Diagnostic notes |
Non refereed |
Update on epidemiology and diagnosis of porcine proliferative enteropathy
Roberto Guedes, DVM, MS, PhD
Department of Veterinary Clinic and Surgery, Veterinary School, Universidade Federal de Minas Gerais, Belo Horizonte, MG 31270-901, PO Box 567, Brazil; E-mail: guedes@vet.ufmg.br.
Cite as: Guedes R. Update on epidemiology and diagnosis of porcine proliferative enteropathy. J Swine Health Prod. 2004;12(3):134-138.
Also available as a PDF.
 Proliferative enteropathy (PE) is an
enteric disease that occurs in pigs1 and
a number of other species.2 The
etiologic agent of PE in swine is the obligatory
intracellular bacterium Lawsonia
intracellularis.3-6 The disease in pigs,
commonly referred to as ileitis, occurs in two major clinical forms. Acute
hemorrhagic diarrhea and sudden death of
replacement animals and finishing pigs close to
market age is known as proliferative hemorrhagic enteropathy (PHE); and chronic,
mild diarrhea with poor growth performance in grower-finisher pigs (Figure
1) is known
as porcine intestinal adenomatosis (PIA).1,7
Proliferative enteropathy (PE) is an
enteric disease that occurs in pigs1 and
a number of other species.2 The
etiologic agent of PE in swine is the obligatory
intracellular bacterium Lawsonia
intracellularis.3-6 The disease in pigs,
commonly referred to as ileitis, occurs in two major clinical forms. Acute
hemorrhagic diarrhea and sudden death of
replacement animals and finishing pigs close to
market age is known as proliferative hemorrhagic enteropathy (PHE); and chronic,
mild diarrhea with poor growth performance in grower-finisher pigs (Figure
1) is known
as porcine intestinal adenomatosis (PIA).1,7
Proliferative enteropathy is widespread among swine herds (30 to 50% are infected) in different types of production systems and in all parts of the world.8-13 It was the most common disease problem in grower-finisher pigs reported in the 2000 National Animal Health Monitoring System survey, occurring on more than a third of all sites and reported on 75% of large sites (10,000 or more total inventory).14 Serologic studies have shown that the prevalence of PE-positive herds ranges from 60 to 90% in different countries.7,15-19 The economic impact of PE on the swine industry is estimated to be very high. It was estimated to cost the industry US$20 per sow annually in Australia,20 and US$20 million annually in the United States.21
The main impact of the disease has been due to increased use of antibiotics and mortality related to the acute form of the disease (PHE). The chronic form (PIA) is seldom detected and diagnosed. In this article, we will discuss some aspects of the epidemiology of the disease, importance of the subclinical-chronic form, and diagnosis of PE.
Transmission
Feces from infected pigs are the main source of new infections in susceptible animals.1 A serologic study performed in 184 herds showed that seropositivity in the breeding herd is an important risk factor for new PIA cases in grower-finisher pigs.22 In addition, seropositivity in grower-finisher animals was a risk factor for PHE in replacement animals.22 In a study of the prevalence of L intracellularis detected in fecal samples by polymerase chain reaction (PCR) in an endemically infected herd in Europe, the highest proportion of positives occurred 10 to 24 days after weaning.23 Only 12.9% of the grower-finisher animals and 0.9% of the mature animals were positive. In this study, the possible epidemiological importance of transmission from breeding sows to young suckling piglets was proposed. Fecal shedding has been reported in pigs as young as 3 weeks of age24 and in pigs 25 and 42 days of age.25 However, despite evidence suggestive of sow-to-piglet transmission, this has yet to be studied and proven.
On the other hand, pig-to-pig contact is an important route of transmission. Rowland and Rowntree26 found an association between a PHE outbreak in young breeding stock and the onset of chronic diarrhea in in-contact weaned pigs a few weeks later. Lawsonia intracellularis infection was transmitted between breeding stock and young adult pigs in a natural PHE outbreak, where movement of sows and boars between units was permitted.27 In an experimental trial, sentinel pigs became infected when housed in contact with pigs experimentally inoculated with a pure culture of L intracellularis.28
Results from a questionnaire survey among British farm owners indicated that slatted and meshed flooring were important risk factors for PE. They suggested that such floors, commonly found in postweaning facilities, are often insufficiently cleaned.29 However, the findings in this report were based on the owners’ opinions, and no diagnostic testing was performed to support the results of the survey. Another study, which included a questionnaire survey, production records, and fecal PCR analysis, reported that the use of new buildings and recent mixing of pigs were associated with PE.30 These findings support a hypothesis that subclinically infected pigs shed L intracellularis in the feces, particularly after stress.
Due to the difficulty in isolating, culturing, and maintaining L intracellularis in vitro, the isolation or re-isolation of viable organisms from diseased intestines is very difficult. Therefore, information about survival and resistance of L intracellularis in the environment is scarce. A unique investigation into this area31 found that intestinal colonization of pigs by L intracellularis was detected after they had been orally inoculated with feces from positive animals. Infected feces had been stored for up to 2 weeks at temperatures between 5 and 15°C. In this same study, pure cultures of L intracellularis were fully susceptible to a quaternary ammonium disinfectant (3% cetrimide), less so to 1% povidone-iodine, but not susceptible to 1% potassium peroxymonosulfate or a 0.33% phenolic mixture.
Other possible mechanisms of transmission that need to be considered in future studies are transmission by mechanical vectors (eg, rubber boots) and biological vectors (eg, mice, small birds, and insects). As a broad range of animal species may be affected by PE,32 interspecies transmission is a real possibility. Proliferative enteropathy was reproduced in hamsters33 and mice34 using homogenized mucosa from PE-affected pigs, and in mice using pure L intracellularis culture extracted from pigs.35 Recently, a natural outbreak of PE was reported in a colony of conventional mice in a University of Missouri research unit.36 Natural PE cases have been reported in ratite birds (eg, emu37 and ostrich32,38,39), but there are no similar reports concerning other bird species.
It seems reasonable to conclude that pig-to-pig contact is probably the main mechanism of transmission, with subclinically infected animals being key elements of this transmission. Future efforts are necessary to obtain further information about sow-to-piglet transmission, resistance of the organism in the environment, and possible mechanical and biological vectors, which will help to explain the high prevalence of the disease among herds worldwide.
 Importance of subclinical proliferative enteropathy
Importance of subclinical proliferative enteropathy
In a field study40 and in a controlled experiment,41 intermittent fecal shedding of L intracellularis, as assessed by PCR, has been detected for a period of up to 12 weeks. In the field study, no clinical disease was observed among either PCR-negative or PCR-positive pigs. In the experimental study,41 diarrhea occurred in challenged pigs only from the second week to the fifth week postinoculation. Growth performance was not evaluated in these studies.
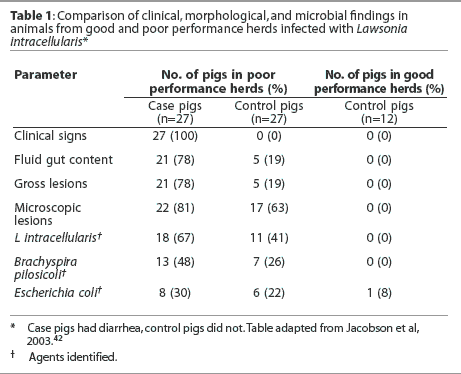
In a recent study,42 clinical, morphological, and microbial findings in animals from good and poor performance herds were compared. The authors concluded that clinically healthy animals from infected herds were often infected with L intracellularis (detected by PCR in fecal samples), and that growth performance in these animals was poor compared to that in uninfected herds (Table 1). Three pigs with diarrhea (case pigs) and three pigs with no signs of clinical disease (control pigs) were selected from each of nine poor performance herds and compared to three control pigs from each of four good performance herds, with pigs matched by age in each case. The average age at which pigs reached a body weight of 25 kg was 85.6 +/- 3.6 days in poor performance herds, and 64.7 +/- 2.6 days in good performance herds. In most case pigs, gross and microscopic lesions were identified that were consistent with PE, colonic spirochaetosis, or both. The most frequently diagnosed enteropathogenic agent was L intracellularis, followed by Brachyspira pilosicoli. An interesting result from this study was the high percentage of control pigs from poor performance herds that were PCR-positive for L intracellularis (41%).
These studies show that growth performance of pigs subclinically infected with a pathogenic isolate of L intracellularis is poor, and that they shed the organism into the environment, resulting in infection of susceptible penmates. It appears that subclinical infection with L intracellularis may result in poor growth performance, unthriftiness, and lost homogeneity in a batch of grower-finisher pigs. These problems have been associated with Mycoplasma hyopneumoniae or circovirus infection, but now an additional possible culprit, L intracellularis, should be considered.
Diagnosis
Diagnostic tools for detecting exposure or infection to L intracellularis (eg, serology, fecal PCR, and immunohistochemistry in tissue samples) have become more available in the last few years. Each diagnostic method evaluates a different epidemiological aspect of PE. Serology, for instance, provides historical information on exposure to the bacteria, while fecal PCR and immunohistochemistry are measures of current infection.
Lawsonia intracellularis is an obligate intracellular organism that infects intestinal epithelial cells. Therefore serum, IgG is not likely to be protective against infection, while secretory IgA and cell-mediated immune responses may play more important roles.41 Nevertheless, detection of serum IgG is a useful tool to evaluate exposure to L intracellularis. Optimization and validation studies of serologic tests for PE have been carried out in recent years, creating new opportunities for a better understanding of the immune response induced by L intracellularis infection.43-45 Indirect immunofluorescent antibody (IFA)44,46 and immunoperoxidase monolayer assay (IPMA)45 are serologic tests that detect L intracellularis-specific serum IgG. Each has a sensitivity of approximately 90% and a specificity of approximately 100%, determined in controlled experimental infection studies. No cross-reactivity was observed when these serologic tests were used on convalescent sera from pigs infected with several Campylobacter species, Salmonella serovar choleraesuis, Salmonella serovar typhimurium, Escherichia coli K88, Brachyspira hyodysenteriae, B pilosicoli, or porcine reproductive and respiratory syndrome virus.45 Serum IgG is first detected in the second week postinfection and persists for 3 to 13 weeks, depending on the form of the disease (PHE or PIA) and its severity.40 Serum IgG was detectable for up to 12 weeks in gilts after a natural outbreak of the acute form of PE, and in 5-week-old pigs infected with high doses of pathogenic L intracellularis. Conversely, seropositivity in grower-finisher pigs in field conditions usually persisted for only 2 to 3 weeks and was detected mainly in 18- to 26-week-old pigs.40 However, age at seroconversion in grower-finisher pigs may vary depending on the feed medication program, pig flow, and type of flooring.30 Although we were unable to demonstrate a statistically significant association between severity of gross lesions and serum titers in pigs 3 weeks after experimental infection,43 we believe that the level of infection correlates with serum titers. Serum IgG titer decays gradually after reaching its peak; therefore, the higher the peak serum titer, the longer detectable serum IgG persists.
Although the specificity of PCR for detection of L intracellularis DNA in fecal samples is virtually 100%,47 the sensitivity of the technique ranges between 39 and 72% in experimentally infected pigs.43,46 In field and controlled experimental studies, animals became positive by fecal PCR 1 to 2 weeks before they seroconverted.41,43 When animals are PCR-positive and seronegative, either they are in the early stage of infection and have not yet had time to seroconvert, or the level of infection is not sufficient to induce a systemic humoral immune response detectable by the serologic test. When animals are PCR-negative and seropositive, either they have been previously exposed to L intracellularis and are no longer shedding the organism, or detection of fecal shedding was limited by the low sensitivity of the PCR technique in fecal samples. Nonetheless, stage of infection with L intracellularis, based on the percentage of seropositive pigs or the percentage of PCR-positive pigs (representing fecal shedding), or both, and observation of a clinical problem represented by diarrhea or poor growth performance, must be evaluated as a whole.
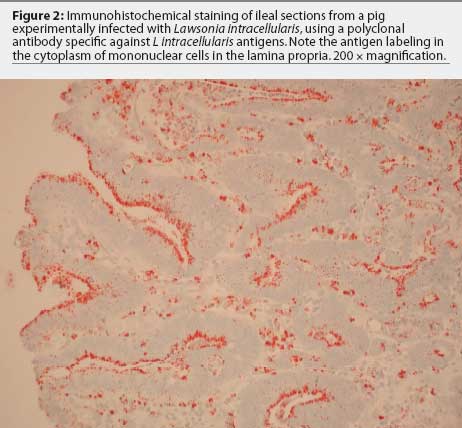 Immunohistochemistry in histologic sections of ileum, using antibodies specific
for L intracellularis
antigens,48,49 has a sensitivity of 87%, compared to histological
examination using hematoxylin and eosin staining (sensitivity 37%) and or
Warthin-Starry silver stain (sensitivity
50%).43 Lawsonia
intracellularis can be detected by immunohistochemistry in just a few
intestinal crypts early in infection and in the cytoplasm of macrophages in the
lamina propria late in the course of the disease (Figure 2). Those two stages of the
disease cannot be differentiated in sections
stained with hematoxylin and eosin or Warthin-Starry silver stains.
Immunohistochemistry in histologic sections of ileum, using antibodies specific
for L intracellularis
antigens,48,49 has a sensitivity of 87%, compared to histological
examination using hematoxylin and eosin staining (sensitivity 37%) and or
Warthin-Starry silver stain (sensitivity
50%).43 Lawsonia
intracellularis can be detected by immunohistochemistry in just a few
intestinal crypts early in infection and in the cytoplasm of macrophages in the
lamina propria late in the course of the disease (Figure 2). Those two stages of the
disease cannot be differentiated in sections
stained with hematoxylin and eosin or Warthin-Starry silver stains.
The PE status of a herd should be evaluated in two common field situations.50 The first one is observation of poor performance, diarrhea, or both in a group of grower-finisher pigs. It is recommended that two or three gaunt pigs with diarrhea be selected from the most severely affected pens for euthanasia, necropsy, and submission of samples of large and small intestine to a veterinary diagnostic laboratory. In addition to the standard bacteriologic and histologic tests, specifically request immunohistochemistry for L intracellularis and bacteriology for Brachyspira (species identification). Collect fecal samples (at least a pea-sized amount of feces) from 20 pigs with loose stools and submit refrigerated samples for PCR testing. Although pooling of fecal samples from two to three animals is acceptable to reduce costs of testing, this does inherently reduce the sensitivity of the test.
The second field situation is the necessity for knowing the probable time of infection in order to determine the optimum time for strategic medication of grower-finisher pigs with growth performance problems, which might be related to L intracellularis infection. Serological testing is recommended in this situation, as it costs less than PCR testing, is suitable for testing large numbers of samples, and provides an estimate of time of exposure. Serum samples from several groups of at least 20 grower-finisher pigs with 3-week age differences should be submitted for serologic testing. Strategic medication, vaccination, or both, are usually recommended 2 to 3 weeks before the age of seroconversion.
Further research focusing on the epidemiology of L intracellularis infection and transmission is imperative in order to design eradication protocols for PE. The presence of subclinically-chronically infected pigs seems to be the major factor contributing to the economic impact of poor growth performance and dissemination of the disease in the herd. Careful interpretation of appropriate diagnostic tests, which may be used to determine the time for treatment or vaccination to be most effective, will help to minimize losses due to PE.
Acknowledgment
I thank Dr Connie Gebhart for the critical review of the manuscript.
References
1. McOrist S, Gebhart CJ. Porcine proliferative enteropathies. In: Straw BE, D’Allaire S, Mengeling W, Taylor DJ, eds. Diseases of Swine. 8th ed. Ames, Iowa: Iowa State University Press; 1999:521-534.
2. Cooper DM. Proliferative enteritis in the hamster, horse, deer and ostrich: detection and characterization of Lawsonia intracellularis [master’s thesis]. St Paul, Minnesota: University of Minnesota; 1996.
3. Gebhart CJ, Barns SM, McOrist S, Lin GF, Lawson GHK. Ileal symbiont intracellularis, an obligate bacterium of porcine intestine showing a relationship to Desulfuvibrio species. Int J Syst Bacteriol. 1993;43:533-538.
4. Lawson GHK, McOrist S, Jasni S, Mackie RA. Intracellular bacteria of porcine proliferative enteropathy: cultivation and maintenance in vitro. J Clin Microbiol. 1993;31:1136-1142.
5. McOrist S, Jasni S, Mackie RA, MacIntyre N, Neef N, Lawson GHK. Reproduction of porcine proliferative enteropathy with pure culture of Ileal symbiont intracellularis. Inf Immun. 1993;61:4286-4292.
6. McOrist S, Gebhart CJ, Boid R, Barns SM. Characterization of Lawsonia intracellularis gen. nov, sp nov, the obligately intracellular bacterium of porcine proliferative enteropathy. Int J Syst Bacteriol. 1995;45:520-525.
7. Lawson GHK, Gebhart CJ. Proliferative enteropathy: review. J Comp Pathol. 2000;122:77-100.
8. Chang WL, Wu CF, Wu Y, Kao YM, Pan MJ. Prevalence of Lawsonia intracellularis in swine herds in Taiwan. Vet Rec. 1997;141:103-104.
9. Kim O, Kim B, Chae C. Prevalence of Lawsonia intracellularis in selected pig herds in Korea as determined by PCR. Vet Rec. 1998;143:567-589.
*10. Pozo J, Carvajal A, De Arriba ML, Lanza I, Rubio P. Presence of Lawsonia intracellularis as an aetiological agent in enteric disorders in Spanish swine herds. Proc IPVS. Birmingham, United Kingdom. 1998:416.
*11. Takghashi K, Kishimoto Y, Yamamoto A, Nose Y, Odagiri Y. Porcine proliferative enteropathy caused by Lawsonia intracellularis in Japan. Proc IPVS. Birmingham, United Kingdom. 1998;3:109.
12. Chiriboga AECN, Guimaraes WV, Vanetti MCD, Araujo EF. Detection of Lawsonia intracellularis in faeces of swine from the main production regions in Brazil. Can J Microbiol. 1999;45:230-234.
13. Stege H, Jensen TK, Møller K, Bkbo P, Jorsal SE. Prevalence of intestinal pathogens in Danish finishing pig herds. Prev Vet Med. 2000;46:279-292.
14. APHIS, USDA, VS. Swine National Animal Health Monitoring System. 2002. Swine 2000, Part II: Reference of swine health and health management in the United States, 2000. Available at http://www.aphis.usda.gov/vs/ceah/cahm/Swine/swine.htm. Accessed February 18, 2004.
*15. Dunser M, Untersperger M, Schweighardt H, Schuh M. Comparative studies by PCR and indirect immunofluorescent antibody test on the occurrence of Lawsonia intracellularis in upper Austrian swine herds. Proc IPVS. Melbourne, Australia. 2000:59.
*16. Hurtado ME, Maestro R, Rolo M, Palencia L, Sandoval J. Seroepidemiologic evaluation of Lawsonia intracellularis in swine farms in Venezuela. Proc IPVS. Melbourne, Australia. 2000:261.
*17. Ohlinger VF, Pesch S, Knittel J. Prevalence of Lawsonia intracellularis in diagnostic samples from Germany, the Netherlands and Belgium. Proc IPVS. Ames, Iowa. 2002:416.
*18. Wendt M, Bonitz A, McOrist S. Prevalence of Lawsonia intracellularis infection in German breeding herds. Proc IPVS. Ames, Iowa. 2002:27.
*19. Prieto C, Pineiro C, Aparicio M. Seroprevalence of L. intracellularis in different production systems. Proc IPVS. Ames, Iowa. 2002:218.
20. Lawson GHK, McOrist S. The enigma of the proliferative enteropathies: a review. J Comp Pathol. 1993;108:41-46.
21. Winkelman NL. Ileitis: an update. Comp Cont Educ Pract Vet. 1996;19:519-525.
22. Bronsvoort M, Norby B, Bane DP, Gardner IA. Management factors associated with seropositivity to Lawsonia intracellularis in US swine herds. J Swine Health Prod. 2001;9:285-290.
*23. Moller K, Jensen TK, Jorsal SE. Detection of Lawsonia intracellularis in endemically infected pig herds. Proc IPVS. Birmingham, United Kingdom. 1998;2:139.
*24. López J, Rodríguez J, Valle R, Alvarez M, Gómez M. A longitudinal study of porcine proliferative enteropathy in a commercial pig farm in Yucatan, Mexico. Proc IPVS. Melbourne, Australia. 2000:68.
*25. Moreno AM, Coutinho LL, Baccaro MR. Detection of DNA of Lawsonia intracellularis using polymerase chain reaction. Proc IPVS. Birmingham, United Kingdom. 1998:113.
26. Rowland AC, Rowntree PGM. A hemorrhagic bowel syndrome associated with intestinal adenomatosis in the pig. Vet Rec. 1972;91:235-241.
27. Love RJ, Love DN, Edwards MJ. Proliferative haemorrhagic enteropathy in pigs. Vet Rec. 1977;100:65-68.
*28. Jordan KM, Knittel JP, Schmoll EM, Schwartz KJ, Roof MB, Larson DJ, Hoffman LJ. A Lawsonia intracellularis transmission study using a pure culture inoculated seeder-pig sentinel model. Proc AASV. Quebec, Canada. 1997:243-248.
29. Smith SH, McOrist S, Green LE. Questionnaire survey of proliferative enteropathy on British pig farms. Vet Rec. 1998;142:690-693.
30. Bane DP, Neumann E, Gebhart CJ, Gardner IA, Norby B. Porcine proliferative enteropathy: a case-control study in swine herds in the Unites States. J Swine Health Prod. 2001;9:155-158.
31. Collins AM, Love RJ, Pozo J, Smith SH, McOrist S. Studies on the ex vivo survival of Lawsonia intracellularis. Swine Health Prod. 2000;8:211-215.
32. Cooper DM, Gebhart CJ. Comparative aspects of proliferative enteritis. JAVMA. 1998;212:1446-1451.
33. McOrist S, Lawson GHK. Possible relationship of proliferative enteritis in pigs and hamsters. Vet Microbiol. 1987;15:293-302.
*34. Chang K, Kurtz HJ, Ward GE, Gebhart CJ. The use of a weaned mouse as a model to study the role of Campylobacter species in proliferative enteritis of swine. Proc Int Workshop Campylobacter Inf. London, United Kingdom. 1985:102.
35. Smith DGE, Mitchell SC, Nash T, Rhind S. Gamma interferon influences intestinal epithelial hyperplasia caused by Lawsonia intracellularis infection in mice. Inf Immun. 2000;68:6737-6743.
*36. Abshier JM, Besh-Williford CL, Franklin CL, Russell SP. Spontaneous infection of Lawsonia intracellularis-like bacteria in the mouse. Proc CRWAD. Saint Louis, Missouri. 2001:49.
37. Lemarchand TX, Tully TN, Shane SM, Duncan DE. Intracellular Campylobacter-like organisms associated with rectal prolapse and proliferative enteroproctitis in emus (Dromaius novae-hollandiae). Vet Pathol. 1997;34:152-156.
38. Cooper DM, Swanson DL, Barns SM, Gebhart CJ. Comparison of the 16S ribosomal DNA sequence from the intracellular agent of proliferative enteritis in a hamster, deer, and ostrich with the sequence of a porcine isolate of Lawsonia intracellularis. Int J Syst Bacteriol. 1997;47:635-639.
39. Cooper DM, Swason DL, Gebhart CJ. Diagnosis of proliferative enteritis in frozen and formalin fixed, paraffin-embedded tissues from a hamster, horse, deer and ostrich using a Lawsonia intracellularis-specific multiplex PCR assay. Vet Microbiol. 1997;54:47-62.
40. Guedes RMC, Gebhart CJ, Armbruster GA, Roggow BD. Serologic follow-up of a repopulated swine herd after an outbreak of proliferative enteropathy. Can J Vet Res. 2002;66:258-263.
41. Guedes RMC, Gebhart CJ. Onset and duration of fecal shedding, cell-mediated and humoral immune responses in pigs after challenge with a pathogenic isolate or attenuated vaccine strain of Lawsonia intracellularis. Vet Microbiol. 2003;91:135-145.
42. Jacobson M, Hård af Segertad C, Gunnasrsson A, Fellström C, de Verdier Klingenberg K, Wallgren P, Jensen-Waern M. Diarrhoea in the growing pig – a comparison of clinical, morphological and microbial findings between animals from good and poor performance herds. Res Vet Sci. 2003;74:163-169.
43. Guedes RMC, Gebhart CJ, Winkelman NA, Mackie-Nuss RAC, Marsteller TA, Deen J. Comparison of different methods for diagnosis of porcine proliferative enteropathy. Can J Vet Res. 2002;66:99-107.
44. Guedes RMC, Gebhart CJ, Winkelman NL, Mackie-Nuss RA. A comparative study of an indirect fluorescent antibody test and an immunoperoxidase monolayer assay for the diagnosis of porcine proliferative enteropathy. J Vet Diag Invest. 2002;14:420-423.
45. Guedes RMC, Gebhart CJ, Deen J, Winkelman NL. Validation of an immunoperoxidase monolayer assay as a serologic test for porcine proliferative enteropathy. J Vet Diag Invest. 2002;14:528-530.
46. Knittel JP, Jordan DM, Schwartz KJ, Janke BH, Roof MB, McOrist S, Harris DL. Evaluation of antemortem polymerase chain reaction and serologic methods for detection of Lawsonia intracellularis exposed pigs. Am J Vet Res. 1998;59:722-726.
47. Jones GF, Ward GE, Murtaugh MP, Lin G, Gebhart CJ. Enhanced detection of intracellular organism of swine proliferative enteritis, ileal symbiont intracellularis, in feces by polymerase chain reaction. J Clin Microbiol. 1993;31:2611-2615.
48. McOrist S, Boid R, Lawson GHK, McConnell I. Monoclonal antibodies to intracellular Campylobacter-like organisms of the porcine proliferative enteropathies. Vet Rec. 1987;121:421-422.
49. Guedes RMC, Gebhart CJ. Preparation and characterization of polyclonal and monoclonal antibodies against Lawsonia intracellularis. J Vet Diag Invest. 2003;15:438-446.
50. Guedes RMC, Gebhart CJ. Proliferative enteropathy (Ileitis): learning how to use and interpret diagnostic tests. Int Pigletter. 2002;22:19-23.
* Non-referred references