Zinc deficiency in swine is characterized by a nonpruritic parakeratotic hyperkeratosis. Acute skin lesions initially present as macules and papules and develop into scales and thick crusts.1 Animals may also present with weight loss, lameness, vomiting, and diarrhea.2 Mortality directly associated with Zn deficiency is rare, though it may be observed in cases where the pig succumbs to secondary bacterial infection due to impaired function of the skin or when the severity of lesions warrants euthanasia. Zinc deficiency may be a result of inadequate intake of Zn through the diet or exposure to high levels of calcium or phytates that act antagonistically and impair Zn absorption.3 Differential diagnoses include exudative epidermitis (Staphylococcus hyicus), ringworm (Microsporum nanum or Trichophyton verrucosum), pityriasis rosea, dermatosis nephropathy syndrome (porcine circovirus associated disease), insect bites, and swine pox (Suipoxvirus).1 Despite an increased focus on nutrition throughout the industry, cases of nutritional deficiency still occur.
Animal care and use
Animals involved in this case report were under the supervision of the herd veterinarian and were cared for in accordance with the Pork Quality Assurance Plus program.
Case description
During summer 2016, veterinarians were asked to examine pigs in a commercial grower-finisher operation in central Iowa because pigs were reported to have skin lesions and diarrhea. Pigs were housed in 4 side-by-side barns with natural ventilation, deep manure pits, and fully slatted flooring. Feeder pigs, averaging approximately 29 kg, were placed between May 4 and May 11 with approximately 800 pigs being allocated to each barn. Staff initially observed skin lesions in the northernmost barn (barn 1) approximately 20 days post placement. Three site visits occurred over the course of 3 months to collect feed, fecal, and tissue (brain, colon, kidney, liver, lung, lymph node, skin, and small intestine) samples for further testing and to monitor both progression and regression of lesions.
Site visits occurred on June 29, July 11, and August 5. During the initial visit, 80%, 65%, 35%, and 35% of the population in barns 1, 2, 3, and 4, respectively, exhibited skin lesions. Lameness, nonuniform pig size, loose stools, nasal discharge, and vomiting were also noted to a lesser degree by veterinarians and caretakers. Lameness appeared to be associated with severe skin lesions. Diarrhea was more evident throughout barns 1 and 2 (5%-10%). Although responsive, the overall activity level of pigs was depressed relative to normal. The skin lesions did not appear to be pruritic; affected pigs were not observed scratching or rubbing against other pigs or facility equipment. Lack of an overwhelming number of flying insects in the environment was noted as caretakers were also concerned that the lesions were the result of insect bites.
During the second visit 12 days later, a reduction in skin lesions was observed in barns 1 and 2 with 40% and 20% of animals being affected, respectively. Between site visits, mineral testing of both feed and liver samples, histopathological examination of skin, and culture and sensitivity of skin samples continued. Feed and water intake and overall activity was noted to have increased. At this time, an increase in lesion severity and number affected relative to the first visit was observed in barn 4. Pigs within barn 4 were reported to have been placed 2 weeks after those in barns 1 and 2. More than 70 of 800 pigs were euthanized in barn 4 for welfare reasons, including the inability to rise or severe lameness. Improvement of skin lesions within barn 4 was observed on July 28 along with a relapse in diarrhea and lethargy in barn 2 as reported by the site manager. Evaluation of the skin samples submitted prior to conducting the field investigation revealed growth of S hyicus that was sensitive to both penicillin and ceftiofur.
In response to these results, oral penicillin was administered in the water while severely affected individuals were treated with ceftiofur crystalline free acid (EXCEDE for Swine; Zoetis Inc) intramuscularly at 5 mg/kg of body weight. A second tissue submission, collected prior to the field investigation, revealed hemolytic Escherichia coli within the colon. Administration of penicillin and neomycin in the water was not administered concurrently. All medication dosages and duration of use were per label instructions using veterinary oversight and diagnostics. A diluted bleach solution lower than the labeled recommendation for reducing Staphylococcus species on surfaces was momentarily administered topically to deter further growth of S hyicus but was discontinued per veterinary instruction. Despite antibiotic treatment with penicillin and ceftiofur, resolution of skin lesions did not occur. On August 4, a Zn methionine complex (Zinpro) was supplemented through the water to all 4 barns for 1 week. This product was an organic form of Zn chelated with an amino acid.
Observations during the third visit included decreased severity and prevalence of skin lesions, minimal diarrhea (<5%), and residual lameness cases, including swollen stifles and elbows of varying severity from toe-tapping lame to nonweight bearing. The site received feed from a single feed mill. No other sites within the associated production system received feed from this mill. No other sites within the system receiving pigs from the same sow farm reported having similar clinical issues.
Gross clinical lesions
Clinically affected animals initially exhibited multifocal to coalescing scabs and crusts surrounded by erythema in a bilateral and symmetrical fashion throughout the ears, hind limbs, and ventral aspect of the abdomen and thorax (Figure 1). Lesions were also noted on the dorsum and face (Figure 2). The size of the initial lesions ranged between 0.5 to 5.0 cm.


Appearance of the lesions varied. Thick, dark-brown, crusting lesions were suspected to be associated with a more chronic presentation. Red-purple to pink multifocal, circular lesions were described as acute lesions. More severely and chronically affected individuals exhibited a dense 0.5 to 1 cm layer of crust that encompassed 60% to 90% of the body. Pigs in this state also exhibited breaks within the crust resulting in hemorrhage and exudation of serosanguinous fluid. Open sores exposing subcutaneous tissue, the result of sloughed skin, were predominately located in both the distal extremities and areas of high mechanical movement (Figure 3). Varying degrees of lameness were observed in association with sores. Individuals believed to be recovering during subsequent visits exhibited multifocal areas of alopecia with seemingly healthy skin (Figure 4). The crust within these areas appeared to have been removed. A rapid response following additional Zn supplementation was observed within 7 days. A full recovery with resolution of skin lesions took between 14 and 21 days. Overall, affected animals in all barns recovered following the supplementation of Zn.


Pathological, microbiological, and nutritional examination
All diagnostic tests were performed at the Iowa State University Veterinary Diagnostic Laboratory. Tissues from affected individuals and feed samples were collected prior to and during site investigations for evaluation. A total of 12 feed samples were collected from feeders from the 4 barns throughout the investigation. The feeders were selected at random as all pens in each barn possessed affected animals. Feed originating from the same bulk bins was collected from several corresponding feeders and pooled. Feed samples from bins corresponding to different bulk bins were not pooled and were noted to be different batches of feed. Tissue samples from brain, colon, kidney, liver, lung, lymph node, skin, and small intestine were immediately collected from animals that were euthanized with a captive bolt and processed for histopathological evaluation. Tissues were placed in 10% buffered formalin for approximately 12 hours, trimmed/processed per regular histologic protocols, embedded in paraffin, sectioned at 5 µm, and stained with hematoxylin and eosin. Sections of lung and lymph node underwent immunohistochemistry staining for porcine circovirus type 2 (PCV2). For microbiological evaluation, samples of fresh skin were cultured to evaluate the presence of S hyicus and fungal agents. Staphylococcus hyicus was cultured from submitted skin samples as previously mentioned. Identification of isolated colonies was performed using matrix-assisted laser desorption ionization time-of-flight mass spectrometer. In addition, analysis of lymph nodes for PCV2, lung tissues for influenza A virus (IAV), and fecal material for porcine epidemic diarrhea virus (PEDV) and porcine delta coronavirus (PDCoV) were completed using polymerase chain reaction (PCR). Multiple fresh liver and feed samples were collected for trace mineral analysis through inductively coupled plasma mass spectrometry. Vitamin A analysis was performed via high performance liquid chromatography on 12 of 17 fresh liver samples collected.
Histopathological results
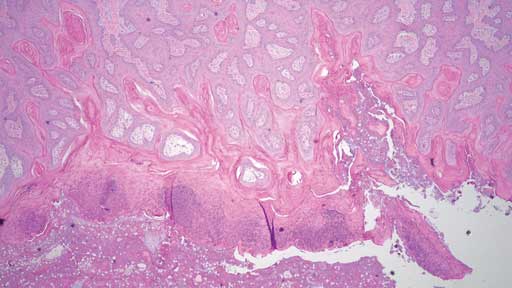
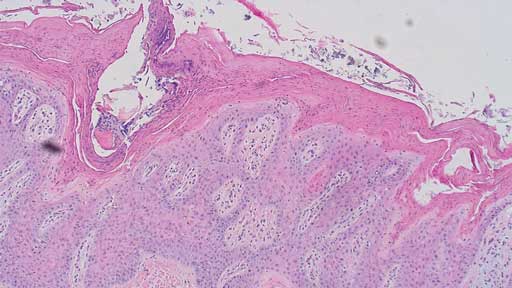
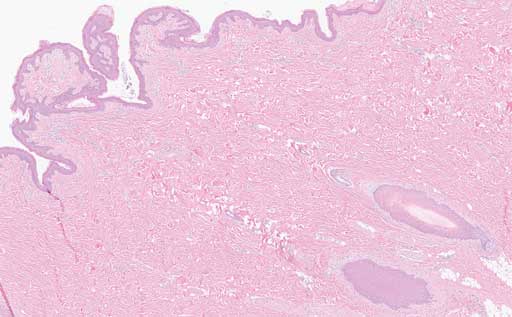
Histopathology revealed hyperplastic and exudative dermatitis characterized by a dense serocellular to suppurative crust with mild to severe parakaratotic hyperkeratosis along with marked acanthosis (Figures 5 and 6). Bacterial colonies and corneal pustules were present. Both sebaceous and apocrine glands within the dermis exhibited neutrophil and macrophage infiltration. Aggregates of lymphocytes and plasma cells were scattered within the superficial dermis. A section of normal haired skin is provided for comparison (Figure 7). Histopathological examination of brain, colon, kidney, liver, and lung tissue was unremarkable.
Nutritional results
Zinc concentrations among the 17 livers analyzed ranged between 15 and 23 ppm with only 3 exceeding 20 ppm. The concentration of Zn within feed ranged between 14 and 117 ppm, with only 4 samples exceeding 50 ppm. The minimum concentration of Zn within feed intended for growing and finishing swine has been established at 50 ppm (mg/kg) by the National Research Council.4 Multiple feed samples contained less than the established recommended levels with several possessing Zn concentrations as low as 14 ppm. These concentrations were significantly lower than those observed in a previous study in which lesions were observed when Zn feed concentrations were 34 to 44 ppm.5 In healthy swine, hepatic Zn concentrations greater than 40 ppm are suggestive of adequate supplementation resulting in suitable stores. Swine may be considered Zn deficient when hepatic concentrations fall below 25 ppm.6 From the liver samples, all 12 pigs were found to be deficient in Zn as hepatic concentrations were all less than 25 ppm. Hepatic Zn concentrations may increase during infection or inflammation. Throughout the investigation, sequestration of Zn within the liver may have occurred. However, even if sequestration did occur, the observed hepatic Zn concentrations remained at a deficient concentration and would further suggest an initial depletion.3 With the exception of 2 samples, calcium concentrations within feed were below 10,000 ppm. Each of the 12 livers analyzed for vitamin A were below 60 ppm with concentrations ranging from 8 to 57 ppm.
Molecular and microbiological results
Porcine circovirus Type 2, IAV, PEDV, and PDCoV antigens were not detected by PCR testing. Moderate amounts of S hyicus and Streptococcus equisimilis, as well as low amounts of Candida albicans and Zygomycetes species were cultured from the skin.
Outcomes
Further investigation identified a feed mixing error as the cause of the clinical signs and the inconsistent mineral levels in the feed and liver samples. The feed mill received pre-prepared mineral packets which were being added at the end of the ration mixing cycle. As a result, the minerals were not spread homogenously throughout the feed. Little to no Zn, regardless of type, was available to pigs. Seeing how little Zn was present, antagonism by other minerals or phytate is likely to have been of little concern. The duration for which the mixing errors had occurred prior to morbidity and mortality is unknown. It is also unknown as to why Zn appeared to be the only deficient mineral following misformulations. Although it is possible that other mineral issues were present, the clinical presentation of signs and lesions and subsequent resolution to Zn administration were significantly pronounced.
Discussion
In this case, we confirmed and described a case of zinc responsive dermatosis in a group of commercial finishing pigs. To the authors’ knowledge, there are limited reports of such cases in literature over the past 50 years. Although rare, an understanding of this condition is useful to veterinarians since it is both high impact and preventable.
Zinc is an essential trace mineral that serves as a critical component of numerous enzymes and proteins necessary for physiological processes.3,4 Although the historical etiology of the skin lesions was not fully understood, resolution of lesions and other clinical signs was shown to occur following transfer of affected swine from dry lots to pasture.2,7 Historically, the role of Zn in parakeratosis was made evident following the resolution of clinical signs when dietary Zn was increased.5 Other etiological agents such as S hyicus can produce similar gross lesions to those observed in this case. However, microscopic lesions of diffuse parakeratosis differentiate this nutritional disease from other likely etiologies.1 It is uncommon and unlikely that S hyicus would affect swine of this age and size to this degree. Microscopic lesions did not implicate a causative role for endemic infectious opportunists such as S hyicus, S equisimilis, or C albicans that were detected by culture. Gross and microscopic lesions present are consistent with those observed in previous studies of parakeratosis and Zn deficiency.2,5,7,8 This case was also made challenging by the diagnosis of multiple pathogens. Other detected infectious agents may have also, to some degree, contributed to morbidity and mortality. This also demonstrates the importance of a complete sample set, including feed, water, and fresh and formalin- fixed tissue, for meaningful diagnostic investigations.
Zinc deficiency can arise or be exacerbated by one or more cofactors. Calcium and phytate both interfere with absorption of Zn. Previous studies have revealed that porcine diets containing greater than 1.1% calcium (11,000 ppm) have led to the development of parakeratotic lesions.9-11 Absence or low amounts of Zn within diets can also lead to deficiency. As dietary calcium increases, the amount of Zn within the diet must increase as well to prevent development of Zn deficiency and parakeratosis.5,9 Phytate is considered to have antagonistic effects on Zn absorption through chelation.3 Although Zn requirements and ensuing development of parakeratosis for gilts and barrows differ slightly,12 the differential number of gilts and barrows affected was not recorded during the case investigation. Addition of dietary Zn can help to restore Zn concentrations within the body and resolve associated lesions.5,10 Increased absorption of Zn during periods of deficiency is accomplished through the upregulation of a number of Zn transporters within the intestine.13
Given that both feed calcium and Zn concentrations were consistently below 11,000 ppm and 50 ppm, respectively, coupled with consistently low hepatic Zn, it was determined that Zn deficiency and the ensuing skin lesions were likely not the result of antagonistic effects but of low dietary Zn. This was further supported through the identification of an error during the feed mixing process and resolution of clinical signs when Zn levels were supplemented and restored. Due to the wide margin of safety with Zn in swine diets, Zn supplementation could have been an effective treatment strategy earlier in the case, despite varying levels of Zn in the tissue and the feed samples tested. Cases that include lesions and other clinical signs that may be associated with either infectious agents or nutritional imbalances can be difficult to diagnose. Practitioners should always consider the possibility of nutritional deficits when encountering such situations.
Errors leading to the misformulation of feeds do occur and can have devastating consequences. Sources of incorrect formulation of feed include factors associated with both mechanical or electrical failure and human error. Protocols implemented by feed mills, as well as producers, aid in reducing such situations. A best practice approach when milling feed includes routinely following all protocols, from evaluation of equipment condition and functionality to adding the correct components at the established amounts. Complacency, a common source of error, is avoided by routinely following set protocols. Due to the high volume of output by mills, ensuring that completed rations are formulated correctly through the testing of every sample is impractical. Periodic testing of feed offers an alternate means by which milling processes can be monitored. Another option that may be considered is the retention of samples by both feed manufacturers and recipients for a period. This allows for retrospective analysis of feed in the event health complications arise and a nutritional or toxic component can be ruled out.
Implications
Under the conditions of this study:
- Parakeratosis associated with Zn deficiency responded to Zn supplementation.
- Severe parakeratosis can lead to varying degrees of lameness.
- Paired feed and liver samples allowed for reliable mineral analyses.
Acknowledgments
We thank the Iowa State University Veterinary Diagnostic Laboratory staff for timely processing of the collected samples.
Conflict of interest
None reported.
Disclaimer
Scientific manuscripts published in the Journal of Swine Health and Production are peer reviewed. However, information on medications, feed, and management techniques may be specific to the research or commercial situation presented in the manuscript. It is the responsibility of the reader to use information responsibly and in accordance with the rules and regulations governing research or the practice of veterinary medicine in their country or region.
References
1. Dritz S, Goodband R, DeRouchey J, Tokach M, Woodworth J. Nutrient deficiencies and excesses. In: Zimmerman JJ, Karriker LA, Ramirez A, Schwartz KJ, Stevenson GW, Zhang J, eds. Diseases of Swine. 11th ed. Wiley Blackwell; 2019:1043-1054. https://doi.org/10.1002/9781119350927.ch68
2. Stevenson J, Earle I. Studies on parakeratosis in swine. J Anim Sci. 1956;15(4):1036-1045. https://doi.org/10.2527/jas1956.1541036x
3. Suttle NF. Zinc. In: Suttle N, ed. Mineral Nutrition of Livestock. 4th ed. CABI; 2010:426-458. https://doi.org/10.1079/9781845934729.0426
4. National Research Council. Minerals. In: Nutrient Requirements of Swine. 11th ed. National Academies Press; 2012:74-103.
*5. Tucker HF, Salmon W. Parakeratosis or zinc deficiency disease in the pig. In: Proc Soc Exp Biol Med. 1955;88(4):613-616. https://doi.org/10.3181/00379727-88-21670
6. Puls R. Mineral Levels in Animal Health. Diagnostic Data. 2nd ed. Sherpa International; 1994.
7. Kernkamp H, Ferrin E. Parakeratosis in swine. J Am Vet Med Assoc. 1953;123(918):217-220.
8. Anderson J, Cooper G, Hoekstra W. The histochemistry of the parakeratotic lesion of swine. J Invest Dermatol. 1967;48(6):521-530. https://doi.org/10.1038/jid.1967.84
9. Oberleas D, Muhrer M, O’dell B. Effects of phytic acid on zinc availability and parakeratosis in swine. J Anim Sci. 1962;21(1):57-61. https://doi.org/10.2527/jas1962.21157x
10. Luecke R, Hoefer J, Brammell W, Thorp Jr F. Mineral interrelationships in parakeratosis of swine. J Anim Sci. 1956;15(2):347-351. https://doi.org/10.2527/jas1956.152347x
11. Lewis P, Hoekstra W, Grummer R, Phillips P. The effect of certain nutritional factors including calcium, phosphorus and zinc on parakeratosis in swine. J Anim Sci. 1956;15(3):741-751. https://doi.org/10.2527/jas1956.153741x
12. Liptrap D, Miller E, Ullrey D, Whitenack D, Schoepke B, Luecke R. Sex influence on the zinc requirement of developing swine. J Anim Sci. 1970;30(5):736-741. https://doi.org/10.2527/jas1970.305736x
13. Cousins R, Liuzzi J, Lichten L. Mammalian zinc transport, trafficking, and signals. J Biol Chem. 2006;281(34):24085-24089. https://doi.org/10.1074/jbc.R600011200
* Non-refereed reference.
 PDF version
PDF version RIS
citation
RIS
citation