Kihlstrom SL. 2001;2:65-69 Assessing the progressive decontamina
Original Research
Peer-reviewed
Assessing the progressive decontamination of farrowing crate floors by measuring the decrease in aerobic bacteria
Stacey L. Kihlstrom, MSc; W.E. Morgan Morrow, BVSc, PhD; Peter R. Davies, BVSc, PhD; Geraldine H. Luginbuhl, PhD
SLK, WEMM: Dept of Animal Science, North Carolina State University, Raleigh, North Carolina 27695; Requests for reprints to WEMM; PRD: College of Veterinary Medicine, North Carolina State University, Raleigh, North Carolina 27606; GHL: Dept of Microbiology, North Carolina State University, Raleigh, North Carolina 27695
Kihlstrom SL, Morrow WEM, Davies PR, et al. Assessing the progressive decontamination of farrowing crate floors by measuring the decrease in aerobic bacteria. J Swine Health Prod. 2001;9(2):65-69. Also available in PDF format (128k).
Summary
Objective: To measure the progressive decontaminationof three farrowing crate floor types and evaluate the efficacy of a surfactant and two disinfectants, povidone iodine (polyvinylpyrrolidone-iodine) and cresylic acid, used in the process.
Method: Using a randomized incomplete block split-plot design, four treatments (two disinfectants, each with or without a surfactant) were applied to three floor types, including concrete slats, triangular metal bar, and plastic-coated expanded wire. Two areas were swabbed with a sterile sponge after low-pressure washing, power washing, and disinfecting. Material collected on the sponge was suspended in sterilesaline, and tenfold dilutions were plated in duplicate onto tryptic soy agar. Plates were incubated aerobically at 37 degrees C (98.6F) for 24 h, and colonies were counted using a counting grid.
Results: After the last step in decontamination, mean log aerobic bacterial counts were similar on all three floor types, regardless of treatment. Low-pressure washing, power washing, and disinfection each contributed to the decrease in contamination, and use of surfactant had no effect on aerobicbacterial counts.
Implications: Sequential low-pressure washing, power washing, and disinfection of farrowing house floors results in a major reduction of aerobic bacteria in the presence of residual organic matter, with each step contributing to decontamination. Afterpower washing, povidone iodine and cresylic acid were equally effective disinfectants, and use of surfactant was not advantageous.
Keywords: swine, disinfection,
farrowing house, flooring
swine, disinfection,
farrowing house, flooring
Received: July 11, 2000
Accepted: October 19, 2000
Environmental contamination is a major source of infection for far-rowing sows and piglets. Improper cleaning and disinfection allows bacteria to persist in dried organic matter such as feces, dust, dirt, feed, and blood.1 To reduce the risk of infection, managers usually remove all weaned sows and clean the room before bringing in another group of sows due to farrow. The cleanliness of the farrowing crate floor is particularly important, because it is usually the first surface piglets are exposed to after birth. Materials used for farrowing crate floors include concrete, triangular metal bar, and plastic-coated expanded wire. Each surface varies in its ability to collect dust and dirt, and the ease with which it can be cleaned.2 Farrowing crates are usually washed with water, first under low and then under high pressure, to physically remove most of the organic matterand microorganisms and prepare the surfaces for disinfection.
Polyvinylpyrrolidone-iodine (povidone iodine, PVPI) is commonly used as a skin and wound antiseptic, for example, in teat dips in the dairy industry,3 and is recognized for its low toxicity4 and activity against a broad spectrum of microorganisms.5 The carrier, PVP, stabilizes and enhances water solubility of iodine. In solution, the PVPI complex forms a reservoir of available iodine in equilibrium with PVP.6 Free iodine (available I2) is slowly liberated from the reservoir as iodine reacts with the thiol groups of enzymes or cytoplasmic proteins of bacteria, viruses, protozoa, and fungi, resulting in microbial death.7 The advantages of using PVPI to disinfect swine farrowing houses include its high residual activity, compatibility with surfactants, broad antimicrobial activity (including bacterial spores), and low toxicity to animals and farm employees.8, 9
Cresylic acid, the combined fraction of cresols and xylenols obtained from the destructive distillation of coal,3 has good biocidal properties except against bacterial spores, excellent resistance to organic debris, and good residual activity. However, it is a general protoplasmic poison, is readily absorbed through the skin, and can cause severe burns. The Environmental Protection Agency (EPA) has determined that cresols are possible human carcinogens.10 The Occupational Safety and Health Administration (OSHA) has set an exposure limit of 22 mg per m3 for cresols in work-place air for an 8-hour work-day, 40-hour work-week,11 and advises avoiding eye and skin contact.
The use of a combination ionic and non-ionic surfactant (Sunlight(TM), Lever Brothers Co., New York, New York) was also evaluated as an aid in decreasing bacterial populations on the floors of farrowing crates. Although the anionic and non-ionic components have little antimicrobial activity, low concentrations of non-ionic surfactants affect the permeability of the outer parts of the gram-negative envelopes, and anionic compounds contribute to the general denaturation of cell proteins, enabling emulsification of organic matter that may protect pathogens.12
We measured numbers of aerobic bacteria that remained after each step of progressive decontamination of three farrowing crate floor types, and evaluated the efficacy of povidone iodine, a "safer" compound compared to cresylic acid, when each was used after power-washing with or without a surfactant.
Materials and Methods
The four farrowing rooms used (North Carolina State University Swine Educational Unit II) each contained twelve crates, with floors of slatted concrete underneath the sow, plastic-coated expanded steel beside the sow in the creep, and triangular metal bar behind the sow. Four three-step decontamination treatments (Table 1) were applied using a randomized incomplete block, split-plot design. For Treatments 3 and 4, a dishwashing liquid surfactant (Sunlight(TM), Lever Brothers Co., New York, New York) was added to the power-washing water at a concentration of 1:10.

Two treatments were blocked per farrowing room (Table 2). At random, one treatment was applied to two adjoining crate floors in the front of the room and the other was applied to two adjoining crates in the back of the room. To minimize cross contamination, the two pairs of treated crates were separated by eight untreated crates. Each treatment was repeated in three trials. Trials were performed after sows and piglets were removed from the room, not simultaneously, but as farrowing rooms became available.
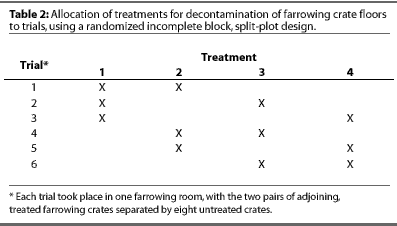
To remove gross contamination, the floors were hosed down with water using a low-pressure booster pump and allowed to dry for 20 minutes prior to sampling. Floors were then power washed, with or without surfactant, using a Karcher(TM) power washer (West Patterson, New Jersey) at 140.61 kg per cm2 (2000 psi), and allowed to dry for 20 minutes prior to sampling. Immediately after power washing, either povidone iodine (Agridine(TM), Novel Pharmaceutical, Hamlet, North Carolina) or cresylic acid (185 Premise Cleaner(TM), BioSentry, Inc., Stone Mountain, Georgia) was diluted accordingto the label and sprayed on the floors, which were allowed to dry for 10 minutes prior to sampling.
A portable frame delineated two sampling areas of 0.094 m2 (1 sq. ft2) on each type of floor (concrete, plastic-coated expanded metal, and triangular metal bar), for a total of six sites per crate. A 5-cm square sponge (Microsponge(TM), MicroNex, Inc., Raleigh, North Carolina) was used to collect the replicate samples, as described by Jones.13 Counts from the corresponding floor sites of the two adjoining crates were averaged, so that for each step in decontamination, there were six measurements from each room (one set of measurements for each treatment of the three floor types). We did not collect swabs from the same areas after each treatment step because swabbing was likely to remove bacteria, and the resulting counts might have been under-estimates. No samples were taken before low-pressure washing, when floors were grossly contaminated with fecal material.
Sponges were placed in sterile Whirlpaks(TM) (Fisher Scientific Co., Pittsburgh, Pennsylvania) and transported on ice to the laboratory within 1 hour of collection. To extract bacteriological samples, 25 ml of sterile saline was poured into the Whirlpak(TM), and the sponge was massaged for about 30 seconds. The sponge was aseptically extracted from the bag, 100 uL of saline suspension was removed, and tenfold serial dilutions were made and plated in duplicate, using a Model C Spiral Plater (Spiral Systems(TM), Inc., Cincinnati, Ohio), onto tryptic soy agar (TSA). Plates were incubated aerobically at 37 degrees C (98.6 degrees F) for 24 h and counted using a counting grid (Spiral Systems(TM), Inc., Cincinatti, Ohio). Results from duplicate plates were averaged. Mean aerobic bacterial populations were calculated for each replicate on each floor type and each crate in each treatment. The final statistical model used for analysis by PROC Mixed procedure (SAS(R) System for Mixed Models, Cary, North Carolina: SAS Institute Inc., 1996) was Yijkl = u + Trial (T)i Surfactant (S)j + Disinfectant (D)k + TSDijk + Floor (F)l + SFjl + DFkl + SDFjkl + Log of the mean aerobic bacterial count after pressure washing (LogP)m + Eijkl m. Ti and TSDijk are random effects. The statistical model we used before power washing logically excluded the effects of surfactant and disinfectant (Y = Treatment (Tx) + Floor (F) + Tx*F) and similarly the model to analyze the effects after power washing logically excluded the disinfectant effects (Y = Treatment (Tx) + log of mean aerobic bacterial count after the low-pressure wash (LogLP) + Surfactant (S) + Floor (F) + Tx*F).
Pairwise comparisons (pdiff) of the least squares means (lsmeans) were used to determine differences between mean aerobic populations on each floor type and reductions in mean aerobic populations after each step of decontamination.
Results
After low-pressure washing, contamination on the three floor types differed (P<.05) (Table 3), with the mean log bacterial count highest on concrete (6.89), intermediate on metal bar (6.01), and lowest on plastic-coated expanded wire (5.32) (Figure 1).
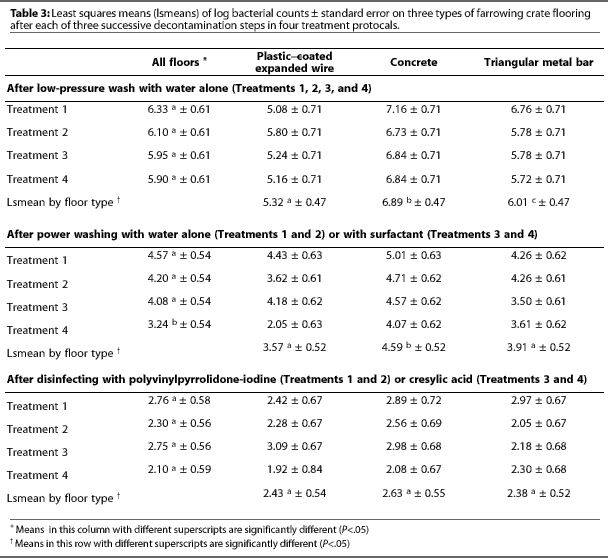
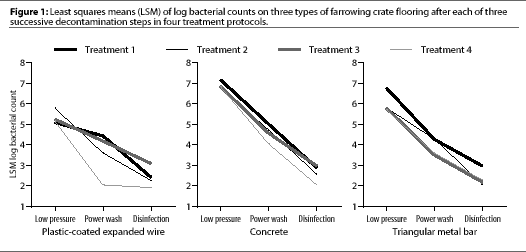
After power washing, the mean log bacterial count was higher on concrete (4.59) than on plastic-coated expanded wire (3.57, P=.009) or triangular metal bar (3.91, P=.03) (Table 3). Power washing significantly (P<.01) reduced aerobic bacterial counts on all floors (Table 4). Surfactant had no effect on aerobic bacterial counts (P=.12).
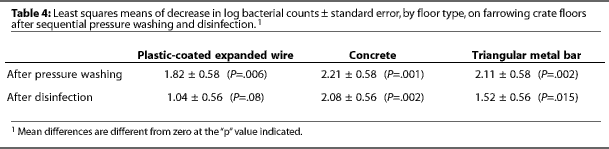
After disinfection, mean log bacterial counts were similar on all three floors (Table 3), regardless of treatment (Figure 1). Mean log bacterial counts on all floor types were reduced by 1.54 after disinfection with PVPI (P=.06) and by 1.55 after cresylic acid (P=.06). Type of disinfectant did not affect the number of aerobic bacteria remaining. Disinfection removed more (P=.03) aerobic bacteria from concrete (2.08) than from plastic-coated expanded wire (1.03).
No interactions (P>.05) were found between use of a surfactant and either PVPI or cresylic acid, floor type and surfactant, or floor type and disinfectant in the final model.
Discussion
Because contamination of flooring was confounded by location in the crate (concrete under the sow where she lies, triangular metal bar behind the sow where she defecates, and plastic-coated expanded wire in the creep where the piglets sleep and defecate), we cannot determine whether the flooring material or the location of the flooring in the crate was responsible for initial contamination. We believe the material was at least partially responsible, because the area behind the sow, triangular metal bar flooring, is usually the most heavily contaminated with sows’ feces, yet it was intermediate in bacterial contamination. In addition, the concrete, intuitively a more difficult surface to clean because of its porosity, had the most bacterial contamination, although this area often had the least gross contamination. None of the treatments totally eliminated bacteria on any of the floors, but in animal accommodation, we do not need or expect to achieve sterility. Bacteria may have been protected in the pores of the material (e.g., concrete), making it more difficult for the disinfectant to come in contact with the organisms.14 Contamination from the walls and ceilings, which were not cleaned at the same time as the floors, may have settled on the flooring in the time between cleaning and sampling.
No treatment was better than any other in reducing the number of aerobic bacteria, and none resulted in a less contaminated surface. All treatments resulted in a cleaner surface and all steps (low-pressure washing, power washing, and disinfection) contributed to the decrease in bacterial contamination. These results suggest that each step in the process is important. To decrease the cost of clean up, managers either decrease the time required (e.g., by deleting some of the steps) or decrease the cost of the process (e.g., by substituting a cheaper disinfectant for a more expensive one). Our results suggest that the surfactant could be deleted, but we were unable to determine whether either the low-pressure wash or power-wash steps of the cleaning process could be deleted. The final mean log bacterial counts were similar on all three floors, indicating that contaminated surfaces can be satisfactorily cleaned by any of the treatments tested.
Implications
- Sequential power washing and disinfection of farrowing house floors results in a major reduction of bacteria, and each step contributes to decontamination.
- Power washing with water alone was as effective as power washing with surfactant in reducing contamination of farrowing crate floors.
- After power washing, a disinfectant that is deactivated by organic material (povidone iodine) was as beneficial as a disinfectant that is effective in the presence of organic material (cresylic acid).
- Povidone iodine is a safer disinfectant than cresylic acid and just as effective against aerobic bacteria on farrowing crate floors.
References — refereed
1. Linton AH. Epidemiology of infectious diseases in animals. In: Linton AH, Hugo WB, Russell AD, eds. Disinfection in Veterinary and Farm Animal Practice. Boston, MA: Blackwell Scientific Publications; 1987:1-11.
2. Sandahl, AM. Cleanability of building materials. Farm Building Progress. 1975;40:19-21.
3. Russell AD and Hugo WB. Chemical disinfectants. In: Linton AH, Hugo WB, Russell AD, eds. Disinfection in Veterinary and Farm Animal Practice. Boston, MA: Blackwell Scientific Publications; 1987:19-20.
4. Shelanski HA, Shelanski MV. PVP-Iodine: history, toxicity, and therapeutic uses. J Inter Coll Surg. 1956;25:727-734.
5. Zamora JL. Chemical and microbiologic characteristics and toxicity of Povidone-iodine solutions. Am J Surgery. 1986;151:400-406.
6. Siggia S. The chemistry of polyvinylpyrrolidone-iodine. J Am Pharm Assoc. 1957;46:201-204
8. Gershenfeld L. Povidone-iodine as a topical antiseptic. Am J Surg. 1957;94:938-939.
9. Gottardi W. Iodine and iodine compounds. In: Block SS, ed. Disinfection, sterilization, and preservation. 4th ed. Philadelphia, PA: Lea and Febiger; 1991:152-166.
14. Morgan-Jones S. Practical aspects of disinfection and infection control. In: Linton AH, Hugo WB, Russell Ad, eds. Disinfection in veterinary and farm animal practice. Boston, MA: Blackwell Scientific Publications; 1987:144-167.
References — non-refereed
7. Krusé WC. Halogen action on bacteria, viruses and protozoan. Proc Natl Spec Conf Disinfection. ASCE, Amherst, MA; 1970:113-137.
10. U.S. Environmental Protection Agency. Integrated risk information system (IRIS) on o-cresol. Cincinnati, OH: Environmental Criteria and Assessment Office, Office of Health and Environmental Assessment, Office of Research and Development; 1993.
11. Chemical Information Manual. July 1, 1991. OSHA Instruction CPL 2-2.43A.
12. Hildebrandt A. Notification concerning the registration of drugs (Preparative monographs for the human medicine area). Berlin: German Federal Department of Health, miscellaneous publication; April 22, 1991.
13. Jones FT. Simple sponge may be best for sampling for Salmonella. Feedstuffs, 1993; 65:33.