 ELISA (test kit serial 288EZ; Idexx Laboratories, Westbrook, Maine) and hemagglutination inhibition (HI) test, using as antigen Texas 98 H3N2 SIV. The ELISA sample-to-positive (S:P) ratios and reciprocal HI titers are reported.
|
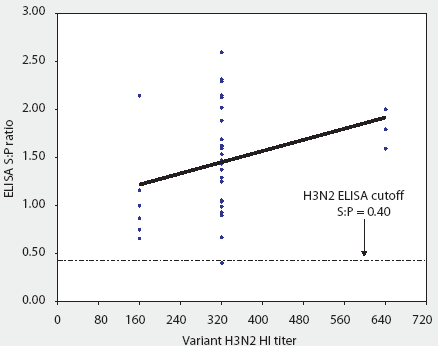
Figure 2: Convalescent sera from 36 pigs naturally infected with a recent antigenic drift variant of IL 99-like H3N2 swine influenza virus (SIV) were tested both by HerdChek H3N2 ELISA (test kit serial 288EZ; Idexx Laboratories, Westbrook, Maine) and hemagglutination inhibition (HI) test, using as antigen homologous IL 99-like H3N2 SIV. The ELISA sample-to-positive (S:P) ratios and reciprocal HI titers are reported.
|
With one exception, all sera were classified as positive by the ELISA, ie, sample-to-positive (S:P) ratio >= 0.40. Figure 1 demonstrates that the reactivity of the assay was linear and that S:P values increased with increasing homologous (IL 99-like) HI titer.
For the two convalescent sera from pigs infected with CO 99 H3N2 SIV, S:P ratios were 0.30 (suspect) and 0.22 (negative), respectively, in four replicate assays.
Summary
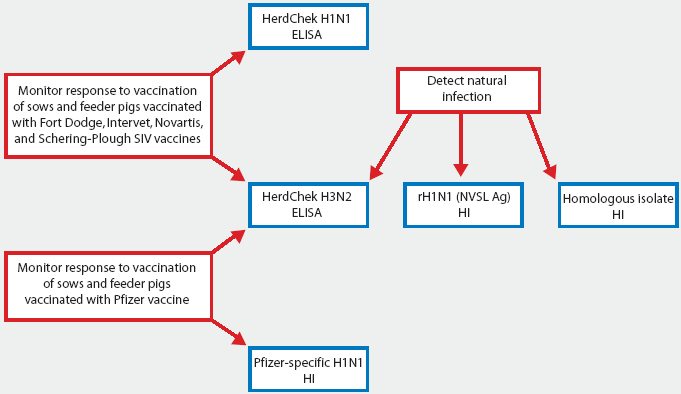
For broadest surveillance against SIV strains currently known to be circulating in US swine, two H1N1 HI tests using different antigens (reassortant and H1N2-like) in combination with the H3N2 ELISA should be utilized. For routine immunization programs utilizing bivalent vaccine, the H3N2 ELISA should be adequate to determine if pigs or sows have been vaccinated according to schedule. Figure 3 shows currently available tests and recent data presented by Jackson et al.5 Reactivity of the H3N2 ELISA with the CO 99 sera was much lower than the responses detected for the IL 99-like outbreak. Further testing with CO 99 sera is needed to evaluate the ability of the assay to reproducibly detect antibodies against CO 99-like strains of H3N2 SIV.
Figure 3: Specific uses of serological tests currently available for swine influenza virus (SIV). The HerdChek H1N1 ELISA and HerdChek H3N2 ELISA test kits are manufactured by Idexx Laboratories, Westbrook, Maine. The reassortant H1N1 (rH1N1) SIV HI test strain, provided by the National Veterinary Services Laboratories (NVSL), is available at the Minnesota Veterinary Diagnostic Laboratory (MVDL), St Paul, Minnesota. Pfizer-specific H1N1 test antigen is provided by Pfizer Animal Health to veterinary diagnostic laboratories upon approval by the Manager of Assay Development, Veterinary Medicine Research and Development, Pfizer Inc, 601 West Cornhusker Highway, Lincoln, NE 68521.
|
Other antigens expressed on the surface of the virus have been proposed as ELISA test antigens. Killed SIV vaccines do not stimulate antibodies against these antigens, which provides for recognition of natural infection in the presence of vaccinal or colostral antibodies. The primary immune response to a killed vaccine is against the dominant surface antigens of the virus, the hemagglutinin and neuraminidase. Ideally, vaccinated sows or their pigs would be seronegative to a test using an alternative antigen unless they had been naturally infected. Due to the broad antigenic spectrum of SIV strains currently circulating in US swine, it is clear that new approaches are needed to monitor herds for recent infection with SIV.
References
*1. Gramer ML, Rossow KD. Epidemiology of swine influenza and implications of reassortment. Proc AD Leman Swine Conf. St Paul, Minnesota. 2004:69-73.
*2. Erickson GA, Gramer ML. North America perplexed by swine influenza. Pig Prog Resp Dis. 2003;October:18-19.
3. Webby RJ, Rossow K, Erickson G, Sims Y, Webster R. Multiple lineages of antigenically and genetically diverse influenza A virus co-circulate in the United States swine population. Virus Res. 2004;103:67-73.
4. Webby RJ, Swenson SL, Krauss SL, Gerrish PJ, Goyal SM, Webster RG. Evolution of swine H3N2 influenza viruses in the United States. J Virol. 2000;74:8243-8251.
*5. Jackson TA, Chandler-Conrey N, Prouty K, Theis D, Ducommun A, Goyal S. Serologic responses to three commercial bivalent swine influenza virus vaccines in juvenile pigs. Proc AASV. Des Moines, Iowa. 2004:235-240.
* Non-refereed references.