Kirkwood RN. 2001;2:77-99 Effect of pig age and autogenous sow v
Case Study
Peer-reviewed
Effect of pig age and autogenous sow vaccination on nasal mucosal colonization of pigs by Haemophilus parasuis
Roy N. Kirkwood, DVM, PhD; Shirley A. Rawluk; Artur C. Cegielski, DVM; Arlene J. Otto
RNK: FAEMS, Royal Veterinary College, Hawkshead Lane, Herts
AL9 7TA, UK,
e-mail: rkirkwoo@rvc.ac.uk; SR,AC,AO: Alberta Agriculture,
Edmonton, Alberta,
Canada
Kirkwood RN, Rawluk SA, Cegielski AC, et al. Effect of pig age and autogenous sow vaccination on nasal mucosal colonization of pigs by Haemophilus parasuis. J Swine Health Prod. 2001;9(2):77-79. Also available in PDF format (108k).
Summary
This report describes an attempt to evaluate the effect of piglet age (with or without weaning), sow parity, and sow vaccination, on the timing of nasal mucosal colonisation of piglets by Haemophilus parasuis. We also determined the serotypes involved and compared serotypes isolated from the nasal mucosa and the trachea. Compared to tracheal swabs, nasal mucosal swabs were more easily performed and also more consistently yielded H parasuis. Serotypes obtained from each site were the same. A lower level of colonisation was found within the litters of young sows and a low level of colonization by H parasuis at weaning likely predisposes piglets to clinical disease in the nursery, assuming the presence of a virulent serotype. We found that more than one serotype of H parasuis was present in the herd and that vaccination of sows did not influence the timing of apparent colonization or the serotypes prevalent within a litter.
Keywords: swine, Haemophilus
parasuis, vaccination
swine, Haemophilus
parasuis, vaccination
Received: May 16, 2000
Accepted: November 28, 2000
Bacteria that colonize
mucosal surfaces during the first two weeks of life have been
termed early colonizers.1 Assuming adequate transfer
of specific colostral immunoglobulins, immunity against early
colonizers (e.g., Haemophilus parasuis) is good in early
lactation but declines progressively. Between about 10 and 15
days of age, antibody levels no longer prevent colonization, but
do limit it such that clinical disease is prevented.1
With time, level of H parasuis colonization increases to
the point where the pigs develop their own active immunity (about
3 weeks of age). Therefore, there is a balance between infection
and resistance such that the piglets are subjected to a relatively
slow and controlled exposure to a potential pathogen that allows
them the time to developtheir immunological defences.
Short lactations, high health status, and the isolated swine populations associated with modern swine production have increased the incidence and severity of disease due to infection with H parasuis (Glasser’s disease).2,3 To be able to implement strategies to control H parasuis requires a knowledge of when pigs become colonized, the effect of piglet age on the proportion of the litter that becomes colonized, and the role of the immune status of the sow. An earlier study has demonstrated that, when the sows are free of H parasuis and hence fail to provide passive immunity to their litters, epizootic Glasser’s disease may occur.4 More recent studies have shown that maternally derived passive immunity is essential for protection against H parasuis challenge3 but that sow immunity (and hence pig immunity via colostral immunoglobulins) following vaccination may be serovar specific.2 While vaccination of sows may improve the level of protection afforded to the litter, it is possible that it may also prolong the period of slow colonization. For early weaned pigs, the effect of this could be a reduction in the proportion of a litter that is colonized at weaning and consequently an increased risk of clinical disease in the nursery. To further examine the relationship between sow immune status and piglet age, studies were undertaken using a herd positive for H parasuis.
Case description
All pigs used in these studies were from the University of
Alberta swine research unit and the experimental procedures were
approved by the University of Alberta Animal Care committee. Herd
composition consisted of a 100-sow commercial farrow-to-finish
herd housed in a single barn, and a second component for reproduction
research where sows were housed in separate breeding-gestation
and farrowing barns and were killed prior to production of a second
litter. Nursery rooms were operated all in-all out, by room. All
buildings were on the same site. Gilts for both herd components
were purchased from the same source and the lactation length was
25 to 28 days,
except where indicated otherwise for specific experiments. Piglets
from both components were co-mingled at weaning.
To confirm that H parasuis was present in the herd,
nasal swabs were obtained from 11 randomly selected pigs in the
nursery after wiping the nasal planum rostrale with alcohol. Swabs
were submitted to the Alberta Agriculture bacteriology laboratory
(Edmonton, Alberta) for culture. Nasal swabs were inoculated into
modified Amies clear transport medium and delivered to the laboratory
within 60 min of collection. Swabs were cultured to both enrichment
(modified Haemophilus Mueller Hinton medium, H, Difco, Detroit,
Michigan; [H]) and selective enrichment (as above with either
3 µg per mL vancomycin [HV] or 0.1 µg per mL crystal
violet and 4.5 µg per mL bacitracin [HCB]) primary plating
media in order to optimize recovery of
H parasuis.Organisms were presumptively identified as H
parasuis if they were V-factor dependent, positive for catalase,
mannose, and saccharose, and negative for indole, urease, lactose,
mannitol, raffinose, sorbitol, and CAMP; and if they grew luxuriously
on H, HV, and HCB plating media.
Haemophilus parasuis was isolated from 11 of 11 swabs cultured. Some colonies cultured from four of 11 swabs were identified as most likely to be H parasuis, since they showed the appropriate biochemical characteristics except that acid production in mannose and (or) saccharose was usually absent and, when present, was observed as a delayed weak reaction. Also, these colonies either failed to grow or grew sparsely on HCB medium.
Study One
The litters from eight sows were used. Four of the litters were derived from multiparous sows housed in the commercial production barn and were weaned at 28 days of age. The remaining four litters were derived from primiparous sows housed in the reproduction research farrowing barn and were weaned at 14 days of age. Within each weaning group, the nasal passages of all piglets from each of the four litters were swabbed at 14 and 28 days of age, and the swabs were cultured for H parasuis. Serotyping of isolates was not done.
All piglets were weaned into the same nursery, but each weaning
age occupied the nursery at a different time. Pig health was monitored
daily during the nursery period. At 14 days of age, 53% of piglets
from multiparous sows were sufficiently colonized by H parasuis
to yield a positive culture, while none of the piglets from primiparous
sows yielded a positive culture (Chi square, P<.001).
At 28 days of age, nearly all (97%) of the piglets that remained
with their dams yielded a positive culture, but only 50% of pigs
weaned at 14 days of age yielded a positive culture at 28 days
of age (Chi square, P<.01). These results indicate that
the level of apparent colonization was reduced either by low parity
or barn and, further, that from 14 days of age, the rate of apparent
colonization of pigs was slower if the pigs were weaned. Weaning
delayed apparent colonization in this study, likely as a consequence
of there being relatively few pigs in the nursery with a sufficient
H parasuis load to allow efficient pig-to-pig infection.
In contrast, unweaned pigs continued to have intimate contact
with their dams, the presumed source of H parasuis. Eighteen
days after weaning, a disease outbreak occurred in the pigs that
had been weaned at 14 days, and three died. Post mortems revealed
polyserositis, but a definitive etiology was not established.
Because the above data was confounded by parity and barn, we performed another replicate using four litters from each barn, weaned at 28 days of age, but all from primiparous sows. Piglets (n=6 or 7 per litter) were nasal swabbed at 14 and 28 days of age and the swabs were cultured for H parasuis. Serotyping of the isolates was not done. At 14 days of age, 39% of piglets in the commercial barn, and 25% of piglets in the reproduction research barn, were culture-positive for H parasuis, and this difference was not significant (Chi square, P=.27). By the time of weaning at 28 days of age, 96 to 100% of all piglets were culture-positive. The reason that the level of apparent colonization in the reproduction research barn was higher in this replicate than in the first was not resolved.
Study Two
This study was undertaken to determine the prevalent serotypes of H parasuis present within the herd and to examine the possibility that the serotypes identified would be different depending on whether the nasal cavity or trachea was swabbed. At 25 days (preweaning) and 50 days of age, 31 pigs were anesthetized with xylazine (2 mg per kg, IM) and ketamine (10 mg per kg, IM), and nasal cavity and tracheal swabs were collected. The tracheal swabs were guarded. If colonies were identified as either H parasuis or H parasuis-like, a representative colony of each bacterial type from each pig was submitted for serotyping (Bayer, Worthington, Minnesota). Additionally, colonies that appeared atypical on growth media and (or) biochemical reactions were also sent for serotyping.
Of the 31 nasal swabs obtained at 25 days of age, serotype 9 was isolated from 19 swabs, a non-typable serotype (NT) from one swab, and serotype 9 plus NT from 11 swabs. Serotype 9 was isolated from 14 tracheal swabs and NT from one tracheal swab. Sixteen swabs were negative for Hparasuis. Only 29 pigs were swabbed at 50 days of age. Of the 29 nasal swabs obtained at 50 days of age, serotype 9 was isolated from 12 swabs, NT serotype from seven swabs, and both serotypes 9 and NT from eight swabs. Two swabs were negative for H parasuis. Tracheal swabs obtained at 50 days of age yielded seven serotype 9, one NT, and two serotype 9 plus NT. Nineteen swabs were negative for Hparasuis.
When the organisms identified as most likely H parasuis
were serotyped, all were
H parasuis serotype 9. With the exception of two pigs at
50 days of age, tracheal swab isolates were always represented
by the same serotype as that isolated from nasal swabs (e.g.,
if the nasal swab yielded either serotype 9 or NT or both, either
serotype 9 and NT or both were isolated from the tracheal swab).
However, the success of isolation from tracheal swabs was considerably
less than that from nasal swabs. A comparison of H parasuis
serotypes recovered from pigs swabbed at the two different ages
revealed that six pigs carrying only serotype 9 at 25 days yielded
only NT at 50 days. A further eight pigs that carried only serotype
9 at 25 days yielded both serotypes 9 and NT at 50 days. In contrast,
ten pigs that carried both serotypes 9 and NT at 25 days yielded
only serotype 9 at 50 days.
Study Three
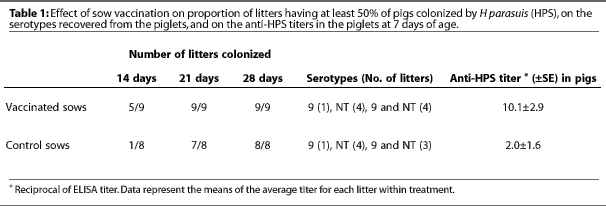
The objective of this study was to determine the effect of maternal vaccination against H parasuis on the timing and cumulative incidence of apparent colonization of pig nasal mucosa by H parasuis. Of 17 sows housed in the commercial barn, nine received an autogenous anti-Hparasuis vaccine at 5 and 2 weeks pre-farrowing, and eight served as unvaccinated controls. The vaccine was prepared commercially (Bayer Laboratories, Worthington, Minnesota) using one serotype 9 and one NT organism isolated during Study Two. The reason for using only one NT organism was that five NTs were determined to have the same outer membrane protein profiles, and so it was reasonable to conclude that they represented a single serotype (VJ Rapp-Gabrielson, personal communication). All pigs in each litter were nasally swabbed at 14, 21, and 28 days of age and the swabs were cultured for H parasuis. Representative colonies from two pigs from each litter were submitted for serotyping.
To confirm a greater intake of colostral anti-H parasuis antibodies by pigs nursing vaccinated sows, each piglet was bled at 7 days of age and serum titers against Hparasuis were determined by ELISA (Biovet, St. Anthony, Minnesota). Since the sow was the experimental unit, the mean titer for each litter was calculated and the treatment titer is the mean of the litter titers. Vaccination of the sow against Hparasuis was associated with elevated anti-H parasuis titers in pigs 7 days of age (Table 1). There appeared to be no association between pig serum titers within a litter and the proportion of the litter that was apparently colonized at 14 days. Overall, there was no apparent effect of sow vaccination on the number of pigs within a litter that were apparently colonized by Hparasuis at any of the ages examined. Similarly, there was no effect of sow vaccination on the serotypes of H parasuis recovered from the litters (Table 1).
Discussion
The present results serve to remind us that pigs nursing young sows will likely have a reduced exposure to early-colonizing normal mucosal flora. The potential clinical significance of this is illustrated by the example of a new herd that was farrowing too many primiparous sows for the available facilities. This necessitated a temporary shift in weaning age from 16 to 18 days to 11 to 14 days and, although a causal relationship was not proven, the nursery soon suffered an outbreak of Glasser’s disease. Further, it is interesting to note that during the first replicate of Study One, an outbreak of disease occurred 18 days into the nursery phase, involving pigs weaned at 14 days of age. Three pigs died and post mortems were suggestive of Glasser’s disease (polyserositis), although a Streptococcus suis etiology could not be ruled out. The occurrence of disease specifically in that group of pigs is consistent with the theory proposed by Pijoan,1 that a low level of colonization by H parasuis (or S suis) at weaning predisposes piglets to clinical disease in the nursery.
The conclusions to be drawn from Study Two are that nasal swabbing provides an effective means of establishing the presence of H parasuis and the prevalent serotypes. Also, as previously shown by others,5,6 a herd may be infected concurrently with different serotypes of H parasuis, which may occur singly or in combination within a pig. Further, within the limits of the serotypes within a herd, the prevalent serotype within a pig may change over time.
Finally, the results of Study Three indicate that vaccination of the sow alone did not change the timing of apparent colonization by H parasuis, or the prevelant serotype. This implies that full protection at an early age requires vaccination of both the sow and litter as demonstrated by Solano-Aguilar et al.3
Implications
- Compared to tracheal swabs, nasal mucosal swabs were more easily performed and more consistently yielded H parasuis. Serotypes obtained from each site were the same.
- A lower level of colonization can be expected within the litters of young sows, and a low level of colonization by H parasuis at weaning likely predisposes piglets to clinical disease in the nursery, assuming the presence of a virulent serotype.
- More than one serotype of H parasuis can be present in the herd.
- Vaccination of sows did not influence the timing of apparent colonization by H parasuis or the serotypes prevalent within a litter.
Acknowledgements
We gratefully acknowledge the excellent technical support provided by Annette Visser, Suzanne Gibson, and Evelyn Bowlby. Financial assistance was generously provided by Intervet Canada.
References — refereed
2. Rapp-Gabrielson VJ, Kocur GJ, Clark JT, Muir SK. Haemophilus parasuis: Immunity in swine after vaccination. Vet Med. 1997;92:83-90.
3. Solano-Aguilar GI, Pijoan C, Rapp-Gabrielson VJ, Collins J, Carvalho LF, Winkelman N. Protective role of maternal antibodies against Haemophilus parasuis infection. Am J Vet Res. 1999;60:81-87.
4. Nielsen R, Danielsen V. An outbreak of Glasser’s disease. Studies on etiology, serology and the effect of vaccination. Nord Vet Med. 1975;27:20-25.
5. Rapp-Gabrielson VJ, Gabrielson DA. Prevalence of Haemophilus parasuis serovars among isolates from swine. Am J Vet Res. 1992;53:659-664.
6. Smart NL, Miniats OP, Rosendal S, Friendship R. Glasser’s disease and prevalence of subclinical infection with Haemophilus parasuis in swine in southern Ontario. Can Vet J. 1989;30:339-343.
References — non refereed
1. Pijoan C. Diseases of high health pigs: some ideas on pathogenesis. Proc AD Leman Swine Conf. 1995; 22:16-17.