Royer RL. 2001;6:281-284 Susceptibility of porcine circovirus ty
Original research
Peer reviewed
Susceptibility of porcine circovirus type 2 to commercial and laboratory disinfectants
Ryan L. Royer; Porntippa Nawagitgul, DVM, MS; Patrick G. Halbur, DVM, PhD; Prem S. Paul, BVSc, PhD
RLR, PN, PGH: College of Veterinary Medicine, Iowa State University, Ames, Iowa; PSP: University of Nebraska, Lincoln, Nebraska
Royer RL, Nawagitgul P, Halbur PG, et al. Susceptibility of porcine circovirus type 2 to commercial and laboratory disinfectants. J Swine Health Prod. 2001;9(6):281-284. Also available as a PDF.
Summary
Objective: To evaluate the virucidal efficacy of 11 commercially available disinfectants against porcine circovirus type 2 (PCV2) using an in vitro model.
Methods: Disinfectants were prepared according to the manufacturers’ label directions and mixed with virus stock. The disinfectant-virus solution was then passed through a detoxification column to remove compounds toxic to cell culture. The filtrate was collected, serially diluted, and inoculated onto porcine kidney cells (PK-15). After a 48-hour incubation period, the cell cultures were fixed and an indirect immunofluorescence assay performed to determine remaining infectious virus titers. Virus titers after disinfection were compared to the negative control (no disinfectant), using Dunnett’s test for statistical analysis.
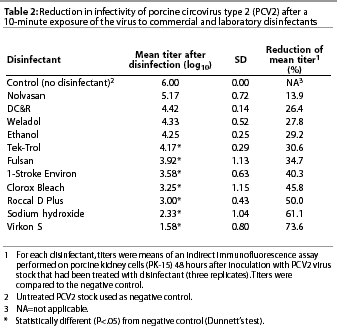
Results: Results demonstrated statistically significant reduction in PCV2 virus titer by several disinfectants, including Virkon(R) S, sodium hydroxide, Roccal(R) D Plus, Clorox(R) Bleach, 1-Stroke Environ(R), Fulsan(R), and Tek-Trol(R). No significant reduction in PCV2 titer was demonstrated using Nolvasan(R), DC&R(R), Weladol(R), or ethanol.
Implications: This study was performed in vitro under controlled laboratory conditions. Variation from these results may occur under field conditions. Nevertheless, these results may aid in the selection of more effective disinfectants to reduce exposure to PCV2 and the incidence of post-weaning multisystemic wasting syndrome or other PCV2-associated diseases.
Keywords:  swine, disinfectants,
porcine circovirus, PCV2
swine, disinfectants,
porcine circovirus, PCV2
Received: January 9, 2001
Accepted: April 19, 2001
Porcine circovirus (PCV), a member of the Circoviridae family, is a non-enveloped, single-stranded, circular-genome DNA virus with a diameter of 17 nm.1,2 Porcine circoviruses are resistant to inactivation when exposed to chloroform, solutions with pH 3, and a temperature of 70 degrees C.2 Two genotypes of PCV exist.3 Porcine circovirus type 1 is non-pathogenic, while PCV type 2 (PCV2) has been associated with post-weaning multisystemic wasting syndrome (PMWS).3-6,13,14 The diagnosis of PCV2-associated PMWS has steadily increased; however, research is lacking with regard to prevention or control of this disease.7 The efficacy of disinfection protocols has not been documented for premises where PMWS has occurred. An effective disinfectant used in conjunction with proper sanitation techniques may prove to be a critical step in controlling exposure to PCV2 and development of PMWS. The objective of this study was to evaluate the anti-PCV2 activity of disinfectants commonly used by producers, veterinarians, and researchers for control of PCV2 associated with PMWS, using an in vitro model.
Materials and methods
Virus preparation
Porcine circovirus type 2 (strain ISU 31) was propagated in porcine kidney cells (PK-15) free of PCV types 1 and 2 (provided by Dr Kelly Lager, National Animal Disease Center, Ames, Iowa). Cell cultures were maintained in minimal essential media (MEM; Gibco BRL, Grand Island, New York) with 5% fetal bovine sera (FBS; Gibco BRL), at 37 degrees C in an atmosphere of 5% CO2. Virus stock was prepared as described elsewhere.8 The infectivity of the virus was determined by an indirect immunofluorescence assay (IFA) using anti-PCV2 rabbit hyperimmune serum.8 A stock virus titer of 1×106 median tissue culture infective doses (TCID50) was attained. Virus stock was aliquoted into 5-mL vials and stored at -70 degrees C.
Disinfectants
Disinfectants were purchased from commercial suppliers or received from the manufacturers directly and were diluted according to manufacturers’ label directions using deionized distilled water. Disinfectants tested included ethanol; polyalkyleneglycol-iodine complex (Weladol; Pitman-Moore, Inc, Mundelein, Illinois); two phenolic compounds (1-Stroke Environ; Steris Corporation, Road Mentor, Ohio, and Tek-Trol; Bio-Tek Industries, Inc, Atlanta, Georgia); two quaternary ammonium compounds (Roccal D Plus; Pharmacia and Upjohn, Peapack, New Jersey, and Fulsan; Fuller Brush Company, Great Bend, Kansas); a formaldehyde and quaternary ammonium compound (DC&R; Hess and Clark, Inc, Ashland, Ohio); chlorhexidine (Nolvasan; Fort Dodge Labs, Fort Dodge, Iowa); sodium hydroxide (Fisher Scientific, Fair Lawn, New Jersey); sodium hypochlorite (Clorox Bleach; Clorox Company, Oakland, California); and a mixture of potassium peroxymonosulfate and sodium chloride (Virkon S; Antec International, Sudbury, Suffolk, UK). Disinfectants, their manufacturers, and the recommended dilutions are listed in Table 1.
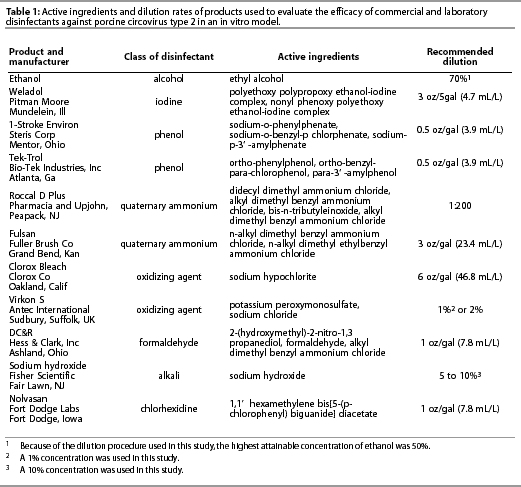
Detoxification column
The detoxification columns were assembled according to the method of Blackwell and Chen.9 The column consisted of a sterile, 30-mL, round-bottom tube wedged within a larger, 50-mL, graduated conical centrifuge tube. The bottom of the inner tube was pierced to make a small hole and a swatch of cotton was placed over the hole. A slurry of 22% Sephadex LH-20 beads (Amersham Pharmacia Biotech AB, Piscataway, New Jersey) and deionized distilled water was added to the inner tube. The Sephadex slurry was clarified by centrifugation at 1000g for 8 minutes, then stored at 4 degrees C until used. The Sephadex slurry forms a gel that traps small molecular weight molecules, while allowing larger molecular weight compounds, such as virus particles, to pass through the gel bed.9,10 In this case, toxic ions in the disinfectants, which are lethal to cells in culture, were trapped in the Sephadex column, while larger viral particles passed through the gel bed into the filtrate.
Procedure for evaluating disinfectant efficacy against PVCV2
The following procedure was performed in triplicate for each disinfectant.
Disinfectants were diluted to 2 x the manufacturer’s recommended
concentration. One mL of double-strength disinfectant was added
to 1 mL of PCV2 virus stock (106 TCID50),
resulting in 2 mL of the virus-disinfectant mixture with the disinfectant
at the manufacturer’s recommended concentration. The disinfectant
remained in contact with the virus at room temperature for 10
minutes, after which the solution was filtered through the detoxification
column by centrifugation at 1000g for 8 minutes. After
centrifugation, the filtrate was collected and five serial ten-fold
dilutions were made. Each dilution was then mixed with PCV-free
PK-15 cells at a concentration of 1×105 cells per mL.
The virus-cell suspension was dispensed into a 96-well microtitration
plate, 100 µL per well, and incubated at 37 degrees C in
an atmosphere of 5% CO2. Untreated PCV2 stock virus,
passed through a detoxification column and serially diluted as
described above, was included in each trial and served as the
negative control. One hour after inoculation, 100µL of MEM
with 5% FBS was added to each well for maintenance. Twelve hours
post inoculation,
30 µL of 300-mM D-glucosamine (Sigma Chemical Co, St Louis,
Missouri) was added to each well to enhance PCV replication in
PK-15 cells.3 Cells were then rinsed with Hank’s Balanced
Salt Solution (Gibco BRL) and maintained in MEM with 5% FBS. Forty-eight
hours post inoculation, the cells were fixed with absolute methanol,
and an indirect IFA, using anti-PCV2 rabbit hyperimmune serum,
was performed to determine PCV2 titers.8
Statistical analysis
The titers of PCV2 virus remaining after disinfection were determined by indirect IFA and transformed to log10 before use in calculations. To test the hypothesis that there was a difference among three replicates of each disinfectant, virus titers (log10) of each of the three disinfectant trials were compared using a generalized linear model procedure. Dunnett’s test was used to compare differences in titers between each disinfectant treatment and the negative control (untreated PCV2). If a mean titer was less than the minimum significant difference as determined by Dunnett’s test, the interpretation was that the mean titer of disinfectant-treated virus was not significantly different from the negative control. All statistical analyses in this study were performed by Statistical Analysis System (SAS) software.
Results
The in vitro effect of the 11 disinfectants as measured by reduction of PCV2 titers in cell culture is summarized in Table 2. The three replicates for each disinfectant treatment did not differ significantly (P=.69), confirming that three replicates were sufficient. Several of the disinfectants were capable of decreasing PCV2 titers (P<.05) compared to the negative control. Those disinfectants included Virkon S, sodium hydroxide, Roccal D Plus, Clorox Bleach, 1-Stroke Environ, Fulsan, and Tek-Trol. Nolvasan, DC&R, Weladol, and ethanol did not significantly decrease PCV2 titers when compared to the negative control.
Discussion
Conditions of this experiment were closely controlled under a laboratory setting and optimized for maximal disinfectant activity. Disinfectants were not compared to each other, but were evaluated only against the negative control and within the triple replicate for the same disinfectant.
Disinfectants evaluated in this study represent products commonly used in swine facilities and research laboratories. Because PCV2 has only recently been identified as a swine pathogen, none of the disinfectants tested in this study have label claims of efficacy against PCV2. Achieving maximum efficacy from disinfectants begins by reading and following the manufacturer’s label instructions and applying the disinfectant to a well-cleaned surface.12
A virus-disinfectant contact time of 10 minutes was used to approximate the amount of contact time a disinfectant might have with surfaces in a swine facility and is also in accordance with many disinfectant manufacturers’ label directions. It may not be correct to assume that increasing the contact period will necessarily increase viral inactivation. Disinfectants that did not perform optimally in the laboratory may be even less effective in a field setting where organic material, contact time, and physical surfaces are less than ideal for optimal disinfectant activity. The use of disinfectants should be regarded as only one component of sanitation and biosecurity strategies for prevention of PCV2-associated diseases.
Information available to producers or veterinarians for control of PMWS is lacking. Reduction of PCV2 in the pig’s environment may reduce or eliminate group-to-group transmission of PCV2. Ability to inactivate PCV2 in this in vitro model differed among the disinfectants evaluated. This information should help guide veterinarians and producers when recommending or purchasing disinfectants to aid in control and management of PCV2-associated diseases.
Another consideration, when choosing disinfectants to combat PCV2-associated PMWS, is that additional disease-contributing pathogens may be found within the same environment. For example, evidence suggests that coinfection with porcine parvovirus (PPV) and PCV2 results in increased incidence and severity of PMWS.6,13,14 Both PCV2 and PPV are small, non-enveloped, single-stranded DNA viruses with similar environmental stabilities.1,2 Porcine parvovirus and PCV are resistant to temperatures up to at least 70 degrees C, pH as low as 3, and multiple anti-viral chemicals, making inactivation of these viruses difficult.2,10 Results obtained by Brown,10 using a similar in vitro model, showed differences among disinfectants in virucidal activity toward PPV when compared to the negative control (no disinfectant).10 Brown reported inactivation of PPV at both 5- and 20-minute virus-disinfectant contact periods for 5% and 10% sodium hydroxide and 1:32 and 1:16 dilutions of sodium hypochlorite, compared to controls. Additionally, PPV was inactivated after 20 minutes but not 5 minutes of contact time with solutions of 2% glutaraldehyde, twice the manufacturers’ recommended dilution of DC&R, or 8% formaldehyde.10 Further research should be conducted both in vitro and especially under field conditions to expand the list of commercial disinfectants effective against specific pathogens. This information would allow for more informed decisions by producers and veterinarians on establishing disinfection protocols for swine facilities.
Acknowledgements
The authors would like to thank Merial Limited and Merck Company Foundation for their funding of this project through the Merck-Merial Veterinary Scholars Research Program at Iowa State University. We also thank Marsha Morgan and Dr Roman Pogranichnyy for their technical input, Dr Chalermpol Lekcharoensuk for statistical analysis, and Dr Steven Sorden for manuscript review.
References — refereed
1. Tischer I, Gelderblom H, Vetterman W, Koch MA. A very small porcine virus with circular single-stranded DNA. Nature. 1982;295:64-66.
2. Allan GM, Phenix KV, Todd D, McNulty MS. Some biological and physico-chemical properties of porcine circovirus. J Vet Med B 1994;41:17-26.
3. Allan GM, Ellis JA. Porcine circoviruses: a review. J Vet Diagn Invest 2000;12:3-14.
6. Morozov I, Sirinarumitr T, Sorden SD, Halbur PG, Morgan MK, Yoon KJ, Paul PS. Detection of a novel strain of porcine circovirus in pigs with post-weaning multisystemic wasting syndrome (PMWS). J Clin Microbiol. 1998;36:2535-2541.
8. Nawagitgul P, Morozov I, Bolin SR, Harms PA, Sorden SD, Paul PS. Open reading frame 2 of porcine circovirus type 2 encodes a major capsid protein. J Gen Virol. 2000;81:2281-2287.
9. Blackwell JH, Chen JHS. Effects of various germicidal chemicals on H.Ep.2 cell culture and herpes simplex virus. J AOAC Int 1971;53:1229-1236.
10. Brown TT. Laboratory evaluation of selected disinfectants as virucidal agents against porcine parvovirus, pseudorabies virus, and transmissible gastroenteritis virus. Am J Vet Res. 1981;42:1033-1036.
11. Tischer I, Mields W, Wolff D, Vagt M, Griem W. Studies on epidemiology and pathogenicity of porcine circovirus. Arch Virol. 1986;91:271-276.
13. Allan GM, Kennedy S, McNeilly F, Foster JC, Ellis JA, Krakowka SJ, Meehan BM, Adair BM. Experimental reproduction of severe wasting disease by co-infection of pigs with porcine circovirus and porcine parvovirus. J Comp Path. 1999;121:1-11.
14. Krakowka S, Ellis JA, Meehan B, Kennedy S, McNeilly F, Allan G. Viral wasting syndrome of swine: Experimental reproduction of postweaning multisystemic wasting syndrome in gnotobiotic swine by coinfection with porcine circovirus 2 and porcine parvovirus. Vet Path. 2000;37:254-263.
References — non refereed
4. Clark EG. Post-weaning multisystemic wasting syndrome. Proc AASP. Quebec, Canada. 1997:499-501.
5. Harding JC. Post-weaning multisystemic wasting syndrome (PMWS): Preliminary epidemiology and clinical presentation. Proc AASP. Quebec, Canada. 1997;503.