Docic A. 2001;1:25-27 effect of short term high feed intake on t
Brief communication
The effect of short term high feed intake on the onset of puberty in transported gilts.
Anton Docic, Dipl Agr; Gabor Bilkei, DVM, PhD
Docic A, Bilkei G. The effect of short term high feed intake on the onset of puberty in transported gilts. J Swine Health Prod. 2001;9(1):25-27. Also available as a PDF (108k).
Bilkei Consulting, Raubbuhlstrasse 4, CH-8600 Dubendorf, SWZ, Phone: (41)1-820-0226.
Summary
We conducted a trial to determine whether energy flushing combined with transport, regrouping, and exposure to boars influences the onset of puberty. In a large pig production unit, 320 incoming gilts approximately 160 days of age were randomly divided into two groups. The FLUSHED group (166 gilts) were both transported and energy flushed, and the TRANSPORTED group (154 gilts) were transported only. After transport, gilts were housed in small groups exposed to boars across an aisle. Boars were moved daily to pens facing different groups of gilts. As estrus detection is difficult in gilts this young, it was not attempted, and the onset of puberty was determined at slaughter one week after transport. Reproductive organs were collected immediately after slaughter and examined that day. There were more follicles >4 mm and uterine mass was larger (P>.05) in the FLUSHED group than in the TRANSPORTED group. Adrenal gland weight, ovarian weight, and uterine length did not differ between treatment groups. In this trial using 160-day old gilts, energy flushing increased follicular growth and uterine weight, which are indicators of puberty in gilts this age.
Keywords : swine, gilt,
puberty, transport, flushing, ovarian follicles
: swine, gilt,
puberty, transport, flushing, ovarian follicles
Received: March 31, 2000
Accepted: October 18, 2000
In commercial breeding units, the interval between entering the unit and the first estrus is often prolonged in replacement gilts. This may be the result of endocrine dysfunction or management failure.1 Other variables that influence the age of puberty include exposure to boars, age, genetic background, climate, season, photoperiod, housing, transport, and energy flushing.2,3 Nutritional management has a major impact on the reproductive performance of a gilt.4 According to Leman et al,5 farrowing rate does not improve as the gilt’s age increases. Therefore, producers try to breed gilts as early as possible to minimize the number of non-productive days. Breeding gilts at their pubertal estrus, but not their second estrus, is often associated with a low ovulation rate and small first litter size.6 Methods that have attempted to induce an early, fertile estrus include the use of exogenous goandotropins, and transport of gilts combined with exposure to boars and energy flushing.7 We conducted the following trial to determine whether energy flushing combined with boar exposure does influence the onset of puberty in transported gilts.
Materials and methods
Pigs used in the trial originated in a large, East European
production unit of 2500 sows. The F1 Large White x Landrace females
were mated to Duroc boars, and the pigs were weaned at 28 +/-
3 days of lactation and fed ad libitum the diets listed in Table
1. Micronutrient supplementation was comparable to that used in
commercial units in the United States.8
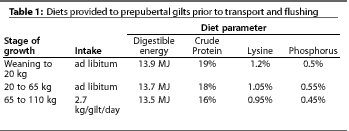
The trial was conducted during the early summer when environmental temperature was ideal. At 110 kg and approximately 160 days of age, 320 gilts were randomly divided into two treatment groups. The TRANSPORTED group (154 gilts) were fed at the rate of 2.7 kg per animal per day (Table 1). The FLUSHED group (166 gilts) were fed the same diet but were allowed ad libitum feeding for 1 week before and 1 week after transport. Average daily intake of the FLUSHED gilts was slightly more than 3.8 kg per animal per day.
Groups of 35 to 40 gilts were transported approximately 40 km, in an open truck, requiring approximately 1 hour. On the day of transport, gilts were provided with water only. On arrival, they were housed in groups of 10 to 12, with a boar housed across the aisle (approximately 1 meter) from each group. The boars were moved every day so that they faced different groups of gilts. Estrus detection was difficult and inconsistent in these young gilts, and occurrence of estrus based on clinical signs was not evaluated statistically.
One week after transport, the gilts were moved to an abattoir about 50 km distant, and slaughtered immediately after arrival. The following parameters were assessed: adrenal gland weight, ovarian weight, uterine weight and length, and number of ovarian follicles >4 mm in diameter. Statistical analysis was performed using the Student 2-Phase t test (Systat program).
Results
A corpus hemorrhagicum was occasionally observed, primarily in gilts in the FLUSHED group, but this was not a consistent finding and it was not recorded.
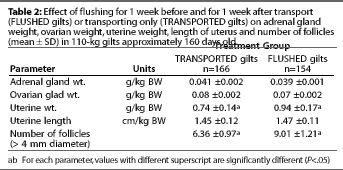
Adrenal gland weight, ovarian weight, and uterine length did
not differ between treatment groups. However, uterine weight and
the number of follicles >4 mm diameter were significantly greater
(P<.05) in FLUSHED than in TRANSPORTED gilts (Table
2).
Discussion
Follicle size is a better indicator of puberty than observation of clinical signs of estrus, which may be "silent" in gilts. Preovulatory follicles in pubertal gilts have a diameter >4 mm.7 Growing follicles and ovulation may be observed in young gilts (approximately 160 days of age) that do not show clinical signs of estrus. Although we did not record the presence of a corpus hemorrhagicum in this trial, we believe that the higher uterine weights in the FLUSHED group indicate that ovulation had occurred in these animals, as an increase in uterine size is associated with ovulation in gilts.7
Nutrition influences the reproductive axis of the gilt.9 When prepubertal gilts are allowed ad libitum access to a previously restricted diet, metabolic changes occur that mediate a significant increase in follicular development.10 An immediate increase in blood insulin concentration increases luetinizing hormone secretion, promotes persistence of medium-sized follicles, and diminishes follicular atresia. Under field conditions, we have found that although increased energy intake prior to breeding increases ovulation rate, litter size is not consistently improved, because increased embryonic mortality accompanies the increase in ovulation rate.
Several micronutrients may be responsible for advancing the onset of puberty and improving ovulation rate and litter size. Kirkwood and Thaker11 reported that supplementing zinc, manganese, copper, and iodine positively influenced litter size and embryonic mortality. Trembly et al.12 reported that supplemental folic acid increased litter size in females that had been flushed to increase ovulation rates. In our experience, supplementing folic acid improves ovulation rate (unpublished data). Mordenti and Marchetti13 reported that ascorbic acid supplementation markedly stimulated the onset of estrus and suggested that biotin may increase uterine size. The flushing accomplished by allowing ad libitum feeding, especially in gilts previously on restricted feed intake, may be effective because levels of energy, trace elements, vitamins, and minerals all increase simultaneously with the high feed intake (>3.8 kg per gilt per day).
Exposure to boars appears to be one of the most important stimuli for initiating the onset of puberty in gilts. Once gilts have reached the threshold age for puberty, the onset of estrus and ovarian activity can be stimulated by pheromones and olfactory stimuli from a present boar,14 but other sensory stimuli, such as visual, tactile, and auditory cues, may contribute to the precipitation of puberty as well.15 Transport and relocation positively affects both onset and synchrony of puberty in gilts.16
As the gilts in this study were all of the same genetic background, and had been exposed to the same conditions of management, boar exposure, and transport, the only difference between the two treatment groups was flushing, which must have been responsible for the observed differences in uterine weight and number of ovarian follicles. However, as we failed to record the occurrence of corpora hemorrhagica in these gilts, further trials would be necessary to establish a clear cause and effect relationship between energy and micronutrient flushing and ovulation.
Implications
- In 160-day old gilts, flushing (intake 3.8 kg feed per gilt per day) increases follicular growth and uterine weight, which are indicators of puberty in gilts this age.
References–refereed
1. Kirkwood RN. Pharmacological intervention in swine reproduction. Swine Health Prod. 1999;7:29-35.
2.Dial GD, Hilley HD, Esbenshade KL. Sexual development and initiation of puberty in the pig. In: DA Morrow, ed. Current Therapy in Theriogenology. Philadelphia: Saunders. 1986: 901-905.
3. Dyck GW. Factors influencing sexual maturation, puberty and reproductive efficiency in the gilt. Can J Anim Sci. 1988;68:1-13.
4.Den Hartog LA, Van Kempten GJ. Relationship between nutrition and fertility in pigs. Neth J Agric Sci 1980; 28:211-227.
6.Vermeer HM, Slijkhuis A. Inseminatie van opfokzeugen bij eerste of tweede bronst. Proef Proefstn Varkens. 1989;1.36-1.56.
7. Hughes PE, Cole DJA. Reproduction in the gilt. 2. The influence of gilt age at boar introduction on the attainment of puberty. Anim Prod. 1976;23:89-94.
9.Hughes PE. Manipulating Pig Prod. A symposium – nutrition, reproduction interactions in the breeding sow. 1989;II:277-280.
11.Kirkwood RN, Thaker PA. Nutritional factors affecting embryo survival in pigs (results and speculations). Pig News & Inf. 1988; 9:1
12. Tremblay GF, Matte JJ, Dufour JJ. Survival rate and development of fetus during the first 30 days of gestation after folic acid addition to swine diet. J Anim Sci. 1989; 67:724-732.
14.Kirkwood RN, Hughes PE, Both WD. The influence of boar related odours on puberty attainment in gilts. Anim Prod. 1983;36:131-136.
16. Zimmerman DR, Carlson R, Lantz B. The influence to the exposure to the boar and movement on pubertal development in the gilt. J Anim Sci. 1974; 39:230.
References–non-refereed
5. Leman AD, Fraser D, Greenley W. Factors influencing farrowing rate in confined Large White X Landrace sows. Proc IPVS Congress, Rio de Janeiro, Brasil. 1988; 288.
8.NCR, National Research Council. Nutrient Requirements of Swine. 10th ed. Washington, DC: National Academy Press. 1998.
10.Foxcroft GR, Cosgrove JR, Aherne FX. Relationship between metabolism and reproduction. Proc 14th IPVS Congress, Bologna, Italy. 1996; 6-9.
13. Mordenti A, Marchetti M. Use of vitamins for unconventional purposes. Proc 14th IPVS Congress, Bologna, Italy.1996; 39-44.
15. Bilkei G. Ed. Sauengeburt und Milchfieber. (Parturition and milk fever of the sow.) Konsulentenbüro 1999; 11-19.