Bane DP. 2001;4:155-158 Porcine proliferative enteropathy: a cas
Original research (Peer-reviewed)
Porcine proliferative enteropathy: a case-control study in swine herds in the United States
David P. Bane, DVM, PhD; Eric Neumann, DVM, MS; Connie J. Gebhart, PhD; Ian A. Gardner, BVSc, PhD; Bo Norby, DVM, MPVM
DPB: Elanco Animal Health, Indianapolis, IN, 46285; EN: Heartland Pork, Inc, Alden, IA, 50006; CJG: Division of Comparative Medicine, Research Animal Resources, University of Minnesota, Minneapolis, MN, 55455; IAG: University of California, Davis, Department of Medicine and Epidemiology, School of Veterinary Medicine, Davis, CA, 95616; BN: Michigan State University, Department of Large Animal Clinical Sciences, College of Veterinary Medicine, East Lansing, MI, 48824
Bane DP, Neumann E, Gebhart CJ, et al. Porcine proliferative enteropathy: a case-control study in swine herds in the United States. J Swine Health Prod. 2001;9(4):155-158. Also available as a PDF.
Summary
Objective: To describe epidemiologic characteristics of outbreaks of porcine proliferative enteropathy (PPE) and evaluate associations between herd production and management factors, and disease occurrence.
Methods: A questionnaire about management factors was administered by telephone to the owners or managers of 30 PPE-affected herds (cases) and 18 unaffected herds (controls). Morbidity, mortality, and demographics of animals in affected herds were recorded. Fecal samples from approximately six pigs in each herd were tested by polymerase chain reaction (PCR) for Lawsonia intracellularis.
Results: Porcine hemorrhagic enteropathy (PHE) occurred in 11 herds, and porcine intestinal adenomatosis (PIA) in 19 herds. In multivariable logistic regression analysis, use of a new building and recent mixing of pigs were associated (P<.05) with PPE. Management stressors such as overheating, chilling, number of times mixed, number of feeders per pen, and group size were not significantly associated with PPE. Fecal samples were more likely (P<.05) to be positive by PCR in case than control herds. Herds with PHE were more likely (P=.05) than herds with PIA to have at least one PCR-positive fecal sample.
Implications: Placement of pigs into new facilities and commingling of groups of pigs significantly increase the risk of PPE. Production and management practices linked with high health status may not be associated with reduced risks of PPE outbreaks. Analysis of fecal samples by PCR is useful to detect L intracellularis in the population, but fecal samples are more likely to be PCR positive in herds with PHE than in herds with PIA.
Keywords: swine, Lawsonia intracellularis, porcine proliferative enteropathy, risk factors
Received: October 4, 2000
Accepted: April 5, 2001
Porcine proliferative enteropathy
(PPE) is a commonly described disease syndrome of growing and
adult swine, and is reported worldwide. The disease is caused
by Lawsonia intracel-
lularis1,2,3 and has two common clinical manifestations:
an acute, hemorrhagic form often termed porcine hemorrhagic enteropathy
(PHE), usually observed in finishing pigs or replacement breeding
animals; and a more chronic, proliferative form often referred
to as porcine intestinal adenomatosis (PIA), typically detected
in growers weighing 20 to 50 kg.
Swine practitioners in North America identified PPE as the most commonly diagnosed intestinal disease of grower-finisher pigs,4 and laboratory evidence supports this contention.5 A national survey of swine producers in 1995 estimated that PPE (ileitis) was diagnosed by a veterinarian or diagnostic laboratory in 7% of swine herds in the previous year.6 Using slaughterhouse examinations, Pointon et al found that 28% of Minnesota swine herds had evidence of PPE lesions.7 It is likely that these surveys have underestimated herd prevalence. Herd prevalence greater than 60% has been found when adequate numbers of pigs have been sampled and sensitive ante mortem tests for L intracellularis infection have been used, such as polymerase chain reaction (PCR) of feces and the serum indirect fluorescent antibody test.8,9
Various farm management factors may influence the development, severity, and therapeutic response of the naturally occurring clinical syndromes, but few published scientific studies have evaluated such risk factors. Smith et al10 studied risk factors for PPE using a postal survey of 319 British herds. Breeding herd size of >500 sows, concurrent enzootic pneumonia, purchase of replacement boars from selected nucleus herds, and use of slatted floors above deep sunken pits were important factors associated with owner-reported PPE on the farm in the 3 years prior to the survey. Movement and mixing of pigs, nutritional changes, feed antibiotic usage, temperature fluctuations, pig density, facility design, and sanitation also have been proposed as factors that precipitate PPE outbreaks in endemically infected herds.4,11
In the present study, we investigated the association of several swine production management variables with clinical PPE in US swine herds, using a case-control study design. We describe clinical findings in 30 PPE-affected herds and indicate factors associated with disease occurrence.
Materials and methods
Case-control study
Veterinary practitioners in the mid-western US were notified by mail and telephone of our study and were asked to nominate PPE case and control herds. Case herds (PPE-affected) were selected on the basis of recent occurrence of clinical signs of PPE and a confirmed histologic diagnosis of PPE from a veterinary diagnostic laboratory within the previous 12 months. Herd veterinarians were consulted by telephone to obtain accurate disease histories of all case herds, and to aid in selection of control herds. Control herds were free of clinical signs consistent with PPE for the previous 12 months. Wherever possible, controls were serviced by the same veterinary practice that provided the case herd.
Between July 1995 and April 1996, farm management data were collected by telephone survey of owners or managers of case and control herds. Farm management data included herd size (sow and growing pig inventory), environment (season, flooring, ventilation system), biosecurity (isolation of herd additions, frequency of herd additions, age segregation) and animal history (genotype, feed and water medications, weaning age, group size and animal density, nutritional history). To facilitate questionnaire completion and allow for ranges of values, many variables (eg, median age group affected, median group weight) were categorized into broad groupings (eg, 5 to 8, 9 to 12, or 13 to 16 weeks of age; 1 to 14, 15 to 27, or 28 to 40 kg). For case herds, morbidity and mortality data and demographics of affected animals were also recorded. A copy of the questionnaire is available from the senior author on request.
The owner or veterinarian collected fecal samples per rectum from approximately six PPE-affected swine in each case herd as soon as possible after a diagnosis of PPE was made. Fecal samples were collected from the same numbers of pigs in the same weight classes on control farms. All samples were refrigerated and shipped within 24 hours to the laboratory of one of the co-authors (CJG) for testing by PCR for L intracellularis.
Polymerase chain reaction
Polymerase chain reaction was performed as previously described,12 using primers derived from the L intracellularis type strain, NCTC 12656 (National Collection of Type Cultures, Collindale, London). Results were interpreted as positive if amplification products of expected size were detected on ethidium bromide-stained gels. All evaluations were performed independent of knowledge of herd PPE status.
Statistical analysis
Characteristics of case herds were summarized by frequencies, medians, and ranges and were compared by chi-square for categorical variables and by Mann-Whitney tests for continuous variables. Strength of association between herd-level risk factors and case or control disease status was measured by odds ratios (OR). Multivariable logistic regression (BMDP Statistical Software, Inc, Los Angeles, California) was used to adjust for the simultaneous effect of several risk factors.13 For comparison of the results of PCR testing, a herd was considered PCR positive if at least one fecal sample was positive for L intracellularis. The proportion of PCR-positive herds was compared among PIA, PHE, and control herds by chi-square tests. Because not all herds provided the requested number of fecal samples, this analysis was restricted to 44 herds from which at least four fecal samples had been collected.
Results
Description of outbreak herds
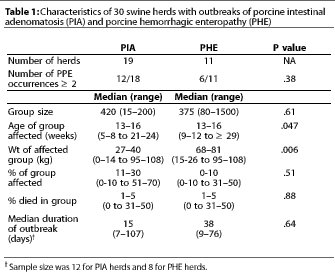 Thirty case herds (19 with PIA
and 11 with PHE) and 18 control herds from 11 states participated
in the study. Table 1 compares the characteristics of herds with
each clinical form of PPE (PIA and PHE). Although the median age
was the same in pigs with PIA and PHE, PHE occurred in heavier
pigs (P<.05). Morbidity was numerically lower in PHE
than in PIA outbreaks, and outbreak duration was numerically longer
for PHE than PIA. Mortality and frequency of multiple occurrences
within a cohort of pigs did not differ between the two forms of
the disease.
Thirty case herds (19 with PIA
and 11 with PHE) and 18 control herds from 11 states participated
in the study. Table 1 compares the characteristics of herds with
each clinical form of PPE (PIA and PHE). Although the median age
was the same in pigs with PIA and PHE, PHE occurred in heavier
pigs (P<.05). Morbidity was numerically lower in PHE
than in PIA outbreaks, and outbreak duration was numerically longer
for PHE than PIA. Mortality and frequency of multiple occurrences
within a cohort of pigs did not differ between the two forms of
the disease.
Risk factors
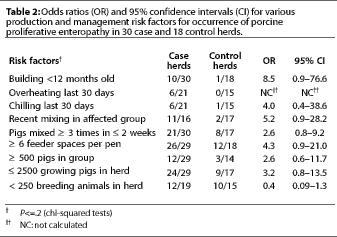 In univariable analysis, risk factors
for PPE which approached significance of association (P<=.2)
are shown in Table 2. These factors were considered for inclusion
in logistic modeling. Only two variables, recent mixing of animals
(<= 2 weeks) and the use of a new building, entered and remained
in the final logistic regression model. Odds ratios, which provide
a measure of association between risk factor and disease, were
calculated for the management variables. High odds ratios (greater
than 3) indicate a strong association between the risk factor
and disease. The odds ratio for mixing animals was 7.2, with a
95% confidence interval of 1.2 to 43. For use of a new building,
the odds ratio was 11.4 with a 95% confidence interval of 1.1
to 115. The interaction term between these two variables was not
statistically significant.
In univariable analysis, risk factors
for PPE which approached significance of association (P<=.2)
are shown in Table 2. These factors were considered for inclusion
in logistic modeling. Only two variables, recent mixing of animals
(<= 2 weeks) and the use of a new building, entered and remained
in the final logistic regression model. Odds ratios, which provide
a measure of association between risk factor and disease, were
calculated for the management variables. High odds ratios (greater
than 3) indicate a strong association between the risk factor
and disease. The odds ratio for mixing animals was 7.2, with a
95% confidence interval of 1.2 to 43. For use of a new building,
the odds ratio was 11.4 with a 95% confidence interval of 1.1
to 115. The interaction term between these two variables was not
statistically significant.
There was no significant association (P>.2) between the following variables and occurrence of PPE: repopulation of the herd in either the previous 4 or 12 months, total or partial confinement, flooring (total slats, partial slats, or solid flooring), cooling system, pig density, dietary factors (pellets or meal; crude protein, lysine, fat, fiber), time on current ration, recently purchased pigs, age weaned, number of moves, continuous pig flow or all in-all out management, lack of washing and disinfection, concurrent disease (atrophic rhinitis, pneumonia, clostridial enteritis, gastric ulcers), isolation of breeding stock replacements (always, sometimes, never) or duration of isolation, frequency of breeding stock additions, number of breeding stock sources, and antibiotic use in rations (breeding, nursery, grower, finisher herds). Insufficient data were gathered to evaluate the association of several variables with PPE (including genetics, use of flush gutters, limit feeding, wet feeding, lack of drinking water, and power failures).
Polymerase chain reaction
At least one fecal sample was positive for L intracellularis
by PCR in 12 of 27 case herds, but in only one of 17 control herds
(P<.01). There was a higher proportion
(P=.05) of PCR-positive results among herds with PHE outbreaks
(seven of ten) than among herds with PIA outbreaks (five of 17).
There was no significant difference (P=.15) between PHE
herds and PIA herds in the number of days from recognition of
the clinical outbreak to the day when the sample was collected
(6 days for PHE herds, 8 days for PIA herds). However, most samples
were collected from PHE herds after the outbreaks ended.
Discussion
Findings from our descriptive analysis and case-control study confirmed several of the suggested epidemiologic patterns and risk factors associated with PPE.4,10,11 The hemorrhagic form of PPE occurred in heavier growing pigs than the proliferative form, but the two forms of the disease did not differ with respect to several other epidemiologic characteristics. The factors which cause this variation in clinical response to infection with L intracellularis are unknown. Possible explanations are that the organism varies in pathogenicity among herds, that herd immunity towards L intracellularis may play a role in age susceptibility, or that other organisms are acting synergistically with L intracellularis in some herds. Some experimental infection studies suggest host factors may play a role in disease susceptibility.3 In our study, it was interesting that both forms of PPE were not documented within a single herd, but our sample of herds was small, and perhaps both forms do occur concurrently in some herds.
It has commonly been suggested that stressors precipitate clinical occurrence of PPE.4,11 In univariable analysis, chilling, overheating, and mixing of pigs were associated with clinical PPE in this study, and the association with recent mixing of pigs remained significant in the logistic regression analysis. Although mechanisms by which these insults may initiate PPE are unknown, we speculate that a disruption of eating patterns caused by environmental or social stressors may alter intestinal motility and render the intestinal mucosa more susceptible to infection by intestinal organisms.
Pigs housed in new buildings (less than 1 year old) were more likely to be diagnosed with PPE than pigs housed in older buildings. Herd owners may reduce the antibiotic usage in new facilities because of the pristine environment of the new building. A reduction in antibiotic usage may increase the likelihood of enteric disease outbreaks.
We found no significant association between several of the production and management variables commonly linked with poor herd health and the occurrence of PPE. Solid flooring systems, continuous pig flow, lack of washing and disinfection, and lack of isolation of breeding stock were not associated with the disease. This finding may be the result of selection bias, of unidentified interactions of several factors, or of misclassification of the status of control herds. Because the number of fecal samples tested by PCR in control herds was small, we were not able to determine unequivocally that these herds were non-infected.
We believe that the PCR test for L intracellularis was acceptable for herd PPE diagnosis, although only 44% of case herds had at least one PCR-positive fecal sample. A greater percentage of case herds would probably have tested PCR positive if more fecal samples had been tested in each herd, because outbreaks of PPE often affect a small proportion of pigs.4,11 Therefore, increasing the sampling size increases the likelihood of finding at least one PCR-positive pig in each case herd. For example, in a study of Danish finisher pigs,9 20 pigs were tested per herd. In our study, sample deterioration prior to PCR testing, or delayed collection of samples relative to the PPE outbreak, may also have generated false negative results. Prompt collection, refrigeration, and analysis of a large number of fecal samples (ideally direct rectal swabs) after detection of clinical PPE would most likely result in highly accurate herd-level PCR test results. Only one PCR-positive fecal sample was found in the 17 control herds. This may have been a false positive result, but we believe that it is more likely that the presence of L intracellularis alone is not sufficient for the development of clinical disease.
The higher proportion of PCR-positive results from PHE outbreaks compared to PIA outbreaks may have occurred because within-herd prevalence of L intracellularis was higher in the PHE-affected herds, or because pigs with PHE shed more L intracellularis organisms for a longer period. Alternatively, PPE-affected animals may be more easily identified, and therefore sampled, when they exhibit the hemorrhagic form of the disease (PHE) rather than the nonhemorrhagic form (PIA).
Our study had a number of limitations. We had initially planned to include at least 40 case and 40 control herds to detect odds ratios of at least 3 with high power and confidence. Control herds were more difficult to obtain than case herds. Accordingly, the lack of significant association for some variables may be attributable in part to the small number of herds in the study. In our analysis, we assumed that the two types of case herds would have identical risk factors. To test this assumption, we performed a stratified analysis using the PIA and PHE cases separately. The two significant risk factors were still significant in the separate analyses (data not shown), although other risk factors might not have been affected similarly.
Implications
- Clinical signs of PPE change as pigs grow and mature.
- Testing of fecal samples by PCR is a useful indicator of herd status regarding L intracellularis infection.
- Placement of pigs within new facilities and recent mixing of pigs (<= 2 weeks) are significant risk factors for PPE.
- Production and management practices linked with high health status may not be associated with reduced risks of PPE outbreaks.
- Common stressors such as overheating, chilling, repeated mixing of pigs, inadequate number of feeders per pen, and group size may be associated with outbreaks of PPE and warrant further investigation.
Acknowledgements
We thank the veterinarians who participated in the study.
The study was funded in part by the Center for Food Animal Health, School of Veterinary Medicine, University of California, Davis, California.
References – refereed
1. McOrist S, Jasni S, Mackie RA, McIntyre N, Reef N, Lawson GH. Reproduction of porcine proliferative enteropathy with pure cultures of ileal symbiont intracellularis. Infect Immun. 1993;61:4286-4292.
2. McOrist S, Gebhart CJ, Boid R, Barns SM. Characterization of Lawsonia intracellularis gen., sp. nov., the obligately intracellular bacterium of porcine proliferative enteropathy. Int J Sys Bacteriol. 1995;45:820-825.
3. Joens LA, Nibbelink S, Glock RD. Induction of gross and microscopic lesions of porcine proliferative enteritis by Lawsonia intracellularis. Am J Vet Res. 1997;58:1125-1131.
4. Connor JF. Diagnosis, treatment, and prevention of porcine proliferative enteritis. Food Anim Compend. 1991;13:1172-1176.
5. Wilson JB, Pauling GE, McEwan BJ, Smart N, Carman PS, Dick CP. A descriptive study of the frequency and characteristics of proliferative enteropathy of swine in Ontario by analyzing routine animal health surveillance data. Can Vet J. 1999;40:713-717.
6. USDA. NAHMS Swine’95: Part I. Reference of 1995 Swine Management Practices. http://www.aphis.usda.gov/vs/ceah/cahm/Swine/swine.htm Accessed May 10, 2001.
7. Bronsvoort M, Norby B, Bane DP, Gardner IA. Management factors associated with seropositivity to Lawsonia intracellularis in US swine herds. J Swine Health Prod. In press.
9. Stege H, Jensen TK, Møller K, Baekbo P, Jorsal SE. Prevalence of intestinal pathogens in Danish finishing pig herds. Prev Vet Med. 2000;46:279-292.
10. Smith S, McOrist S, Green L. Questionnaire survey of proliferative enteropathy on British pig farms. Vet Rec. 1998;142:690-693.
11. Winkelman NL. Ileitis: an update. Compend Cont Educ. 1996:18(1):S19-S25.
12. Cooper DM, Swanson DL, Gebhart CJ. Diagnosis of proliferative enteritis in frozen and formalin-fixed, paraffin-embedded tissues from a hamster, horse, deer and ostrich using a Lawsonia intracellularis-specific multiplex PCR assay. Vet Microbiol. 1997;54:47-62.
13. Hosmer DW, Lemeshow S. Applied logistic regression. New York, NY: Wiley and Sons; 1989.
References — nonrefereed
8. Pointon AM. Industry disease rates: assessment of impact of veterinarians. Proc Minnesota Swine Conf Vet. 1990:46-57.
This page last updated .