Bronsvoort M. 2001;6:285-289 Management factors associated with
Original research
Peer reviewed
Management factors associated with seropositivity to Lawsonia intracellularis in US swine herds
Mark Bronsvoort, BVSc, MPVM; Bo Norby, DVM, MPVM; David P. Bane, DVM, PhD; Ian A. Gardner, BVSc, PhD
MB: University of Liverpool, Department of Clinical Science
and Animal Husbandry, Leahurst, Nr Neston L64 7TE, UK; BN: Michigan
State University, Department of Large Animal Clinical Sciences,
College of Veterinary Medicine, East Lansing, MI 48824; DB: Elanco
Animal Health, Indianapolis, IN 46285; IG: University of California,
Davis, Department of Medicine and Epidemiology, School of Veterinary
Medicine, Davis, CA 95616. Tel: 530-752-6992;
Fax: 530-752-0414; E-mail: iagardner@ucdavis.edu
Bronsvoort M, Norby B, Bane DP, et al. Management factors associated with seropositivity to Lawsonia intracellularis in US swine herds. J Swine Health Prod. 2001;9(6):285-290. Also available as a PDF.
Summary
Objective: To determine risk factors for Lawsonia intracellularis seropositivity in the breeding and grower-finisher units of US farrow-to-finish swine herds.
Methods: Serum was collected from 15 breeding females and 15 grower-finisher pigs per herd in 184 farrow-to-finish herds, a subset of 405 herds in the National Animal Health Monitoring System (NAHMS) Swine ’95 Study that examined management, health, and productivity in herds with at least 300 finisher pigs. Sera were tested by indirect fluorescent antibody test for L intracellularis. Test results were linked with NAHMS questionnaire data, and a logistic regression model of management factors associated with L intracellularis serological status was developed. Separate models were used for breeding and grower-finisher units.
Results: Risk factors for seropositive breeding units were L intracellularis-seropositive status of the grower-finisher unit, use of a continuous system of management for the farrowing unit, and a young parity structure (<75% multiparous sows). Risk factors for seropositive grower-finisher units were L intracellularis-seropositive status of the breeding unit, the number of pigs entering the grower-finisher stage, raising pigs on concrete slats, and intensive management compared with raising pigs on outdoor lots.
Implications: Use of all in-all out management in the farrowing house and an older parity structure in the sow herd were associated with a lower risk of L intracellularis seropositivity in the breeding unit, and slatted concrete flooring in grower-finisher houses was associated with a greater risk. Alteration of these management factors might improve control of L intracellularis infection in farrow-to-finish herds.
Keywords: swine, risk factors, porcine proliferative enteropathy, Lawsonia intracellularis, indirect fluorescent antibody test
Received: October 4, 2000
Accepted: February 15, 2001
Lawsonia intracellularis is recognized as the primary cause of porcine proliferative enteropathy (PPE), which is characterized by proliferation of crypt enterocytes and thickening of the intestinal mucosa.1-4 Infection with L intracellularis may cause chronic enteritis, manifested clinically as reduced growth rate and diarrhea in weaned pigs approximately 6 to 20 weeks of age. An acute syndrome characterized by intestinal hemorrhage and sudden death occurs in pigs more than 5 months of age. Porcine proliferative enteropathy is estimated to result in direct losses of $3 to $11 per pig ($US), attributable to increased feeding costs and time to reach slaughter weight.5
Porcine proliferative enteropathy is recognized worldwide as a significant cause of enteritis in pigs. The National Animal Health Monitoring System (NAHMS) Swine ’95 survey reported that clinical PPE occurred in 7% of US finisher herds, on the basis of a confirmed laboratory result or the diagnostic opinion of the herd veterinarian.6 Herd prevalence in other countries ranges from 20 to 94%,5,7-9 but this variation might be attributable in part to differences in the sensitivities of the diagnostic methods used and in the numbers of pigs and age groups sampled in different studies. Until recently, investigation of herd-level risk factors has been hampered by lack of an accurate ante mortem test. Development of a polymerase chain reaction (PCR) assay10 has facilitated ante mortem diagnosis and prevalence surveys, but the PCR is less sensitive than the indirect fluorescent antibody test (IFAT) that detects serum antibodies to L intracellularis.11
The objective of this cross-sectional study was to assess management risk factors associated with the L intracellularis serological status of breeding and grower-finisher units in 184 farrow-to-finish herds surveyed as part of the NAHMS Swine ’95 study.
Materials and methods
Study population and data collection
A total of 1477 swine operations from 16 major swine-producing states (representing 91% of the US swine inventory) participated in Phase 1 of the NAHMS survey. In Phase 2, questionnaires were used to collect data about various management practices and health issues in a sub-sample of 405 herds that had at least 300 finisher pigs. A detailed description of the design of the NAHMS Swine ’95 survey is published elsewhere.12
Of the 405 operations that provided questionnaire data, 285 participated in serologic sampling of 15 breeding animals and (or) 15 grower-finisher pigs per herd. Samples from females were distributed among parities (mean 2.7, range zero to 13) and wherever possible, finisher samples were obtained from pigs within 30 days of slaughter (mean 159 days of age, range 90 to 260 days). Blood samples were collected by herd veterinarians and shipped to the National Veterinary Services Laboratory of the USDA. Serum was stored in 0.4-ml aliquots at -40 degrees C until tested.
Serological testing and herd classification
Sera from the 184 farrow-to-finish herds that had provided breeding and grower-finisher samples were tested for L intracellularis by IFAT, as described by Knittel et al.11 Briefly, an anti-porcine IgG-fluorescein-isothiocyanate conjugate (diluted 1:10 in PBS) was bound to porcine IgG (diluted 1:30 in PBS) that was bound to L intracellularis-infected cell cultures in the wells of 72-well microtitration plates. Plates were examined by fluorescent microscopy, and wells with fluorescing bacteria were interpreted as positive.
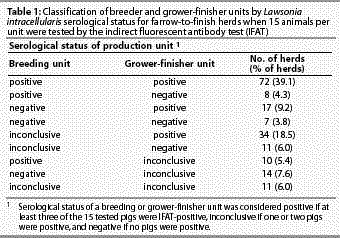
On the basis of a study by Knittel et al,11 the sensitivity of the IFAT was estimated to be 0.90 and the specificity was estimated to be 0.99. These estimates were used in combination with our best guess of prevalence of infection in each herd to guide choice of an appropriate number of test-positive pigs to designate the unit status for L intracellularis. Units were classified as positive if three or more of 15 tested pigs were IFAT-positive; inconclusive if one or two pigs were IFAT-positive; and negative if no pigs were IFAT-positive. Breeding and grower-finisher units in each herd were classified separately.
Statistical analysis
Questionnaire data and IFAT results were matched using the swine herd’s identification number in an Access database (Microsoft Corporation, Redmond, Washington). We selected for risk factor analysis a subset of questions that we considered were relevant a priori to infectious enteric diseases such as PPE. Statistical analyses were performed using BMDP 2D, 4F and LR (BMDP Statistical Software, Inc, Los Angeles, California). Descriptive statistics were calculated for each variable, and continuous variables were categorized for use in analyses on the basis of the median and quartiles. For each potential risk factor, associations with the L intracellularis status of the breeder and grower-finisher units were determined. Variables with P<.2 (chi-square) were considered for inclusion in multivariable logistic regression models.
Separate logistic regression models were developed for the L intracellularis serological status of breeder and grower-finisher units. Data for breeder and grower-finisher units of inconclusive L intracellularis status were excluded from analysis to increase the herd-level specificity of our classification without compromising herd-level sensitivity.13,14 Variables were added to the model when the chi-square P value for the variable was <.10. Interactions between factors in the final best-fitting models were also assessed. For the final models, adjusted odds ratio (OR) and 95% confidence intervals were obtained to quantify the strength of association with the different risk factors. Overall model fit was assessed using the Hosmer-Lemeshow (H-L) goodness-of-fit statistic.15
Results
Indirect fluorescent antibody testing
The median number of L intracellularis-seropositive samples was five (range zero to 14) for breeding units and two (range zero to 15) for grower-finisher units. Of 184 breeding units, 90 (48.9%) were positive, 38 (20.7%) were negative, and 56 (30.4%) were inconclusive for L intracellularis using our interpretive thresholds. Of the 184 growing units, 123 (66.9%) were positive, 26 (14.1%) were negative, and 35 (19.0%) were inconclusive. A total of 141 herds (76.6%) were classed as positive because their breeding unit, grower-finisher unit, or both were positive (Table 1).
Risk factors for seropositive breeding units
Eleven variables passed the initial screening criterion for risk factors (P<.2) for L intracellularis serologic status of breeding units (Table 2). Management variables related to the grower-finisher unit were excluded from analysis except for grower-finisher serological status. This variable was considered to represent the combined effect of all grower-finisher risk factors on the L intracellularis status of the breeding unit. The total number of pigs in the herd was considered a potential confounder on the basis of a prior study16 and was also included in the initial modeling. Though a statistically significant variable, the age when pigs left the nursery could not be biologically justified as a risk factor for breeding unit serologic status and was not considered in the final model.
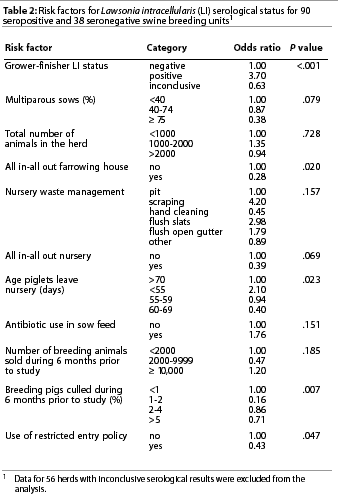
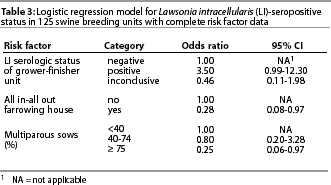
The final multivariable model, which excluded 56 units with inconclusive serologic test results, is given in Table 3 with the OR for each variable. Records for three breeding units tested and recorded in Table 1 were not used in the final model because of missing risk factor data. The odds of the breeding unit being positive were 3.5 times greater when the grower-finisher unit was positive. Use of an all in-all out farrowing policy and having 75% multiparous sows each reduced the odds of being seropositive about fourfold. Two-way interactions between risk factors were not significant (P>.05). Overall fit of the model was good (H-L goodness-of-fit c2=2.27, 8 df, P=.97).
Risk factors for seropositive grower-finisher units
Twelve variables passed the initial screening criterion for risk factors (P<.2) for L intracellularis serologic status (Table 4). Sow serologic status was used as a surrogate variable to represent the combined effects of risk factors in the sow herd that might have influenced the risk of transmission of L intracellularis to the grower-finisher herd. The L intracellularis serological status of sows, percentage of animals on concrete slats, rearing some pigs on outdoor lots, and the number of pigs entering the unit were included in the multivariable modeling. Waste management in the grower-finisher, though potentially a risk factor, was not evaluated further because of sparse data that created instability in the model. In addition, when categories were combined, the association with seropositivity was no longer evident. Five health indices for growers (number of deaths, diarrhea, total disease problems, cull rate, and culled because of diarrhea) were not considered further because it appeared that these were outcomes of L intracellularis infection rather than risk factors for it. The variable ‘treat sick pig and remove to separate pen’ improved model fit but was not included because of difficulty interpreting the categories.
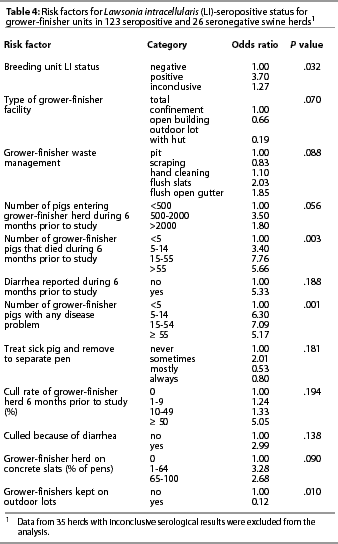
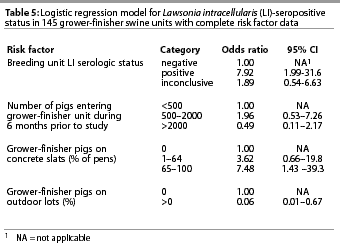
The final logistic model, which excluded 35 units with inconclusive serologic results, included four variables (Table 5). Records for an additional four herds tested and reported in Table 4 were not used in the final model because of missing risk factor data. The serologic status of the breeding unit was the most influential variable, with the odds of the grower-finisher herds being positive almost eight times greater when the breeding unit was also positive. Grower-finisher seropositivity was also associated with the percentage of pigs housed on concrete slats, with the odds of being positive 7.5 times higher for units where more than 65% of pigs were on concrete slats compared to all other floor types. Finally, there was a strong association between grower-finisher status and whether the pigs were on outdoor lots, with the odds of being seropositive substantially lower for pigs on outdoor lots compared with all other surfaces. The overall fit of this model was adequate, with the H-L goodness-of-fit c2=7.68 with 8 df (P=.465).
Discussion
In this study, we identified three factors (L intracellularis serological status of grower-finisher unit, farrowing house management, and percentage of multiparous sows) that best explained the variation in L intracellularis serologic status of breeding units, and four factors (L intracellularis status of breeding unit, number of pigs entering the unit in the previous 6 months, percentage of pigs housed on concrete slats, and rearing some pigs on outdoor lots) that best explained the variation in L intracellularis serologic status in grower-finisher units.
We found that the L intracellularis serologic status of the grower-finisher herd was strongly associated with the serological status of the breeding herd and vice versa. This finding is consistent with evidence that L intracellularis is primarily transmitted by contact with infective fecal material and that the minimal infectious dose for pigs is low.17 The sow herd probably acts as an important source of infection for unweaned pigs, although the potential for transmission of L intracellularis among pigs might be greater in nursery and grower-finisher units because of increased pig-to-pig contact and larger population sizes. However, questionnaires did not have sufficient detail to document the proximity and management relationship (including specific replacement stock practices) between the units in individual herds. For many herds with single-site production, it is possible that replacement gilts and humans or other fomites might have contributed to spread of the agent between the grower-finisher unit and the breeding unit.
The ‘protective effect’ of a higher proportion of multiparous sows suggests that either there may be a greater resistance to L intracellularis with age, or that there is a lower turnover of breeding animals in these herds and thus less contact between susceptible and infected animals and (or) a lower risk of introduction of the pathogen. The association of an all in-all out farrowing policy with a reduced risk of being seropositive suggests that transmission of L intracellularis within breeding units is reduced by minimizing the direct and indirect contact between infected and susceptible animals. Alternatively, it may reflect a generally higher level of management, with better cleaning and disinfection of the farrowing room.
Our results are broadly consistent with those reported by Smith et al,16 who studied risk factors for owner-reported occurrence of PPE in 319 British pig herds. These authors found that use of all in-all out policies and breeding herd sizes of <500 sows had a protective effect. The association between small herd size and clinical PPE was attributed to management differences between small and large herds. In the British study, it is also possible that owners of smaller herds (<500 sows) might have been less familiar with PPE and did not identify occurrences of mild and subclinical disease. In contrast, we did not find an association with herd size, whether herd size was measured as the total number of pigs or the total number of breeding pigs. The results of the British study may have differed from our results in part because we evaluated risk factors for L intracellularis seropositivity, while the British study recorded the occurrence of clinical disease. In our study, serologic evidence of L intracellularis infection was nearly ten times more likely to occur than clinical disease, which was reported in about 7% of grower units during the 12 months prior to serologic testing.6
Risk factors that we identified in our grower-finisher unit model were similar to those reported by Smith et al,16 who found a greater risk of disease when pigs were kept on slats or meshed flooring. The question in the NAHMS questionnaire was worded differently to the one used in the survey by Smith et al, and categorization of slatted flooring varied. However, in our study, there was an association between housing on concrete slats in >64% of the pens on the farm and an increased risk of the grower-finisher herd being seropositive. Smith et al16 suggested that retention of feces on the flooring (possibly between slats) between batches of pigs might be the biological reason for the relationship. Because our study was cross-sectional, we could not exclude the possibility that farms had switched to slatted flooring in response to a clinical PPE problem. We found that use of outdoor lots had a strong protective effect, but this most likely is a proxy for lower stocking density, reduced stress, and a more natural digestive flora from rooting in the soil.
To our knowledge, there are no published studies of risk factors for L intracellularis infection in US swine herds, and only one study of risk factors for PPE outbreaks. Bane et al18 used a case-control study to evaluate risk factors for clinical PPE in mid-western herds, and found that placement of pigs in new facilities and recent mixing of pigs (£2 weeks) were important risk factors for PPE. However, because the NAHMS questionnaires did not address these factors, it was not possible to directly compare findings from the two studies. Bane et al also reported that management factors, such as flooring type, continuous pig flow, lack of washing and disinfection, and lack of isolation of breeding stock, were not associated with clinical disease. Although these results appear to contradict ours and those of Smith et al,16 Bane et al18 used a small sample size and did not evaluate the serologic profiles of the case or control herds. Consequently, they were unable to determine unequivocally whether or not control herds were infected with L intracellularis.
It was difficult to determine the optimal cut-off value (number of test-positive pigs) for classification of L intracellularis serologic status because of the lack of reliable estimates of the sensitivity and specificity of the IFAT. Knittel et al11 suggested indirectly that the sensitivity of the IFAT for an individual test was approximately 0.9 and the specificity was 0.99. However, test sensitivity in field studies is often lower than in experimental studies, and there is evidence that sensitivity varies with factors such as age, and stage and severity of disease, which might also vary among herds. Moreover, in the field, there might be an increased risk of exposure to organisms that induce cross-reacting antibodies.19 Our solution to this potential problem was to require a minimum of three test-positive pigs to designate a positive unit compared with the threshold of one or two seropositive pigs that is commonly used in epidemiologic studies. We excluded units with one or two seropositive pigs from the logistic models. Although it reduced the sample size for our analyses, we believe that this approach reduced misclassification of a unit’s true status and resulted in less bias in estimated odds ratios.
The risk factors identified in this study were non-specific, with the exception of the use of concrete slats. In part, this was because the questionnaires were not designed specifically to study risk factors for L intracellularis infection or clinical PPE. Moreover, the cross-sectional design of the study precluded us from differentiating factors associated with the introduction of L intracellularis and factors associated with transmission of L intracellularis once infection had been established in the herd.
Implications
- Use of all in-all out management in the farrowing house and an older parity structure in the sow herd were associated with a lower risk of seropositivity to L intracellularis in the breeding unit.
- Slatted concrete flooring in grower-finisher houses was associated with an increased risk of seropositivity to L intracellularis, but further studies are needed before making specific flooring recommendations.
- Rearing pigs on outdoor lots might reduce the risk of transmission of L intracellularis in grower-finisher herds, reflecting a less intensive production system and less stressful environment for the pigs.
- Alteration of these three management factors might improve control of L intracellularis infection in farrow-to-finish herds.
Acknowledgements
The Wellcome Trust (UK) generously supported the senior author through a Research Training Fellowship in Tropical Clinical Epidemiology at the University of Liverpool (UK). We thank Dr Eric Bush, Centers for Epidemiology and Animal Health, USDA-APHIS, VS, for providing the questionnaire data and for his helpful discussions, and Dr Kenton Morgan, University of Liverpool, for technical guidance.
References — refereed
1. McOrist S, Lawson GH, Roy DJ, Boid R. DNA analysis of intracellular Campylobacter-like organisms associated with the porcine proliferative enteropathies: novel organism proposed. Fems Microbiol Lett. 1990;57:189-193.
2. McOrist S, Roberts L, Jasni S, Rowland AC, Lawson GH, Gebhart CJ, Bosworth B. Developed and resolving lesions in porcine proliferative enteropathy: possible pathogenetic mechanisms. J Comp Pathol. 1996;115:35-45.
3. Joens LA, Nibbelink S, Glock RD. Induction of gross and microscopic lesions of porcine proliferative enteritis by Lawsonia intracellularis. Am J Vet Res. 1997;58:1125-1131.
4. Gebhart CJ, Barns SM, McOrist S, Lin GF, Lawson GH. Ileal symbiont intracellularis, an obligate intracellular bacterium of porcine intestines showing a relationship to Desulfovibrio species. Int J Sys Bacteriol. 1993;43:533-538.
5. McOrist S, Smith SH, Green LE. Estimate of direct financial losses due to porcine proliferative enteropathy. Vet Rec. 1997;140:579-581.
6. USDA. NAHMS Swine’95: Part II. Reference of 1995 U.S grower-finisher health and management practices. http://www.aphis.usda.gov/vs/ceah/cahm/Swine/sw95des2.pdf . Accessed August 17, 2001.
7. Kim O, Kim B, Chae C. Prevalence of Lawsonia intracellularis in selected pig herds in Korea as determined by PCR. Vet Rec. 1998;143:587-589.
8. Stege H, Jensen TK, Møller K, Baekbo P, Jorsal SE. Prevalence of intestinal pathogens in Danish finishing pig herds. Prev Vet Med. 2000;46:279-292.
9. Møller K, Jensen TK, Jorsal SE, Leser TD, Carstensen B. Detection of Lawsonia intracellularis, Serpulina hyodysenteriae, weakly beta-hemolytic intestinal spirochetes, Salmonella enterica and Escherichia coli from swine herds with and without diarrhea among growing pigs. Vet Microbiol. 1998;62:59-72.
10. Jones GF, Ward GE, Murtaugh MP, Lin G, Gebhart CJ. Enhanced detection of intracellular organism of swine proliferative enteritis, ileal symbiont intracellularis, in feces by polymerase chain reaction. J Clin Microbiol. 1993;31:2611-2615.
11. Knittel JP, Jordan DM, Schwartz KJ, Janke BH, Roof MB, McOrist S, Harris DL. Evaluation of antemortem polymerase chain reaction and serologic methods for detection of Lawsonia intracellularis-exposed pigs. Am J Vet Res. 1998;59:722-726.
12. Losinger WC, Bush EJ, Hill GW, Smith MA, Garber LP, Rodriguez JM, Kane G. Design and implementation of the United States National Animal Health Monitoring System 1995 National Swine Study. Prev Vet Med. 1998;34:147-159.
13. Jordan D, McEwen SA. Herd-level test performance based on uncertain estimates of individual test performance, individual true prevalence and herd true prevalence. Prev Vet Med. 1998;36:187-209.
14. Martin SW, Shoukri M, Thorburn MA. Evaluating the health status of herds based on tests applied to individuals. Prev Vet Med. 1991;14:33-43.
15. Hosmer DW, Lemeshow S. Applied Logistic Regression. New York: John Wiley; 1989:82-144.
16. Smith S, McOrist S, Green L. Questionnaire survey of proliferative enteropathy on British pig farms. Vet Rec. 1998;142:690-693.
17. McOrist S, Jasni S, Mackie RA, McIntyre N, Reef N, Lawson GH. Reproduction of porcine proliferative enteropathy with pure cultures of ileal symbiont intracellularis. Infect Immun. 1993;61:4286-4292.
19. Greiner M, Gardner IA. Epidemiologic issues in the validation of veterinary diagnostic tests. Prev Vet Med. 2000;45:3-22.