Lameness in swine is a frequent cause for compromised animal welfare and reduced efficiency. Lameness results in behavioral changes such as reduced activity, social behavior, and feeding behavior due to reduced locomotion ability and pain, thereby decreasing welfare.1 Furthermore, retaining healthy sows and gilts is vital to avoiding unnecessary losses and incurring unwanted costs. Involuntary culling, or removal of animals from the herd due to poor health, injury, or incurable disease before the end of their productive lifespan, is generally less profitable than voluntary culling because the producer is not prepared for it.2 Lame sows are frequently unable to attain their ideal breeding efficiency and are often culled before they reach their peak production.3 Production costs can exist even if the animal is not culled, as feed intake may decrease in the days prior to the presentation of lameness, potentially impairing productivity.4 Using sow lameness models, attempts have been made to determine objective measurements for detecting lameness earlier in order to treat these sows while they still have value.5 Because multiple factors such as parity, gestation stage, and housing characteristics can influence detection of lameness, it is important to use a reliable indicator of sow lameness for treatment or voluntary culling.6
In breeding sows, the most common causes of lameness include hoof lesions, trauma, musculoskeletal disease, fractures, skin lesions, and arthritis.1 Studies have shown that regardless of housing type, lameness severity, and other lesions (body or limb), a majority of sows will have at least one hoof lesion.7 The cause of lameness may not be apparently evident since physical examinations are difficult to perform, and oftentimes there is more than one lesion causing the lameness. The most common hoof lesions seen are overgrown hooves, torn dewclaws, hoof cracks, white line cracks, cracks at the heel-sole junction, and sole ulcers.7-9
The basic anatomy of the porcine hoof has been described in various anatomy texts, but detailed descriptions have yet to be reported. Most of the assumed information has been extrapolated from studies on equine and bovine hooves, which bear many similarities to those of swine. Regardless of the species, an important function of the hoof is protecting the terminal limb structures. The hard epidermal hoof wall lies just over the supportive tissue layer, the corium. The corium, or the dermal part of the hoof, contains blood vessels and nerves, making it sensitive to pain once exposed to the external environment. When the integrity of the hoof capsule becomes compromised and the sensitive dermis has been exposed, the sow may develop lameness.
Areas of the hoof where hard horn meets soft horn (where wall meets sole) are also prone to injury. Interaction of the epidermis and the corium occurs at the laminar junction, where primary epidermal laminae project from the innermost layer of the hoof wall, the stratum internum. These primary epidermal laminae interact with the similarly-structured primary dermal laminae in order to maintain the attachment of the hoof wall to the distal phalanx enclosed within the hoof.10 One previous study showed that there was a significant difference in lesion severity on the abaxial wall as well as the white line in lame sows and sound sows,11 suggesting that further study into these areas may be warranted.
A better understanding of the porcine hoof capsule depth at different locations, as well as determining the density and laminar structure in sows, may provide an anatomical correlation to previously documented hoof lesions and aid producers and swine veterinarians in formulating preventative measures or treatment plans for lame sows. The purpose of this study was to create a basic reference for normal porcine hoof measurements as well as quantify the density of epidermal laminae.
Animal care and use
All samples were obtained post mortem from a federally inspected abattoir subject to the US Humane Methods of Slaughter Act.
Materials and methods
Measurement of hoof wall dimensions
For this study, 40 forelimbs and 40 rear limbs were obtained from mixed-breed sows participating in an Agriculture and Food Research Initiative lameness trial. The sows were not clinically lame when they were sent to the abattoir and the distal limb was disarticulated in the carpal or metacarpal region. Most of the limbs were labeled right vs left and front vs rear at the time of death. If they were not differentiated right from left, the carpal or tarsal bones were used to identify the left from right limb. In cases where the disarticulation was distal to the carpus or tarsus, the tendons of the long or common digital extensor and lateral digital extensor muscles were used. Feet with severe lesions, as defined by the Zinpro Feet First hoof lesion scoring system,12 were discarded from the study. The weight, age, and parity of these sows were unknown.
The limbs were frozen until they were ready for use. They were thawed either at room temperature for approximately 12 hours or in a cooler for 24 to 36 hours prior to obtaining measurements. Measurements of length, width, and sole depth were taken in a manner similar to a study evaluating cows post mortem.13 All holes were drilled with a Hollymatic HY16 electric drill and all depth and length measurements were taken with a ProGrade electronic digital caliper.
Three, approximately 6-mm holes were drilled into each digit using a 0.25-inch drill bit. The holes were used to measure the dorsal wall depth, the abaxial wall depth, and the sole depth. The dorsal wall depth was measured at the most proximal aspect, while the abaxial wall depth was measured at the most palmar or plantar aspect of their respective holes. The sole was measured in 4 sites: cranial, caudal, axial, and abaxial.
The dorsal wall length was measured from the most proximal aspect of the perioplic segment to the most distal aspect of the toe. The same location on the distal aspect of the toe to the caudal-most aspect of the bulb was used to determine the ground surface. The palmar or plantar aspect of the ground surface was used as the initial measurement for the abaxial wall height. The calipers were placed approximately perpendicularly to the ground surface and measured from there to the haired skin (Figure 1).
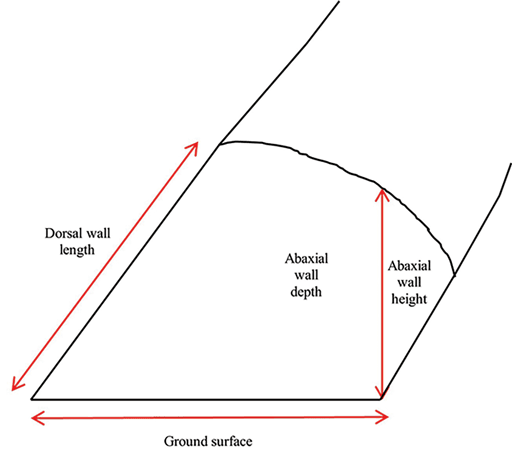
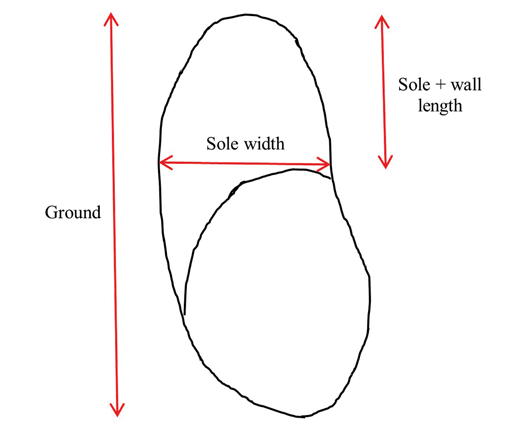
Sole width was measured at the caudal-most aspect of the axial sole in the interdigital space across to the abaxial surface at an approximate 90° angle to the ground surface measurement. The sole + wall length was measured in the same manner as the ground surface, from the center of the distal toe, but only extending the length of the sole, where hard and soft horn meet (Figure 2). This measurement includes the sole, the white line, and the wall. For each sample, 3 measurements were taken at each location and the mean of the 3 measurements was used for statistical analysis.
Quantification of epidermal laminae
For the second part of the study, 69 forelimbs and 74 rear limbs from sows were obtained from a federally inspected abattoir. These limbs were all counted individually, and no grouping was made per sow or per digit. The limbs were disarticulated at the distal metacarpus or the proximal phalanges. These limbs were not differentiated right from left, and both digits from each limb were regarded in the same manner. The purpose of this study was to create a basic reference for normal porcine hoof measurements as well as quantify the epidermal laminar density. Limbs were initially frozen, keeping the forelimbs separate from the rear limbs, and then thawed for approximately 12 hours at room temperature until the digits could be manipulated separately.
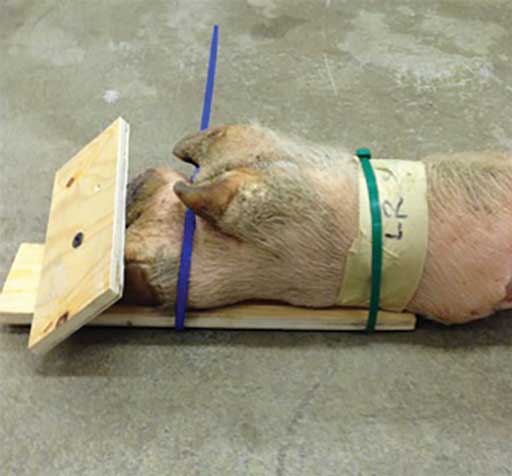
Commercially-obtained cable ties were used to secure them to pieces of plywood in a weight-bearing position. To accomplish this, the dorsal aspect of the distal limb was laid on a rectangular 15- to 20-cm wide and 30- to 40-cm long piece of plywood and 1 to 2 cable ties were used to secure it. A second piece of plywood, approximately 10 × 20 cm, was placed on the ground surface of the hoof and secured to the first piece of plywood with a screw in the interdigital space. After the feet were once again frozen, the cable ties and wood were removed (Figure 3).
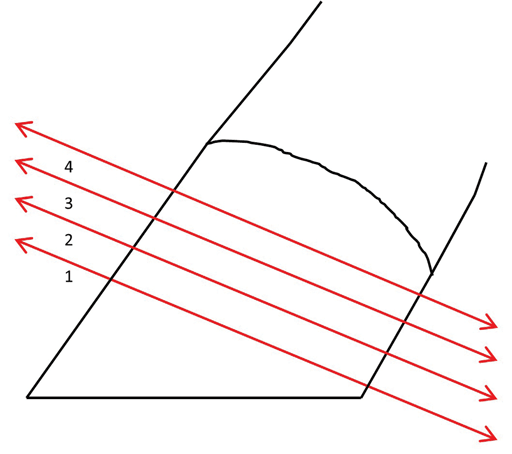
The feet were sliced with a band saw at an approximate 30° to 35° angle from the ground surface. Each slice was approximately 5-mm thick. The slices were labeled 1 to 4, with 1 being the most distal slice and 4 being the most proximal slice (Figure 4). The distal and proximal aspects of each slice were inspected to determine the best sample to visualize the epidermal laminae.
The laminar junction was stained with a 5% methylene blue stain solution and immediately placed in 70% alcohol for 5 minutes, rinsed with distilled water, and placed back in the 70% alcohol for another 5 minutes. The slice was blotted dry with a paper towel and then allowed to air dry.
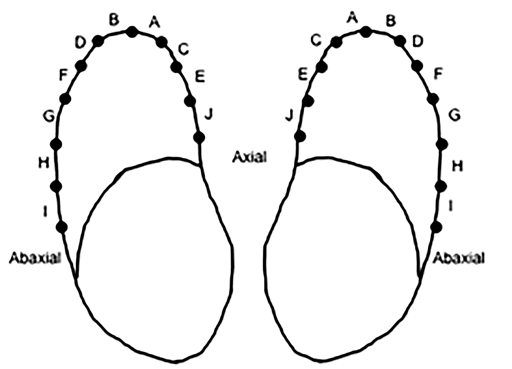
Under a dissecting microscope, the epidermal laminar density was analyzed in a manner similar to that reported by Barreto-Vianna et al.14 The most dorsal aspect of the chosen slice, or the location where the epidermal laminae turned away from each other, was selected as point 1. From this point, the laminar junction was divided into zones of 25 laminae each. The zones started at the dorsal aspect of the toe, and moved axially and abaxially with A, C, E, and J on the axial surface and B, D, F, G, H, and I on the abaxial surface (Figure 5). Zones I and J were not observed on toes from pelvic limbs. Pins were used as markers to differentiate between each zone. The same ProGrade electronic digital caliper was used to make all the measurements from the shaft of the adjacent pins. This “zone width” was used to determine epidermal laminar density, with smaller widths being more dense and larger widths being less dense. For each sample, 3 measurements were taken at each location and the mean of the 3 measurements was used.
Statistical analysis
Variances in hoof wall measurements and density of epidermal laminae were quantified and analyzed with JMP Pro 11 using a one-way analysis of variance followed by Student’s t-test with the significance level set at P < .05 to determine if significant differences in measurements existed between digits 3 and 4 of right and left thoracic and pelvic limbs.
Results
Hoof wall measurements
Overall, the limbs from 48 sows were evaluated. Only normal limbs were evaluated and those with severe hoof wall lesions or abnormalities according to the Zinpro Feet First scoring system12 were excluded. These sows were all obtained from the same source and 1 person made all the measurements. All data appeared roughly normally distributed.
The dorsal wall length of the lateral digit (digit 4) on both right and left rear limbs was significantly longer than that of the medial digit (digit 3) on the right and left rear limbs, as well as all 4 digits on the forelimbs. In addition, the sole + wall length of the lateral digit of both rear limbs was significantly longer than those of all 4 digits on the forelimbs, which were significantly longer than that of the medial digit on the rear limbs (Table 1). The ground surface (sole + wall and volar surface of the bulb) was significantly longer for both lateral digits of the rear limb than the remaining 6 digits (the medial rear limb digit and all 4 forelimb digits, Table 1). Furthermore, the width of the sole when measured among the 4 digits of the forelimbs was very similar. These measurements were all significantly greater than all 4 digits on the rear limbs, showing that the digits of the forelimb are wider than those of the rear limbs (Table 1).
| Digit | Dorsal wall length, mm | Abaxial wall length, mm | Sole width, mm | Sole + wall length, mm | Ground surface, mm |
|---|---|---|---|---|---|
| LF digit 3 | 43.04 (2.82)c | 30.82 (3.90)a | 31.91 (3.75)a | 23.89 (3.21)b | 57.62 (4.54)c,d |
| LF digit 4 | 44.21 (3.60)b,c | 30.08 (4.48)a,b | 32.43 (3.08)a | 24.70 (2.82)b | 60.82 (7.61)b |
| RF digit 3 | 43.04 (3.15)c | 28.57 (4.29)b,c | 31.53 (3.48)a | 23.45 (2.94)b | 58.65 (5.37)b,c |
| RF digit 4 | 44.45 (3.82)b,c | 31.63 (4.71)a | 32.24 (2.62)a | 23.99 (3.03)b | 60.80 (7.38)b |
| LR digit 3 | 45.87 (3.80)b | 28.01 (4.93)b,c | 24.06 (2.45)c | 21.55 (2.31)c | 55.00 (5.49)d,e |
| LR digit 4 | 48.92 (5.66)a | 27.44 (5.51)c,d | 29.90 (2.69)c | 27.06 (4.18)a | 64.75 (7.97)a |
| RR digit 3 | 45.35 (3.81)b | 25.48 (5.15)d | 24.43 (3.06)b | 22.44 (2.96)c | 54.54 (6.64)e |
| RR digit 4 | 49.67 (5.95)a | 29.76 (5.48)a,b | 30.03 (3.55)b | 27.48 (4.28)a | 66.67 (8.75)a |
a-e Superscript letters denote a connecting letters report of the Student’s t-test. Values within columns with differing letters are statistically different.
LF = left front; RF = right front; LR = left rear; RR = right rear.
The left and right rear digit 4 had a thicker dorsal wall than any digit on the forelimb. There was no significant difference when comparing any of the 4 digits of the forelimb (Table 2). Multiple measurements were made when comparing depths of the sole (cranial, caudal, axial, and abaxial sole depth), but overall, digit 4 of the rear limb had the deepest sole measurements with forelimb digit 3 being the thinnest (Table 2).
| Digit | Dorsal wall depth, mm | Abaxial wall depth, mm | Sole cranial depth, mm | Sole caudal depth, mm | Sole axial depth, mm | Sole abaxial depth, mm |
|---|---|---|---|---|---|---|
| LF digit 3 | 3.24 (0.54)c | 2.77 (0.95)a | 3.71 (0.94)c | 3.80 (1.01)b,c | 3.69 (0.97)c | 3.94 (0.90)c,d |
| LF digit 4 | 3.21 (0.51)c | 2.64 (0.59)a,b | 4.02 (1.04)b,c | 4.07 (1.16)b,c | 4.07 (1.08)b,c | 4.10 (0.98)c,d |
| RF digit 3 | 3.14 (0.58)c | 2.56 (0.80)a,b | 3.71 (0.94)c | 3.80 (0.99)c | 3.92 (1.01)b,c | 3.80 (0.97)d |
| RF digit 4 | 3.34 (0.60)b,c | 2.77 (0.72)a | 4.10 (1.15)a,b,c | 4.30 (1.07)b | 4.05 (1.03)b,c | 4.39 (1.05)b,c |
| LR digit 3 | 3.30 (0.62)c | 2.38 (0.82)b | 3.91 (1.17)b,c | 4.15 (1.18)b,c | 3.84 (1.26)c | 4.15 (1.19)c,d |
| LR digit 4 | 3.62 (0.79)a | 2.52 (0.89)a,b | 4.37 (1.10)a,b | 4.83 (1.19)a | 4.36 (1.29)a,b | 4.71 (1.18)a,b |
| RR digit 3 | 3.28 (0.56)c | 2.50 (0.81)a,b | 3.89 (1.18)b,c | 4.04 (1.02)b,c | 3.92 (1.06)b,c | 4.04 (1.07)c,d |
| RR digit 4 | 3.61 (0.71)a,b | 2.74 (0.92)a | 4.51 (1.32)a | 5.11 (1.46)a | 4.60 (1.26)a | 5.04 (1.33)a |
a-d Superscript letters denote a connecting letters report of the Student’s t-test. Values within columns with differing letters are statistically different.
LF = left front; RF = right front; LR = left rear; RR = right rear.
The most significant differences in hoof capsule depth came when comparing the dorsal wall and the sole to the abaxial wall depth. The dorsal wall depth of all digits (digits 3 and 4 of the forelimbs and rear limbs) was significantly thicker than the abaxial wall depth on all 8 digits. The same was true when comparing the abaxial wall depth of each digit to all 4 sole measurements (cranial, caudal, axial, and abaxial): the abaxial wall is significantly thinner than that of the sole (Table 2).
Quantification of epidermal laminae
On the thoracic limb, zones A and B were significantly narrower than all of the remaining zones. Zones C and D were narrower than E, F, G, H, and I. Zones E and F were narrower than G, H, and I (Table 3). This demonstrates that zones located at the most dorsal aspect of the toe are the narrowest and the zones become wider moving axially and abaxially toward the heel. Since the narrowest zones represent the most densely packed epidermal laminae, the dorsal aspect of the hoof capsule has the most dense epidermal laminae and the least densely packed areas are located at the axial and abaxial hoof walls.
| Zone* | Thoracic limb width, mm | Pelvic limb width, mm | Thoracic limb branching, mm | Pelvic limb branching, mm |
|---|---|---|---|---|
| Zone A | 5.28 (0.86)d | 4.75 (0.71)e | 1.08 (1.20)c | 1.03 (1.18)d |
| Zone B | 5.17 (0.84)d | 4.88 (0.77)e | 1.28 (1.28)b,c | 1.09 (1.22)c,d |
| Zone C | 5.82 (0.90)c | 5.49 (0.88)e | 1.47 (1.43)b | 1.37 (1.47)b,c |
| Zone D | 5.87 (0.83)c | 5.90 (0.77)d | 1.47 (1.30)b | 1.68 (1.36)a,b |
| Zone E | 6.80 (1.05)b | 6.30 (0.95)c | 2.08 (1.52)a | 1.58 (1.49)a,b |
| Zone F | 6.84 (0.98)b | 6.75 (0.93)b | 1.38 (1.21)b,c | 1.71 (1.30)a |
| Zone G | 7.70 (1.12)a | 7.11 (1.14)a | 2.10 (1.48)a | 1.72 (1.16)a |
| Zone H | 7.48 (1.10)a | 7.19 (1.36)a | 2.20 (1.75)a | 1.41 (1.42)a,b,c,d |
| Zone I | 7.89 (0.88)a | NA† | 1.45 (1.51)a,b,c | NA† |
| Zone J | 7.11 (0.78)a,b | NA† | 0.43 (0.79)b,c | NA† |
* Zones comprised 25 laminae each.
† Zones I and J were not present on the pelvic limbs.
a-e Superscript letters denote a connecting letters report of the Student’s t-test. Values within columns with differing letters are statistically different.
LF = left front; RF = right front; LR = left rear; RR = right rear; NA = not applicable.
In the pelvic limb, zones A and B were significantly narrower than the remaining zones, and zones C and D were significantly narrower than E, F, G, and H. The widest zones were G and H (Table 3). Like the thoracic limb, the pelvic limb epidermal laminae are most dense in the dorsal region of the hoof and least dense at the far plantar region. When the thoracic and pelvic limbs are compared to one another, the zones maintain the same pattern of highest density (narrowest zones) at the dorsal part of the hoof, and lowest density (widest zones) at the abaxial wall. The pelvic limb is significantly less dense in the abaxial wall region than the thoracic limb (Table 3).
Discussion
The main objective of this study was to further investigate the anatomy of the porcine hoof wall and draw conclusions about predispositions to foot lesions based on this inherent anatomy. A second goal was to establish known values of various measurements (lengths and depths) of swine hooves for future studies to build on. Some of the most significant findings in this study reaffirmed research that has been done previously, such as the size disparity between the lateral and medial digits on the rear limb and the equal ground surface of the forelimbs.15 An important finding from the present work showed that the thinnest portion of the hoof capsule was located at the abaxial wall, which had not been reported in the scientific literature previously. This corresponded with the least dense region found in the epidermal laminae.
Isolating the differences in thickness of the hoof capsule can point to areas that may be predisposed to cause lameness if foot lesions occur there. A majority of the sow’s weight is born by the heel, one of the most frequent places to see cracks and erosions. One of the subsequent highest weight-bearing regions is where the heel meets the abaxial hoof wall of the lateral digit.15-17 Data from the present work shows that the junction of heel and hoof wall is where the hoof capsule’s thinnest region is located when compared to the sole and the dorsal wall, making it easier for minor cracks to reach the corium, the sensitive layer of the hoof.
The current findings are in agreement with previously reported data concluding that the lateral digits of the rear limbs were longer, both dorsally and on the ground surface, than both the medial digits of the rear limb and the digits of the forelimb. Severe overgrowth of the hooves is associated with lameness, particularly when sows are housed on slatted floors where claws may be trapped between slats and suffer cracks when the sow attempts to free itself.18 The dorsal wall length, ground surface length, and sole width of the forelimbs were more comparable between lateral and medial digits than the greater disparity in size seen in the rear limbs. It has also been reported that hoof wall lesions are more frequently seen on the lateral hooves of the rear limb than on the medial hooves. Previous findings have reported that the lateral digits on the rear limbs carry more weight than the medial digits, and are therefore possibly more prone to developing lesions.19 Additionally, in pigs raised with access to concrete flooring, the rate of claw horn growth and wear are greater on the rear feet. The more rapid growth rate may result in the exposure of less mature horn to the walking surface, potentially predisposing the rear feet to the development of lesions.20 Further studies into the anatomy of these regions in particular may be warranted.
The present results show that the abaxial region has the least dense epidermal laminae when compared to the dorsal toe region. Because laminae function to increase the surface area for attachment, the paucity of laminae means there is less epidermal-dermal interaction, perhaps making this region more susceptible to white line disease. Separation of the corium and epidermis commonly occurs on the abaxial border, frequently at the heel-sole junction.17 Due to the low density of laminae in this area and the thin abaxial wall, it is easier for minor damage to affect the sensitive corium and, due to the location of the lesion, the damage may lead to infections as well.
In the present study, sows were obtained from different sources, and the premortem lameness status was unknown for each individual animal. It would have been ideal to have a truly random sample of clinically sound sows with differentiation between the left and right limb in order to determine the lateral and medial digit when quantifying epidermal laminae. Furthermore, information about the breed, age, parity, weight, and housing of the individual sows used in this study was unavailable. Without knowing these potentially confounding factors, it is not possible to suggest a causal relationship between hoof wall thickness and either lesion prevalence or lameness.
A second limitation in this present study is the lack of comparison sows. This study would have been more complete if the measurements of clinically sound sows were compared to those of lame animals.
Despite these limitations, it is hoped that this manuscript will serve as a descriptive baseline to guide further research into the anatomy of the porcine hoof capsule that will lead to better animal welfare management and decreased cull rates for lameness in breeding sows.
Implications
Under the conditions of this study:
- The thinnest portion of the hoof capsule was located at the abaxial wall.
- The abaxial hoof wall had the lowest density of epidermal laminae.
- Hoof anatomy is related to the most common previously reported lesion sites.
Acknowledgments
Conflict of interest
None reported.
Disclaimer
Scientific manuscripts published in the Journal of Swine Health and Production are peer reviewed. However, information on medications, feed, and management techniques may be specific to the research or commercial situation presented in the manuscript. It is the responsibility of the reader to use information responsibly and in accordance with the rules and regulations governing research or the practice of veterinary medicine in their country or region.
References
1. Heinonen M, Peltoniemi O, Valros A. Impact of lameness and claw lesions in sows on welfare, health and production. Livest Sci. 2013;156(1-3):2-9. https://doi.org/10.1016/j.livsci.2013.06.002
2. Dagorn J, Aumaitre A. Sow culling: Reasons for and effect on productivity. Livest Prod Sci. 1979;6(2):167-177. https://doi.org/10.1016/0301-6226(79)90018-6
3. D’Allaire S, Stein TE, Leman AD. Culling patterns in selected Minnesota swine breeding herds. Can J Vet Res. 1987;51(4):506-512.
4. Munsterhjelm M, Heinonen M, Valros A. Effects of clinical lameness and tail biting lesions on voluntary feed intake in growing pigs. Livest Sci. 2015;181:210-219. https://doi.org/10.1016/j.livsci.2015.09.003
5. Abell CE, Johnson AK, Karriker LA, Rothschild MF, Hoff SJ, Sun G, Fitzgerald RF, Stalder KJ. Using classification trees to detect induced sow lameness with a transient model. Animal. 2014;8(6):1000-1009. https://doi.org/10.1017/S1751731114000871
6. Conte S, Bergeron R, Grégoire J, Gete M, D’Allaire S, Meunier-Salaun MC, Devillers N. On-farm evaluation of methods to assess welfare of gestating sows. Animal. 2014;8(7):1153-1161. https://doi.org/10.1017/S1751731114000949
7. Calderón Díaz JA, Fahey AG, Boyle LA. Effects of gestation housing system and floor type during lactation on locomotory ability; body, limb, and claw lesions; and lying-down behavior of lactating sows. J Anim Sci. 2014;92(4):1675-1685. https://doi.org/10.2527/jas.2013-6279
8. Dewey CE, Friendship RM, Wilson MR. Clinical and postmortem examination of sows culled for lameness. Can Vet J. 1993;34(9):555-556.
9. Nalon E, Conte S, Maes D, Tuyttens FAM, Devillers N. Assessment of lameness and claw lesions in sows. Livest Sci. 2013;156:10-23. https://doi.org/10.1016/j.livsci.2013.06.003
10. Pollitt CC. Anatomy and physiology of the inner hoof wall. Clin Tech Equine Pract. 2004;3(1):3-21. https://doi.org/10.1053/j.ctep.2004.07.001
*11. Anil L, Anil SS, Deen J. Claw lesions predict lameness in breeding sows. Paper presented at: Allen D. Leman Swine Conference. September 15, 2007; St Paul, MN. https://hdl.handle.net/11299/156239
*12. Zinpro Feet First Swine Locomotion Scoring System. Zinpro. 2021. Accessed September 3, 2023. www.zinpro.com/performance-solutions/production-optimization-tools/feet-first/.
13. Nuss K, Paulus N. Measurements of claw dimensions in cows before and after functional trimming: A post-mortem study. Vet J. 2006;172(2):284-292. https://doi.org/10.1016/j.tvjl.2005.04.031
14. Barreto-Vianna ARC, Oliveira LS, Leonardo AS, Santana MI, Godoy RF, de Lima EMM. Density of primary and secondary epidermal laminae of equine hoof. Pesq Vet Bras. 2013;33(4):543-548. https://doi.org/10.1590/S0100-736X2013000400020
15. Cameron R. Integumentary System. In: Zimmerman JJ, Karriker LA, Ramierez A, Schwartz KJ, Stevenson GW, eds. Diseases of Swine. 10th ed. Wiley-Blackwell; 2012:251-269.
*16. Anil SS, Anil L, Deen J. Association of parity, body condition and lactation feed intake with claw integrity in sows. Paper presented at: Allen D. Leman Swine Conference. September 15, 2007; St Paul, MN. https://hdl.handle.net/11299/157064
17. König HE, Liebich HG, Bragulla H. Common Integument. In: König HE, Liebich HG. Veterinary Anatomy of Domestic Mammals: Textbook and Colour Atlas. 3rd ed. Schattauer; 2007:585-635.
18. Lisgara M, Skampardonis V, Kouroupides S, Leontides L. Hoof lesions and lameness in sows in three Greek swine herds. J Swine Health Prod. 2015;23(5):244-251.
19. Mouttotou N, Hatchell FM, Lundervold M, Green LE. Prevalence and distribution of foot lesions in finishing pigs in south-west England. Vet Rec. 1997;141(5):115-120. https://doi.org/10.1136/vr.141.5.115
20. Van Amstel S, Doherty T. Claw horn growth and wear rates, toe length, and claw size in commercial pigs: A pilot study. J Swine Health Prod. 2010;18(5):239-243.
* Non-refereed references.
 PDF version
PDF version RIS
citation
RIS
citation