Walter D. 2001;3:109-113 Treatment and control of porcine prolif
Original Research (Peer-Reviewed)
Treatment and control of porcine proliferative enteropathy using different tiamulin delivery methods
Don Walter, DVM; Jeff Knittel, PhD; Kent Schwartz, DVM, MS; Jeremy Kroll, BS; Mike Roof, PhD
DW: Boehringer Ingelheim Vetmedica Inc., 1051 Barrington Road, Iowa City, Iowa 52245, Email: dwalter@bi-vetmedica.com; JK, MR, JK: Boehringer Ingelheim Vetmedica Inc., Ames, Iowa 50010; KS: Veterinary Diagnostic Laboratory, Iowa State University, Ames, Iowa 50011.
Walter D, Knittel J, Schwartz K, et al. Treatment and control of porcine proliferative enteropathy using different tiamulin delivery methods. J Swine Health Prod. 2001;9(3):109-115. Also available in PDF format.
Summary
Objective: To determine the clinical and pathological effects when pigs infected with Lawsonia intracellularis and showing clinical signs of gastrointestinal disease are treated with tiamulin hydrogen fumarate (THF) administered in feed or water.
Methods: All pigs in Study 1 (47 pigs) and Study 2 (48 pigs) were inoculated with a virulent pure culture of L intracellularis. After clinical signs became apparent, treated pigs were medicated for 28 days with THF administered in the feed at 35 g per ton (Study 1), or for 5 days with THF administered in the drinking water at 60 mg per liter (Study 2). Necropsies were performed on remaining control and treated pigs in Study 1 on Day 37 post-inoculation, and in Study 2 on Day 23 post-inoculation.
Results: Medication in the feed or water significantly reduced development of gross and microscopic lesions, severity of clinical signs, and prevalence of fecal shedding of L intracellularis in treated pigs. Feed treatment with THF (2.0 mg per kg daily) had no effect on seroconversion, whereas water treatment (7.3 mg per kg daily) significantly reduced prevalence of seroconversion against L intracellularis.
Implications: Tiamulin hydrogen fumarate in the feed (35 g per ton) or water (60 mg per liter) was effective in treating and controlling PPE. Timing of medication relative to time of infection with Lintracellularis may affect the immune response (seroconversion), fecal shedding, and development of lesions. Antibiotic dosage may also affect the immune response to L intracellularis infection, and may reduce fecal shedding, interfering with polymerase chain reaction tests for Lintracellularis.
Keywords: swine, proliferative enteropathy, Lawsonia intracellularis, tiamulin
Received: July 5, 2000
Accepted: January 2, 2001
Porcine proliferative enteropathy (PPE, ileitis) is a common enteric disease of grow-finish swine caused by a recently identified, novel taxonomic genus and species, Lawsonia intracellularis.1 The disease may cause poor growth rate, diarrhea, and stunting, or it may present as acute death or bloody diarrhea, particularly in late finishing pigs and replacement gilts.2
Historically, postmortem examination and histopathology have been used for diagnosis of PPE. Polymerase chain reaction (PCR) tests are now available to detect DNA specific for L intracellularis in feces and tissues.3 In addition, an indirect fluorescent antibody (IFA) serological test has been developed to detect IgG antibodies specific for L intracellularis.4
In 1993, Koch’s postulates were fulfilled when pure cultures of Lawsonia intracellularis were administered to pigs.5 Pure culture inoculation eliminates confounding effects of other potentially pathogenic microflora and tissue factors present in crude inoculum preparations.5 In addition, pure culture inoculation provides a uniform infective dose, thus avoiding potentiallysignificant variation in the individual challenge dose when crude intestinal inoculum from one or more donor animals is administered.
Tiamulin hydrogen fumarate (THF; Denagard(TM), Boehringer Ingelheim Vetmedica, Inc, St. Joseph, Missouri) is a member of the pleuromutilin class of antibiotics, which are selectively reserved for use in food producing animals and are not used in human medicine. When administered to pigs orally in feed or drinking water,or parenterally by injection, tiamulin achieves high tissue concentrations in the enteric mucosa, lung, and tonsil.6 It penetrates polymorphonuclear leukocytes and achieves intracellular concentrations up to 18 times greater than extracellular concentrations.7 Tiamulin hydrogen fumarate has good in vitro activity against mycoplasmas, anaerobes, spirochetes such as Brachyspira (formerly Serpulina) spp, and Gram-positive bacteria (eg, streptococci, staphylococci), and selective activity against Gram-negative pathogens, including Lintracellularis,8,9 Actinobacillus pleuropneumoniae, Haemophilus parasuis, and Pasteurella multocida.
In a previous study, THF prevented PPE in pigs challenged with a virulent, pure culture inoculum of a US isolate of Lintracellularis when the medication was administered continuously in the feed before,during, and after exposure.10 In a study conducted in the UK, a British Lintracellularis isolate was used, and the investigators did not wait for clinical signs to be apparent before initiating tiamulin treatment.11 In the two studies described here, pigs were challenged with the US Lintracellularis isolate, but clinical signs of PPE were apparent in most pens of inoculated pigs before THF was administered in the feed or water.
Materials and methods
Experimental design
Both studies were of randomized complete block design with blocks stratified by weight. Treatments were randomly assigned to pens within each block before pigs were inoculated with L intracellularis. Investigators were blinded throughout Study 1. In Study 2, investigators could not be blinded until necropsies were being performed becauseof the physical plumbing requirements for the water medication delivery system.
Animals
The pigs in Studies 1 and 2 were of mixed breed and sex, and were weaned at 3 to 4 weeks of age. They originated from a closed herd with no clinical or diagnostic evidence of PPE, Salmonella, or Brachyspira (formerly Serpulina) spp infections. Offspring from this herd are susceptible to Lintracellularis infection and have previously been used to reproduce PPE.
The 47 pigs in Study 1 were from five litters,ranging in weight from 4 to 9 kg (8 to 20 lb), with a mean weight of 6 kg (13 lb) at approximately 5 weeks of age when the study was initiated. The 48 pigs in Study 2 were from six litters, ranging in weight from 6 to 15 kg (13 to 33 lb), with a mean weight of 10 kg (22 lb) at approximately 6 weeks of age when the study was initiated. No antibiotics had been administered to these pigs before purchase.
Treatments
Study 1. All pigs were identified by ear tag, weighed, and allocated to eight weight-ordered blocks. Six pigs were then randomly allocated to two pens of three pigs within each block. An injured pig was removedfrom one pen before the study began, leaving only two pigs in that pen. Pens within blocks were randomly assigned to the NON-MEDICATED or the MEDICATED treatment group.
All pigs were inoculated with a virulent pure culture of L intracellularis strain N343. NON-MEDICATED pigs received no medication throughout the study. When one or more animals in > 60% of all pens showed clinical signs consistent with PPE (diarrhea or abnormal body condition), MEDICATED pigs received feed containing 35 g per ton (38.5 ppm) THF, administered continuously for 28 days.
Study 2. All pigs were identified by ear tag, weighed, and allocated to eight weight-ordered blocks. Six pigs were then randomly allocated to two pens of three pigs within each block. Pens within blocks were randomly assigned to the NON-MEDICATED or the MEDICATED treatment group.
All pigs were inoculated with a virulent pure culture of L intracellularis strain N343. NON-MEDICATED pigs received no medication throughout the study. When one or more pigs in > 60% of all pens showed clinical signs consistent with PPE (diarrhea or abnormal body condition), MEDICATED pigs received drinking water containing 60 mg per liter THF (60 ppm, 227 mg per gal), administered continuously for 5 days. The treatment period was followed by a 10-day post-medication observation period.
Water and feed
Water and feed were available to all pigs ad libitum for the duration of the study. A corn-soymeal-based mash diet was formulated to meet or exceed NRC requirements for growing pigs.
Study 1. The same diet was fed to all pigs, except that during the treatment period, feed for MEDICATED pigs contained THF at a calculated inclusion rate of 35 g per ton.
Study 2. All animals received nonmedicated feed for the entire study. During the treatment period, MEDICATED pigs received drinking water containing THF at a calculated inclusion rate of 60 mg per liter. The medicated water solution was prepared fresh daily in a tank and delivered to each MEDICATED pen via a single nipple waterer.
Infection
Lawsonia intracellularis strain N343 is a recent field isolate from the ileum of a Minnesota sow that died of the hemorrhagic form of PPE. The in vitro minimum inhibitory concentration (MIC) of THF for this isolate was low (2 µg per ml intra-cellular activity assay, 0.25 µg per ml extracellular activity assay). Low-passage cultures of L intracellularis strain N343 were grown for 8 to 12 weeks using continuous cell culture techniques. Cultures were harvested, processed as previously described, and quantified by the Tissue Culture Infectious Dose 50 percent (TCID50) using the Reed-Meunch method.12 On Day 0, all pigs were inoculated by gastric intubation. Study 1 pigs each received 107.2 organisms, and Study 2 pigs each received 108.2 organisms.
Facilities
For both experiments, the test facility was a totally confined, environmentally controlled single room with twenty 1.5 m x 2.1 m (5 ft x 7 ft) pens separated by solid partitions, with woven wire flooring over a shallow pit. Ambient temperatures were held at approximately 29C (85F) for the first week and lowered incrementally each week to 24C (75F) during the final week. Heat lamps provided supplemental zone heating. A single-sided, adjustable, stainless steel self-feeder and one automatic nipple waterer serviced each pen.
Measurements and observations
Study 1. Pigs were weighed when allocated to pens (Day -1), at the beginning of the treatment period (Day 9), and at subsequent weekly intervals (Days 16, 23, 30, and 37) to test end, 4 weeks after treatment began. Feed disappearance was measured at the same intervals. Fecal swabs were taken on the day of challenge (Day 0), at the beginningof the treatment period (Day 9), and at weekly intervals to the end of the study. Polymerase chain reaction, using primers specific for L intracellularis,3 was used to detect L intracellularis on the fecal swabs. Results were reported as positive or negative. Blood samples were collected at study termination for indirect fluorescent IgG antibody assay for L intracellularis (seroconversion).4 Each pig was observed and scored daily for diarrhea (0 = normal, 1 = mild, 2 = moderate, or 3 = severe), clinical appearance (0 = normal, 1 = gaunt, and 2 = moribund) and presence or absenceof fecal blood (0 = absent, 1 = present). A composite clinical signs score was derived for each pig by adding scores for diarrhea severity, fecal blood, and clinical appearance.
On Day 37, all remaining pigs were euthanized. Necropsies were performed and tissue samples were collected. Histologic sections of jejunum, ileum, and colon were stained with hematoxylin and eosin and examined for lesions. Sections were stained with Warthin-Starry silver stain to identify and estimate relative numbers of intracellular curved bacteria and to score the degree of crypt cell hyperplasia (0 = normal, 1= mild, 2 = moderate, 3 = severe).
Mesenteric lymph node and samples of ileum and colon from all pigs were submitted to the Iowa State University Veterinary Diagnostic Laboratory for isolation of Salmonella and Brachyspira spp. Indirect fluorescent antibody tests were performed on histological sections of ileum and colon to detect and confirm the presence of Lintracellularis.
Study 2. Pigs were weighed when allocated to pens (Day 1), at the beginning of the treatment period (Day 8), at the end of the treatment period (Day 13), and at termination of the study (Day 23). Feed disappearance was measured at the same intervals. Fecal swabs were taken on the day of challenge (Day 0), at the beginning of the treatment period (Day 8), at the end of the treatment period (Day 13), and at termination of the study (Day 23). Blood samples were collected Day 23 for indirect fluorescent IgG antibody assay for Lintracellularis (seroconversion).4 Measurements were taken and samples collected at necropsy as in Study 1.
Statistical analysis
Exact stratified Mantel-Haenszel tests were performed on all
comparisons of rates and proportions. Thus, pigs are compared
only to pigs within the same block. Exact
P values are two-tailed for baseline and pre-treatment
analyses and one-tailed for post-initiation-of-treatment analyses
comparing treatments. Exact stratified logrank tests are nonparametric
survival analyses that test for the incidence of clinical signs,
taking into account the sequencing of clinical events rather than
the exact times of these events. Exact P values are two-tailed
for baseline and pre-treatment analyses and one-tailed for post-initiation-of-treatment
analyses comparing treatments.
Exact stratified Wilcoxon-Mann-Whitney tests were used to compare severity levels of clinical signs and severity levels of lesions. The severity of clinical signs score is a composite score, the sum of scores for general appearance, diarrhea, and fecal blood. Similarly, the severity of lesions score is the sum of all scores across sites and types. All statistical tests with pig-specific categorical data are stratified by block, thus pigs are compared only to pigs within a block and are therefore of similar baseline weight and in close proximity within the barn.
Exact stratified Wilcoxon signed rank tests are used to compare the continuous outcomes associated with growth performance. These outcomes are pen-specific and the analyses retain the pen match within blocks. Pre-treatment comparisons are two-tailed. Post-initiation-of-treatment comparisons are one-tailed, expecting the median differences in average daily feed consumption, average daily weight gain, and feed efficiency to be greater than 0.0.
Results
Initiation of medication
Study 1. Medicated feed was initiated 9 days after inoculation, when pigs in ten of 16 pens (63%) showed clinical signs consistent with PPE (diarrhea or clinical appearance scores >0). Tiamulin hydrogen fumarate was added to the feed of MEDICATED pigs at a calculated inclusion rate of 35 g per ton for the next 4 weeks. Assays on retained feed samples indicated that actual levels of THF averaged 30 g per ton (86% of target). Based on feed THF assays, feed disappearance, and average body weights over the trial period, and assuming no feed disappearance due to wastage, the calculated daily dosage of medication received was 2 mg per kg (0.9 mg per lb).
Study 2. Medication in the drinking water was initiated 8 days after inoculation when pigs in 14 of 16 pens (88%) showed clinical signs consistent with PPE (diarrhea or clinical appearance scores > 0). Tiamulin hydrogen fumarate was added to the drinking water of MEDICATED pigs at a calculated inclusion rate of 60 mg per liter (60 ppm, 227 mg per gal) and continued for 5 days. Assays on retained water samples indicatedthat actual levels of THF averaged 41 mg per liter (68% of target). Based on water THF assays, water disappearance, and average body weights over the trial period,and assuming no water disappearance due to wastage, the calculated daily dosage of medication received was 7.3 mg per kg (3.33 mg per lb).
Bacteriology
Salmonella and Brachyspira (Serpulina) spp were not cultured from samples of mesenteric lymph node, ileum, or colon from any pigs in either study.
Lesions
Study 1. Two NON-MEDICATED pigs were euthanized due to severe clinical disease 34 days post challenge. At necropsy, gross and microscopic lesions typical of PPE, and no other lesions, were observed in both pigs.
The remaining NON-MEDICATED pigs and the MEDICATED pigs were euthanized 28 days after treatment initiation (Day 37). Significantly more gross lesions of PPE were identified in the small intestine in the NON-MEDICATED group (seven pigs) than in the MEDICATED group (one pig). Differences in prevalence of gross lesions in the large intestine were not significant (P= .075) (Figure 1).
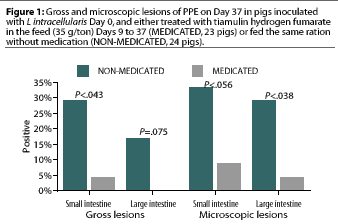
Significantly more microscopic lesions of PPE were identified in the large intestine in the NON-MEDICATED group (seven pigs) than in the MEDICATED group (one pig). Differences in prevalence of micoscopic lesions in the small intestine were not significant (P< .056) (Figure 1).
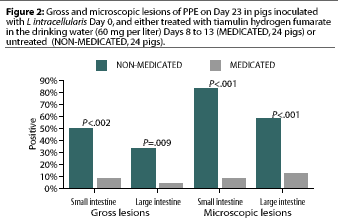
Study 2. Significantly more gross lesions of PPE were identified in the small intestine (12 pigs) and large intestine (eight pigs) in the 24 NON-MEDICATED pigs than in the small intestine (two pigs) and large intestine (one pig) in the 24 MEDICATED pigs (Figure 2). Significantly more microscopic lesions of PPE were identified in the small intestine (20 pigs) and large intestine (14 pigs) in the NON-MEDICATED group than in the small intestine (two pigs) and large intestine (two pigs) in the MEDICATED group (Figure 2).
Serology
In Study 1, the seroprevalence of antibodies to L intracellularis at necropsy was similar for the two treatment groups. Twenty-one of the 23 MEDICATED pigs (91%) and 23 of the 24 NON-MEDICATED pigs (96%) seroconverted.
In Study 2, seroprevalence of antibodies against L intracellularis at necropsy was significantly greater (P<.05) in the NON-MEDICATED pigs. Twenty-three of the 24 NON-MEDICATED pigs seroconverted (96%), compared to nine of the 24 MEDICATED pigs (38%).
Fecal shedding
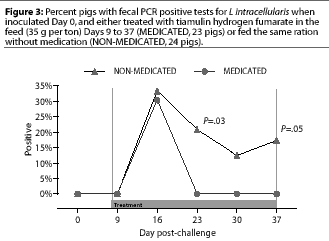
Study 1. Fecal swabs were positive by PCR for the first time Day 16, 7 days after the initiation of treatment; fecal swabs from eight NON-MEDICATED pigs and seven MEDICATED pigs were positive. In the NON-MEDICATED group, fecal PCR results were positive in five pigs on Day 23, in three pigs on day 30, and in four pigs on Day 37. There were no positive fecal PCR results in MEDICATED pigs on these days, which were 14, 21, and 28 days after treatment was begun. These differences were significant on Day 23 (P=.03) and Day 37 (P=.05), but not on Day 30 (P=.10) (Figure 3).
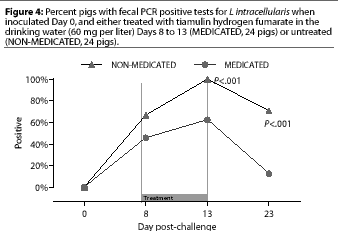
Study 2. Fecal swabs were positive by PCR for the first time on Day 8 in 16 of the 24 NONMEDICATED pigs and 11 of the 24 MEDICATED pigs. On Day 13 (the last day of the treatment period), fecal PCR was positive in more (P=.0004) NON-MEDICATED pigs (24 of 24) than MEDICATED pigs (15 of 24), and on Day 23 (the end of the 10-day post-treatment observation period), fecal PCR was positive in more (P<.00005) NON-MEDICATED pigs (17 of 24) than MEDICATED pigs (three of 24) (Figure 4).
Clinical scores
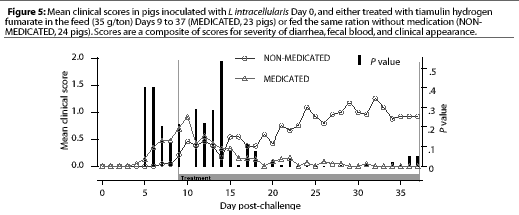
Study 1. On Day 9, when THF feed medication was begun for the pigs in the MEDICATED group, four NONMEDICATED pigs and seven MEDICATED pigs were showing clinical signs. The clinical signs scores for the MEDICATED pigs had decreased significantly (P=.003) 7 days after feed medication with THF had begun, while the NON-MEDICATED pigs had persistent clinical signs throughout the study period, with the maximum levels of severity increasing (Figure 5). No new clinical cases developed in previously asympto-matic MEDICATED pigs after 4 days of medication, while new clinical cases continued to develop in the NON-MEDICATED pigs throughout the study.
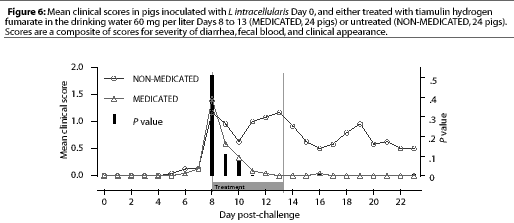
Study 2. On Day 8, when THF drinking water medication was initiated for the MEDICATED group, 12 NONMEDICATED pigs and eight MEDICATED pigs were showing clinical signs. The clinical signs scores for the MEDICATED pigs significantly decreased (P=.0003) within 3 days. However, clinical signs persisted in the NON-MEDICATED pigs throughout the study period (Figure 6). No new clinical cases developed in previously asymptomatic MEDICATED pigs after 3 days of water medication, while new clinical cases continued to develop in the NON-MEDICATED pigs throughout the study.
Growth performance
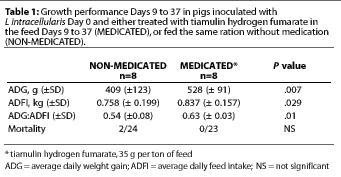
Study 1. For the overall 28-day treatment period, the average daily feed intake (ADFI) was significantly greater (P=.029) for the MEDICATED group (Table 1); the estimated median difference in ADFI was 95 g per pig-day (0.21 lb per pig-day). Average daily weight gain (ADG) was also significantly greater (P=.007) for the MEDICATED group; the estimated median difference was 123 g per pig-day (0.27 lb per pig-day). Feed efficiency (G:F) was also greater for the MEDICATED group (P=.01) (Table 1).
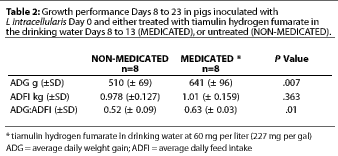
Study 2. For the combined treatment and post-treatment period (Days 8 to 23), the average daily feed intake (ADFI) was not significantly different between treatment groups (P=.3). Average daily weight gain (ADG) was significantly greater (P=.007) for the MEDICATED group (Table 2); the estimated median difference was 132 g per pig-day (0.29 lb per pig-day). Feed efficiency (G:F) was also greater for the MEDICATED group (P=.01) (Table 2).
Discussion
Unlike previously reported studies,10,11 these two studies were designed to mimic field conditions where treatment is not initiated until clinical signs are apparent. Attempts to demonstrate other enteric pathogens were unsuccessful, suggesting an uncomplicated L intracellularis infection. Studies such as these and previous studies10,11 that used virulent pure culture inoculum should be less confounded by other disease variables than studies which relied on gut homogenate inocula or natural infections. However, the infections induced simultaneously in all of these pigs, with large numbers of pure culture L intracellularis, may have produced results not typical of the disease process in natural infections.
Two separate studies are reported here and comparisons between studies should be made with caution. Nevertheless, our results show the effectiveness of THF in reducing lesion development, clinical disease, fecal shedding, and productivity losses associated with PPE, when the drug is administered in feed or water after clinical disease is already evident in the pig population.
In a previous study, rate of seroconversion to L intracellularis was significantly lower in pigs continuously medicated in the feed at 35 g THF per ton before, during, and after infection compared to nonmedicated controls.10 However, in Study 1, the seroprevalence rate in treated pigs, infected with L intracellularis and clinically affected by PPE prior to treatment with THF at 35 g per ton of feed, was comparable to the seroprevalence rate in control pigs. In contrast, the seroprevalence rate of Study 2 pigs treated with THF in the drinking water was lower than for the control pigs.
While serological results in different studies must be compared with caution, possible explanations for the apparent differences in serological response between the two studies are offered.
These apparent differences may reflect a dosage-related effect. Water medication at 60 mg per liter THF delivered approximately 7.3 mg THF per kg body weight (3.3 mg per lb) daily, compared to feed medication at 35 g THF per ton, which delivered approximately 2 mg THF per kg body weight (0.9 mg per lb) daily. The total dose of THF (55.4 mg per kg; 25.2 mg per lb) was greater for the pigs that received medicated feed for 28 days than for the pigs that received medicated water for 5 days (36.7 mg per kg; 16.7 mg per lb). However, it would take 18.5 days on feed medication to get the same total dosage that was achieved with water medication in 5 days. It is possible that the higher daily dosage of medication delivered by water treatment, combined with achieving the total dosage sooner post infection, inhibited bacterial replication and stimulation of the immune system more quickly than is possible with feed medication at the relatively low inclusion rate of 35 g per ton. As the timing of blood sampling relative to time of challenge was different in the two studies reported here (Day 37 post-inoculation in Study 1, Day 23 in Study 2), and since medication may delay seroconversion to some pathogens under certain conditions,13,14 it is possible that the seroconversion rates in the two studies might have been more similar if Day 37 samples had been available for testing in Study 2.
Although the protective immunologic mechanisms for PPE remain undefined, antibody response (seroconversion) is a useful indicator of exposure and immune response to Lawsonia infection. Strategic manipulation of medication selection, timing, and dosage may facilitate the design of more effective PPE medication strategies that allow for capturing the benefits of natural exposure and immunity rather than relying solely upon continuous medication to control the disease.
Continuous medication has been shown to significantly reduce the immune response to L intracellularis infection.10,14 The seroconversion data generated from our studies suggest that antibiotic treatment may confound serological surveys, such as the NAHMS survey,15 causing possible underestimates of the prevalence of Lintracellularis exposure within medicated populations of pigs in the tested herds.
Our results also demonstrate the potential impact of effective medication on the results of fecal PCR diagnostic tests. Medication may interfere with expected fecal shedding and may confound interpretation of these tests in medicated populations.
Finally, tiamulin hydrogen fumarate is reported to possess in vitro activity against Lintracellularis.8,9 The results of our experiments confirm the in vivo effectiveness of THF for treating and controlling PPE.
Implications
- Tiamulin hydrogen fumarate in the feed at 35 g per ton or water at 60 mg per liter is effective in treating and controlling PPE.
- Timing of antibiotic treatment relative to time of infection with Lintracellularismay affect the immune response (seroconversion) and fecal shedding as well as the development of lesions.
- Antibiotic dosage may affect the immune response to L intracellularis infection.
- Antibiotic treatment may reduce fecal shedding and interfere with polymerase chain reaction tests for Lintracellularis.
Acknowledgements
We thank Dr Connie Gebhart and Ms Rebecca Mackie of the University of Minnesota for the MIC testing of the challenge isolate and Dr Jon Lemke of the University of Iowa for statistical analysis.
References — refereed
1. McOrist S, Gebhart CJ, Boid R, Barns SM. Characterization of Lawsonia intracellularis gen. Nov., sp. Nov., the obligately intracellular bacterium of porcine proliferative enteropathy. Int J Syst Bact. 1995;45:820-825.
2. McOrist S, Gebhart C. Porcine proliferative enteropathies. In: BE Straw, et al, eds. Diseases of Swine. 8th ed. Ames, IA: Iowa State University Press; 1999:521-534.
3. Jones GF, Ward GE, Murtaugh MP, Lin G, Gebhart CJ. Enhanced detection of intracellular organism of swine proliferative enteritis Ileal Symbiont intracellularis in feces by polymerase chain reaction. J Clin Micro. 1993;31:2611-2615.
4. Knittel JP, Jordan DM, Schwartz KJ, Janke BH, Roof MB, McOrist S, Harris DL. Evaluation of ante-mortem PCR and serological methods for the detection of Lawsonia intracellularis exposed pigs. Am J Vet Res. 1998;59:722-726.
5. McOrist S, Jasni S, Mackie RA, N. MacIntyre, Neef N, Lawson GHK. Reproduction of porcine proliferative enteritis with pure cultures of Ileal Symbiont intracellularis. Inf Imm. 1993;61:4286-4292
9. McOrist S, Mackie RA, Lawson GHK. Antimicrobial susceptibility of Ileal Symbiont intracellularis isolated from pigs with proliferative enteropathy. J Clin Micro. 1995;22:1314-1317.
11. McOrist S, Smith SH, Shearn MFH, Carr MM, Miller DJS. Treatment and prevention of porcine proliferative enteropathy with oral tiamulin. Vet Rec. 1996;139:615-618.
12. Reed LJ, Meunch H. A simple method of estimating fifty percent endpoints. Am J Hyg. 1936;27:493-497.
References — non-refereed
6. Anderson M, Stroh, S, Rogers, S. Tiamulin (Denagard(TM)) activity in certain swine tissues following oral and intramuscular administration. Proc AASP Ann Meet. 1994;115-117.
7. Nielsen B, Szancer J. Uptake and intracellular activity of tiamulin in human polymorphonuclear leukocytes compared with norfloxacin. Proc Int Pig Vet Soc. 1998;(3)241.
14. Collins A, van Dijk M, McOrist S, Love R. Strategic medication and development of immunity to Lawsonia intracellularis. Proc Int Pig Vet Soc. 2000;30.
15. Bane D, Norby B, Gardner I, Roof M, Knittel J, Bush E, Gebhart C. The epidemiology of porcine proliferative enteropathy. Proc Int Pig Vet Soc. 1998; (3)107.