swine, progressive atrophic rhinitis,
Pasteurella multocida, tilmicosin
Received: December 10, 1999
Accepted: August 20, 2000
Progressive atrophic rhinitis (PAR) remains a significant global economic problem in the swine
industry.1 Typically more severe than
nonprogressive atrophic rhinitis (NPAR), which is caused by toxigenic
Bordetella bronchiseptica,1,2 PAR is a multifactorial
disease complex that is caused by coinfection with toxigenic
Pasteurella multocida types A and D and B.
bronchiseptica.1-3 The etiology of PAR is attributed to concurrent
infections with B. bronchiseptica and toxigenic
Pasteurella multocida types A and
D;1-4 nontoxigenic strains of P.
multocida types A and D have also been observed to
contribute to the syndrome.1,5 Although
infectious organisms play an important role in the progression of the disease, the barn
environment and management also contribute to its
severity.1
Infection with B. bronchiseptica usually results in an inflammatory reaction in
the mucosa and submucosa of the turbinates, causing a mild turbinate atrophy
that allows toxigenic P. multocida to
colonize and damage osteoclasts and osteoblasts. Thus, concurrent infection with
B. bronchiseptica and toxigenic P.
multocida can result in a more severe and progressive
atrophy and septum deviation than infection with
P. multocida alone.1,3,4 Overall,
PAR has a detrimental impact on growth performance and also provides opportunities
for more severe secondary pathogens to
invade.6
Concurrent infections with B. bronchiseptica
and P. multocida (toxigenic and nontoxigenic strains of both types A and
D) remain a reality in modern United States production systems, whether they use
a conventional one-site system1 or wean prior to 21 days and move the nursery
pigs to an off-site unit.7
Tilmicosin is a long-acting macrolide antibiotic, closely related to erythromycin
and tylosin. It was approved under the Veterinary Feed Directive (VFD) 1996 for use
in swine for treatment of pneumonia caused by Actinobacillus pleuropneumoniae
and P. multocida.
In an earlier pilot study, tilmicosin fed
at 200 g per ton for 6 weeks appeared to minimize AR in nursery
pigs.8 According to the current label instructions, the
drug’s use is limited to 183-363 g per ton for 3 weeks. The current experiment
was designed as a controlled continuous disease transmission study to determine
whether tilmicosin, fed at 363 g per ton for 3 weeks, would protect nursery pigs
exposed to infected pigs from contracting atrophic rhinitis and prevent a decrease in
growth rate. Previous investigators have
reported the natural transmission of AR from positive donor pigs to negative recipient
pigs.9
Materials and methods
Donor pigs
A 100-sow, farrow-to-finish herd with severe endemic PAR served as the
"donor" farm for this study.
One month before the initiation of the trial, a slaughter check of 27 market
hogs from this farm revealed gross AR lesions in 91% of the hogs (36% had mild
lesions and 55% had moderate to severe lesions). Pneumonia lesions were observed in 14
of these market hogs. Seven of the 27 market hogs were bled and serological analysis
was performed (Bayer Veterinary Diagnostic Center, Worthington, Minnesota) using
an indirect ELISA to measure antibody titers to
A. pleuropneumoniae, Haemophilus parasuis, Mycoplasma hyopneumoniae,
and swine influenza virus (SIV). A
microagglutination test was used to test for Streptococcus
suis. All seven of the bled hogs were positive
for H. parasuis and M.
hyopneumoniae. Six of these seven were positive
for S. suis and one was positive for SIV. Ten of the 27
market hogs were nasal swabbed; all were positive for nontoxigenic
P. multocida type A and one was positive for toxigenic
P. multocida type D.
After the initial screening of the donor herd, 24 pigs with clinical signs of
atrophic rhinitis were selected to serve as the
donor pigs in this study. Nasal swab cultures
were performed on eight of the 24 pigs on day 0 and all 24 donor pigs on day
21. Blood samples were also collected from the 24 donor pigs on day 21 to test for
the same respiratory pathogens analyzed in prescreening of the donor herd. The
donor pigs received no medication during the trial.
Recipient pigs
Seventy-two crossbred barrows, free of AR on the basis of herd history, nasal
cultures, and slaughter checks, were purchased
from a high-health facility at weaning (12-14 days of age) and designated as
"recipient" pigs. Neither the recipient pigs nor
their dams had been vaccinated against B.
bronchiseptica or toxigenic P.
multocida.
Upon arrival, the recipient pigs were allowed a 7-day acclimation period prior
to the trial. For the first 3 days of this acclimation period, the pigs were treated with
4 mg per kg IM of ceftiofur hydrochloride
(Excenel(R) Pharmacia & Upjohn
Animal Health, Kalamazoo, Michigan) to minimize respiratory infections at the start
of the trial. Also, recipient pigs were fed a commercial diet containing 140
g/ton Neomycin and 100 g/ton Terramycin (Land O’ Lakes, Inc., Minneapolis,
Minnesota) for the entire 7 days, until the trial began. All 72 pigs were negative for
B. bronchiseptica and P. multocida by culture
at day 0 of the trial.
At the start of the trial (study day 0), each of the 21-day-old recipient pigs was
allotted to one of three treatment groups:
- an "NME" group (n = 24) that
was exposed to the donor pigs and was not medicated with tilmicosin,
- an "ME" group that was exposed
to the donor pigs and medicated with tilmicosin, or
- a Control group that was neither exposed to the donor pigs
nor medicated with tilmicosin.
Facilities
All pigs used in this study were housed in isolation rooms at the University of
Wisconsin Animal Research Center. The donor pigs were randomly distributed to pens
in six rooms (two pens per room) with four pigs per pen. The second pen in each
room housed eight 3-week-old recipient pigs. A randomized design of treatments to
rooms was used to eliminate room differences. The pen dividers allowed
nose-to-nose contact. In addition, the gate doors
were opened for approximately 1 hour each day to maximize the contact between
donor and recipient pigs. Three groups of eight recipient pigs were housed in the
remaining two rooms as the nonexposed Controls.
Nutrition
Diets were formulated to meet or exceed NRC 98 requirements for nursery
pigs. The Control and NME groups were fed the same diet. The diet for the ME
pigs included an addition of 363 g per ton of tilmicosin. At the start of the trial,
diets were sampled and analyzed to ensure drug concentrations were within target
concentrations. Feed consumption was monitored during the entire trial, and all recipient
pigs were weighed on study days 0, 21, and 42.
Bacterial culture
During the trial, nasal swab cultures were collected on days 0, 21, and 42.
Control animals were sampled first, after which
the NME and ME groups were sampled. Strict biosecurity was maintained to
prevent cross-contamination between rooms.
All swabs were cultured for B.
bronchiseptica and P. multocida types A and D.
Pasteurella multocida type D isolates were toxin
tested using the standard protocol at the Bayer Veterinary Diagnostic Center using a
fetal feline lung cell line, previously described
by Rutter and Luther.10 Toxin testing was
conducted only for P. multocida type D
isolates because they are more commonly found than are the type A
isolates.1-3
Postmortem examination
On study day 21, all donor pigs were slaughtered and examined for AR
and other lesions. From each room, three of the recipient pigs were randomly selected
and euthanized, and postmortem data was collected on study day 21. The remaining
five recipient pigs, regardless of treatment group, were maintained on
the nonmedicated diet for an additional 21 days. On day 42, the remaining
recipient pigs were euthanized and necropsied.
Visual snout scores, pneumonia lesions, and any other postmortem findings
were recorded.11,12 Pneumonia was scored
from 0-3 based on an estimate of the total percentage of lungs showing gross
pneumonic consolidation, as previously
described.12
Pleuritis was scored 0-3 where:
- 1= mild, local pleuritis with no adhesions to the chest or pericardium;
- 2= moderate adhesions to the chest or pericardium;
- 3= widespread pleuritis with severe adhesions
as previously described.11,12
Snouts were cut transversely between the first and second premolars. After
gently cleaning the turbinates with water, the visual gross scores for atrophy and
deviation were recorded. The visual atrophic rhinitis score (0-9: 0 = no lesions, 9
= severe lesions) puts twice the weight on atrophy as it does on
deviation.9,13
Snouts were then photographed with a 35-mm camera and the photographs used
to measure the turbinate perimeter ratio (TPR) according to the scoring
system described by Collins, et al.14 (a ratio
of >=1.4 = no lesions, a ratio of <1.0 =
severe lesions). Measurements were performed using a digital image processing and
analysis program (NIH Image version 1.59, National Institute of Health,
Bethesda, Maryland).
Statistical analysis
Total feed intake (FI) and average daily gain (ADG) were calculated for
each treatment group and for the different time periods. ADG (g per day) and F:G
was calculated on a pen basis. Descriptive statistical analysis of the continuous
variables (ADG, Feed efficiency [FE], FI, TPR)
were determined using an univariate ANOVA in SAS (PC/SAS Version 7-1, SAS
Institute, Cary, North Carolina). Categorical data including culture results, pneumonia,
pleuritis, and visual snout scores were analyzed using the CATMOD procedure in
SAS. Data were considered to be significantly different if the
P value was <.05.
Results
Growth performance
All of the treatment groups had similar average starting weights (5.74 +/- 0.99 kg).
Among all three treatment groups, FI did not differ
(P > 0.1) over the first 21 days (Figure 1).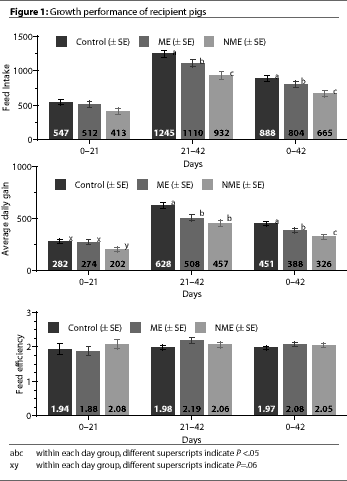 From day 21 to day 42, FI
was significantly higher for Control pigs than ME or NME pigs
(P<.05), and was significantly higher for ME pigs than NME
pigs (P <.05).
From day 21 to day 42, FI
was significantly higher for Control pigs than ME or NME pigs
(P<.05), and was significantly higher for ME pigs than NME
pigs (P <.05).
During the first 21 days of the trial, ADG for NME pigs tended to be less
(P=.06) than the Control and ME pigs (Figure
1). There were no significant differences between Control and ME pigs.
From days 21 to 42, ADG in the Control pigs was significantly higher
(P<.05) than in the NME and ME pigs. When
overall ADG was calculated for the entire 6-week trial, ADG differed significantly among
all three treatment groups (Figure 1).
FE did not differ significantly among the three treatment groups throughout the
trial (Figure 1).
Serologic testing of donor pigs
Donor pigs had antibody titers for A.
pleuropneumoniae (10 of 24), Mycoplasma hyopneumonia
(13 of 24) H. parasuis (2 of 24), SIV (6 of 24), and
S. suis (7 of 24).
Bacterial culture
On day 0, six of eight of the donor pigs were culture positive for
P. multocida type A, and two of the eight were culture
positive for toxigenic P. multocida type D.
All recipient pigs were culture negative for B.
bronchiseptica and P. multocida types A
and D (Figure 2).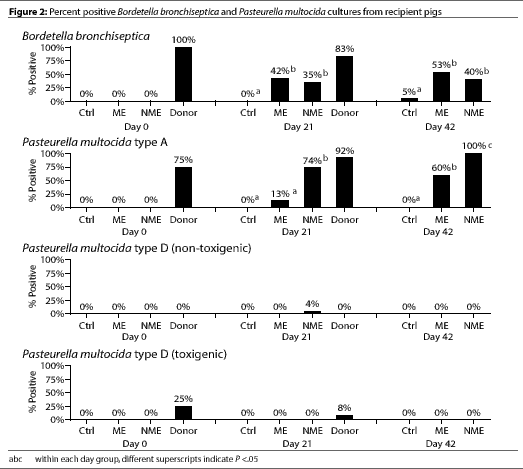
The Control pigs remained culture negative for
P. multocida types A and D throughout the entire study, although
one Control pig was culture positive for B.
bronchiseptica on day 42 (Figure 2).
By day 21, most of the donor pigs were culture positive for
B. bronchiseptica and P. multocida type A (Figure 2). Only two
of the 24 donor pigs were culture positive for toxigenic
P. multocida type D. Significantly more of the NME pigs were culture
positive for P. multocida type A compared
to ME pigs (P <.05) (Figure 2). However,
culture results for P. multocida type D
(toxigenic or nontoxigenic) and for B.
bronchiseptica did not differ between the NME and ME groups.
By day 42, the percentage of pigs culture positive for
B. bronchiseptica and P. multocida type D (toxigenic or nontoxigenic)
did not differ between the ME and NME groups. However, there were still
significantly more pigs in the NME group than in the ME group that were culture
positive for P. multocida type A (P <.05).
Postmortem examination and snout scores
Most donor pigs had pneumonia and pleuritis lesions, and also had a median
visual AR score of 5 (Table 1). 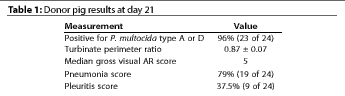 At necropsy, no pneumonia or gross pleuritis lesions
were observed in any of the Control pigs (Figure 3).
At necropsy, no pneumonia or gross pleuritis lesions
were observed in any of the Control pigs (Figure 3). 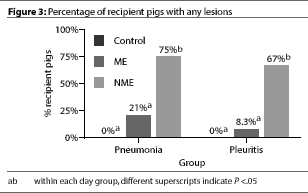 Both pneumonia and pleuritis
scores were significantly (P <.05) higher in
the NME pigs than in the ME or in the Control pigs; ME and Control groups did
not differ.
Both pneumonia and pleuritis
scores were significantly (P <.05) higher in
the NME pigs than in the ME or in the Control pigs; ME and Control groups did
not differ.
The median visual snout score for both the Control pigs and the ME pigs was zero
(0). However, the median visual snout score of the NME pigs (3) was significantly
worse (P <.05) than in the other two
treatment groups.
TPR measurements of the donor pigs at day 21 showed evidence of PAR (Table
1). Whether recipient pigs were euthanized at day 21 or 42, the TPR
measurement differed significantly among groups
(P<.05) (Figure 4).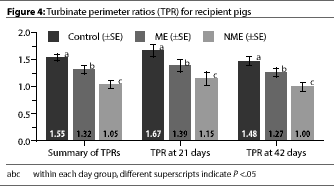
Discussion
The study’s primary focus was to determine what effect tilmicosin would have
on transmission of progressive atrophic rhinitis. The model was designed to
simulate the natural transmission of disease via
animal contact similar to what would occur in a commercial herd, where animals can
be simultaneously exposed to different pathogens.
In our study, disease characterized by turbinate atrophy, pneumonia, and
pleuritis appeared to be effectively transmitted
from 12-week-old donor pigs with clinical signs of PAR to healthy 3-week-old
recipient pigs. Our biosecurity and isolation methods were also effective in preventing
transmission of P. multocida to the Control
pigs. However, we could not verify that toxigenic
P. multocida type D had been transmitted from donor pigs to recipient
pigs, since only two of the 24 donor pigs cultured positive for toxigenic
P. multocida type D at any point in the trial.
Although by day 21, 22 of the 24 donor pigs were culture positive for
P. multocida type A, toxin testing on this serotype was
never performed. Therefore, we lack the bacterial evidence that would allow us to
definitively differentiate between progressive or nonprogressive atrophic rhinitis in the
recipient pigs.
The significant differences in ADG and FI between the ME and NME pigs, and
the significantly more severe lesions observed at necropsy in the NME pigs compared
to the ME pigs, are highly suggestive of progressive atrophic rhinitis. Growth and
lesion data were not consistent, however, with the bacterial results. No toxigenic
P. multocida type D was ever cultured from any of the ME or NME pigs,
and nontoxigenic P. multocida type D was
cultured from only one of the NME pigs. It is possible that the discrepancy between
the culture results and growth rate and necropsy data could be explained by a
failure to detect P. multocida type D and
its toxins. However, two donor pigs were found to be culture positive for toxigenic
P. multocida type D on day 21, suggesting that the tests used were adequate.
We based our standard laboratory protocol to test only the
P. multocida type D isolates for toxin on reports that toxigenic
P. multocida type A is rarely cultured in cases
of progressive atrophic rhinitis.1-3 In
retrospect, it would have been more informative to test all
P. multocida isolates for toxin production.
Although ADG differed significantly among all three treatment groups
when calculated for the overall study period, it was only during the 21-42 day
period, when no tilmicosin was administered, that all three groups became significantly
different. It seems likely that the Control pigs, because they had been isolated,
maintained maximum health and continued to grow well from days 21-42. After the
tilmicosin was withdrawn from the feed of the ME pigs, their growth differed
significantly from the Control pigs (P <.05) and
not from the NME pigs.
Tilmicosin appeared to limit the number of pigs testing positive for
P. multocida types A and D in the ME group. After
tilmicosin was discontinued and all of the donor
pigs were removed from the study, the number of pigs testing positive for
P. multocida continued to increase (Figure 2). This is
another indication that the drug may not be 100% protective against infection, but is able
to minimize the effects of disease and to improve the growth performance
of exposed treated pigs compared to exposed nontreated pigs. Our findings were
consistent with those of a study that evaluated tilmicosin’s effectiveness against
respiratory disease in 12- and 21-day-old weaned
pigs, in which it was observed that the clinical expression of mycoplasmal pneumonia
was delayed in treated pigs.15 Two other
studies examined tilmicosin’s efficacy against
A. pleuropneumoniae and observed that
treated pigs had an increase in ADG and a decrease
in the severity of A. pleuropneumoniae
pneumonia.16,17
TPR has been shown to be an effective tool in evaluating AR in
pigs.14 The TPR values we observed in the Control pigs were
consistent with the findings of Collins, et
al.14 The TPRs of the NME pigs at day 42
were similar to the TPRs of the donor pigs that were euthanized at day 21. The TPR
measurements in our study further support the likelihood that the donor pigs had
transmitted progressive atrophic rhinitis to the exposed recipients.
It is important to keep in mind that our donor pigs came from a commercial
herd and had been exposed to a variety of other pathogens typical for such
operations. Therefore, the role of the few
respiratory pathogens identified in this study
should not be overstated. The possibility remains that the other respiratory
pathogens present in the donor pigs may have influenced the outcome of the study.
Implications
- When determining the role of P.
multocida in atrophic rhinitis (progressive and nonprogressive), it is
important to test for toxin production regardless of serotype.
- Tilmicosin, used as a feed additive at 363 g per ton for 3 weeks in a
pig starter diet, appeared to minimize the lesions and improve the
growth performance of pigs exposed to various respiratory pathogens.
References–refereed
1. De Jong MF. Progressive and Nonprogressive Atrophic Rhinitis. In: BE Straw, S D’Allaire,
WL Mengeling, DJ Taylor, eds. Diseases of
Swine. 8th ed. Ames, Iowa: Iowa State University
Press;1999:355-384.
2. Chanter N, Rutter JM. Pasteurellosis in pigs
and the determinants of virulence of toxigenic
Pasteurella multocida. In: C. Adlam and J.M. Rutter, eds.
Pasteurella and Pasteurellosis. San Diego, CA:
Academic Press Limited;1989:161-195.
3. Rimler RB, Brogden KA. Pasteurella
multocida isolated from rabbits and swine: Serologic types
and toxin production. Am J Vet Res.
1986;47(4):730-737.
4. Pedersen KB, Barfod K. Effect on the
incidence of atrophic rhinitis of vaccination of sows with
a vaccine containing Pasteurella multocida toxin.
Nord Vet Med. 1982;34:293-302.
5. Chung W-B, Bäckström LR, Conrad T,
Collins MT. A comparison of different challenge
methods for induction of atrophic rhinitis in pigs.
APMIS. 1990;98: 442-452.
6. Cowart RP, Bäckström L, Brim TA.
P. multocida and Bordetella
bronchiseptica in atrophic rhinitis and pneumonia in swine.
Can J Vet Res. 1989;53:295-300.
8. Bäckström L, McDonald J, Collins M,
Chung W-B, Shyrock TR, Ose EE. Efficacy of
tilmicosin, and a combination of tylosin and
sulfamethazine, for control of swine atrophic rhinitis involving
infection with toxigenic P. multocida type D.
Swine Health Prod. 1994; 2 (4):11-14.
9. Bäckström L, Brim TA, Collins MT.
Development of turbinate lesions and nasal colonization
by Bordetella bronchiseptica and P.
multocida during long-term exposure of healthy pigs to pigs
affected by atrophic rhinitis. Can J Vet
Res. 1988;52:23-29.
10. Rutter JM, Luther PD. Cell culture assay
for toxigenic Pasteurella multocida from atrophic
rhinitis of pigs. Vet Rec. 1984; 114:393-396.
11. Kiorpes AL, Bäckström LR, Collins MT,
Kruse GOW. Comparison of conventional and
long-acting oxytetracyclines in prevention of induced
Actinobacillus (Haemophilus)
pleuropneumoniae infection of growing swine.
Can J Vet Res. 1989;53:400-404.
13. Bäckström LR, Hoefling DC, Morkoc
AC, Cowart R. Effect of atrophic rhinitis on growth
rate in Illinois swine herds. JAVMA. 1985;187:712-715.
14. Collins MT, Bäckström LR, Brim T.
Turbinate perimeter ratio as an indicator of conchal
atrophy for diagnosis of atrophic rhinitis in pigs.
Am J Vet Res. 1989; 50 (3):421-424.
15. Clark LK, Wu CC, Van Alstine WG, Knox KE. Evaluation of the effectiveness of a macrolide
antibiotic on reduction of respiratory pathogens in
12-day and 21-day weaned pigs. Swine Health
Prod. 1998.
References–nonrefereed
7. Amass S. The effect of weaning age on
pathogen removal. Comp Cont Ed-Food An.
1998; September:196-203.
12. Bäckström L, Kruse G, Collins M. The
preventative effect of long-acting oxytetracycline on
induced infection with A. pleuropneumoniae in
swine. Proc IPVS Cong. 1988:96.
16. Paradis MA, Vessie G, Merrill JK, Dick CP, Moore, C., Charbonneau G, Gottschalk M,
Higgins R, Mittal KR. Efficacy of tilmicosin phosphate
in feed (Pulmotil(R), Elanco) for the prevention
of Actinobacillus pleuropneumoniae infection in
swine. Proc AASP Ann Meet; 1998:105-107.
17. Fleck Veenhuizen M, Moore GM, Zimmermann AG. Evaluation of the clinical
efficacy of Pulmotil(R) for swine respiratory disease
control. Proc AASP Ann Meet. 1997:45-49.
 From day 21 to day 42, FI
was significantly higher for Control pigs than ME or NME pigs
(P<.05), and was significantly higher for ME pigs than NME
pigs (P <.05).
From day 21 to day 42, FI
was significantly higher for Control pigs than ME or NME pigs
(P<.05), and was significantly higher for ME pigs than NME
pigs (P <.05).

 At necropsy, no pneumonia or gross pleuritis lesions
were observed in any of the Control pigs (Figure 3).
At necropsy, no pneumonia or gross pleuritis lesions
were observed in any of the Control pigs (Figure 3).  Both pneumonia and pleuritis
scores were significantly (P <.05) higher in
the NME pigs than in the ME or in the Control pigs; ME and Control groups did
not differ.
Both pneumonia and pleuritis
scores were significantly (P <.05) higher in
the NME pigs than in the ME or in the Control pigs; ME and Control groups did
not differ.
