Respiratory disorders are widely recognized as the leading cause of financial losses to the pig industry due to veterinary care costs, decreased performance, and increased mortality. The etiology of porcine respiratory disorders is usually complex, with several pathogens acting together to determine the clinical and pathological outcomes. As a consequence, the term porcine respiratory disease complex (PRDC) has been introduced to indicate a multifactorial respiratory disease in growing and finishing pigs.1,2
Mycoplasma hyopneumoniae is among the most important causative agents of PRDC and is recognized as the primary pathogen of the so-called enzootic pneumonia (EP), a chronic and worldwide diffuse respiratory disease usually showing high morbidity and low mortality rates. Dry cough is the main clinical sign, which is greatly exacerbated by physical activity and may last for weeks to months. In EP-affected pigs, pulmonary lesions are bilateral consisting of slightly red or grey areas of bronchopneumonia, which affect the cranio-ventral parts of both lungs. Such gross findings are not pathognomonic for M hyopneumoniae as other pathogens (eg, swine influenza virus) may induce similar lesions. Microscopically, bronchointerstitial pneumonia and hyperplasia of the bronchial associated lymphoid tissue are the most relevant lesions.3,4
The present study aimed to assess the strengths and weaknesses of thoracic ultrasonography in pigs to provide suitable information to diagnose respiratory diseases, a special emphasis being placed upon EP-like lung lesions.
Materials and methods
This investigation was conducted in a farrow-to-finish pig herd (about 200 sows) with a recent history of severe respiratory disease. Pigs were not vaccinated for M hyopneumoniae and cases of EP complicated by Pasteurella multocida had been repeatedly diagnosed during the previous weeks.
Ultrasonography was performed using a linear multifrequency ultrasound transducer (probe Chison L7V-A, 5.3-10 MHz; Chison Medical Technologies Eco 3 Expert). A pregnancy-check ultrasound machine (3.5 MHz convex probe; manufacturer unknown) was also used.
Lung ultrasonography
Pigs (n = 8; 12-16 weeks of age) that spontaneously died after showing respiratory distress were necropsied. Lungs were removed from the chest and carefully examined. Thereafter, ultrasonography was performed by applying the transducer on the lung surface in both healthy and diseased areas. This step confirmed the ultrasound pattern of normal and diseased lungs. In this case, the linear multifrequency ultrasound transducer was used at 10 MHz.
Ultrasonography on pig cadavers and lungs
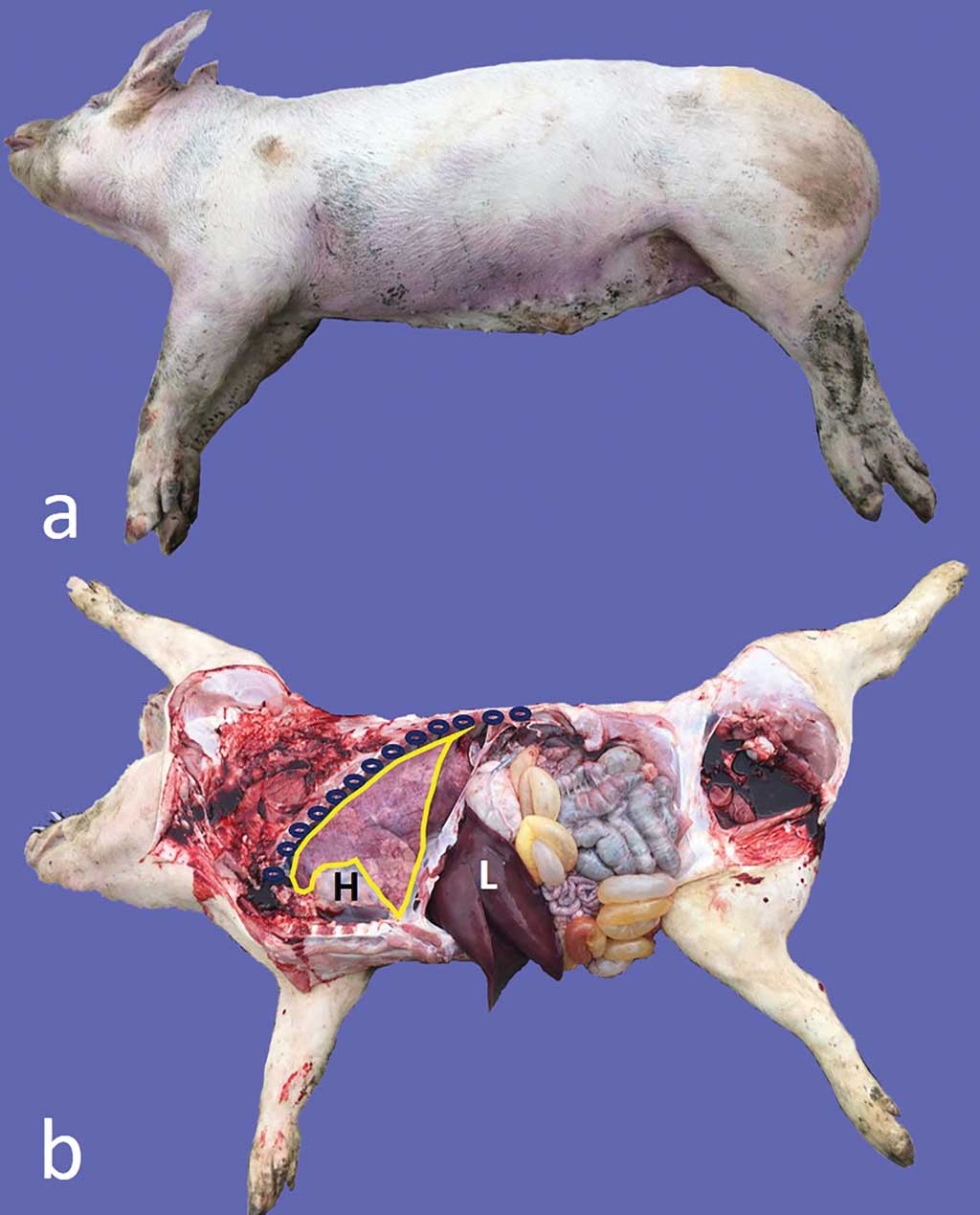
Transcutaneous ultrasonography was conducted along the left and right thoracic walls of pig carcasses (n = 8; 12-16 weeks of age) using some anatomic sites (ie, the elbow, the heart, and the intercostal spaces) as landmarks (Figure 1). Subsequently, the pigs were necropsied, and the ultrasound findings compared with the pathological findings. Once again, lungs were removed from the chest, carefully inspected, and further examined by ultrasonography. The linear multifrequency ultrasound transducer was used at 5.3 MHz on carcasses and at 10 MHz on lungs.
Ultrasonography on clinically healthy and diseased live pigs
Ultrasonography was carried out on live, clinically healthy pigs aged 4 (n = 8), 8 (n = 8), and 12 (n = 8) weeks, randomly selected from two batches. Likewise, ultrasonography was performed on live pigs (n = 8; 12-16 weeks of age) showing prominent respiratory clinical signs (dry coughing) and, therefore, confined in the recovery pen. To this aim, pigs were placed in the lateral decubitus position with no sedation or hair clipping required. Specifically, the pig was placed with its back facing an operator, who restrained the pig by holding the two forelimbs with one hand, and the two hindlimbs with the other hand. The linear multifrequency ultrasound transducer was used at 5.3 MHz.
Results
Lung ultrasonography
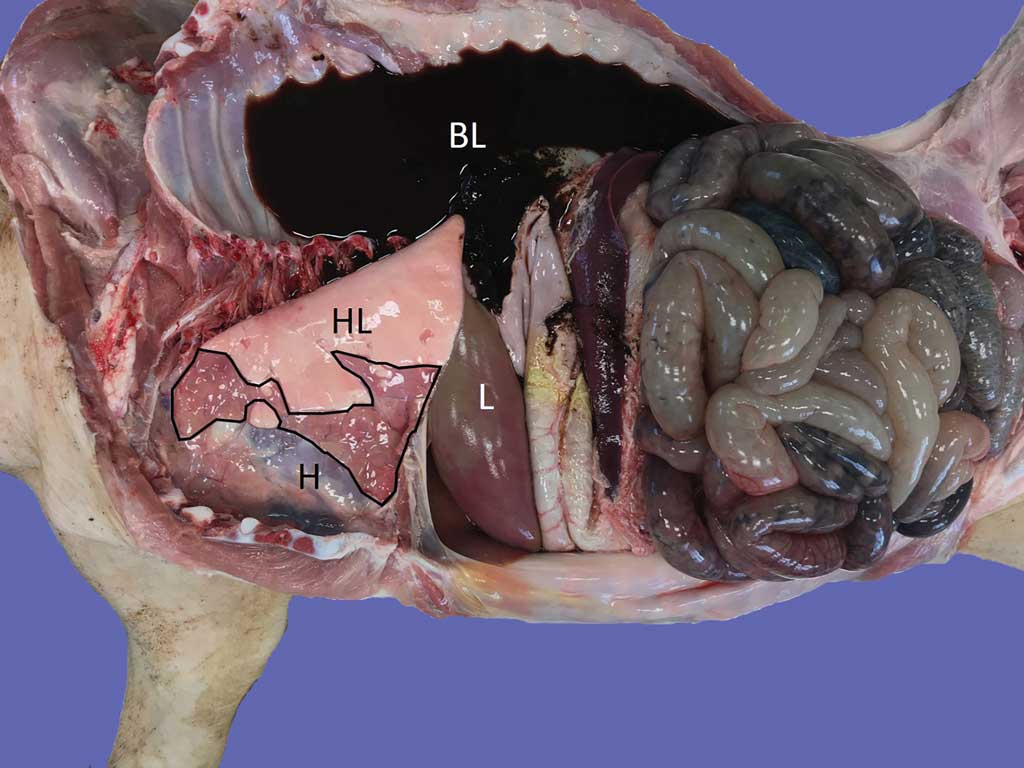
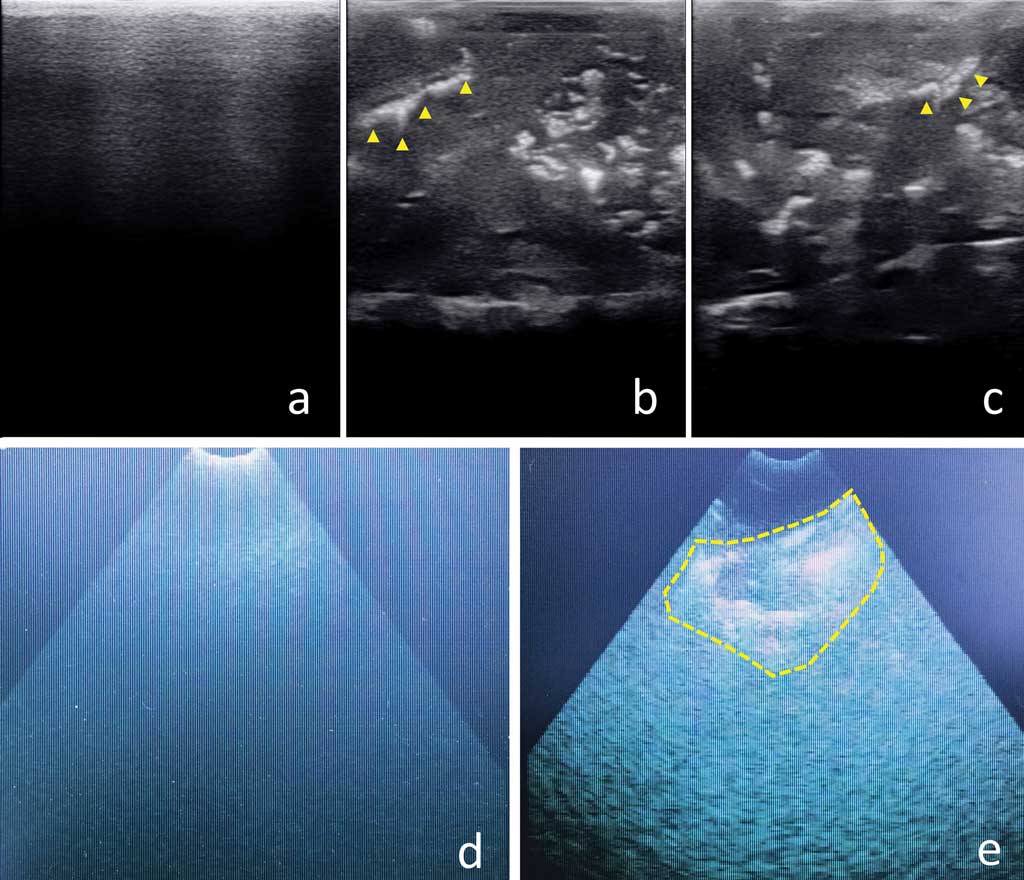
All lungs under study showed gross lesions compatible with EP (Figure 2). Ultrasonography of diseased areas showed a homogeneous, “liver-like” appearance, with hyperechogenic spots and lines of variable size and shape (Figure 3). The healthy lung parenchyma could not be shown by ultrasonography. Its air content induced the appearance of the so-called A-lines, ie, reverberation artefacts running parallel to the pleural surface. The images obtained by the pregnancy-check ultrasound machine were of lower quality because they were photographs of the device screen (this tool was unable to store and download images; Figure 3).
Ultrasonography on pig cadavers and lungs
The aforementioned ultrasonographic patterns were easily recognized also on pig carcasses. The intercostal spaces corresponding to disease ultrasonograms were recorded before necropsy. Gross findings consisted of bilateral foci of EP-like bronchopneumonia and were consistent with ultrasonograms.
Ultrasonography on clinically healthy and diseased live pigs
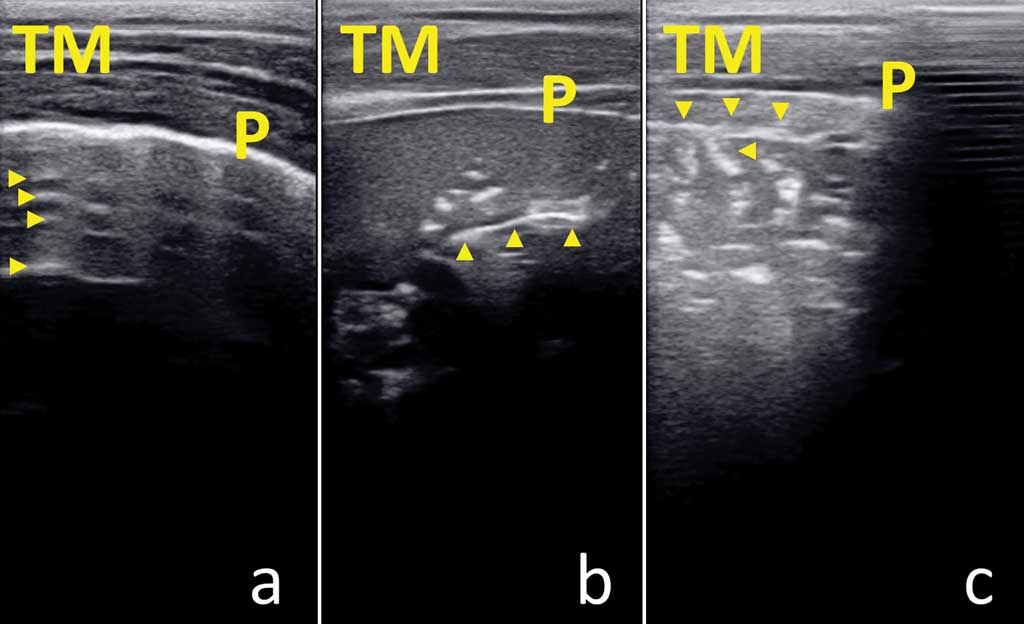
Thoracic ultrasonography took about 5 min/pig. No disease ultrasonogram was observed in clinically healthy pigs at 4, 8, and 12 weeks of age. On the contrary, ultrasonograms compatible with bronchopneumonia were always detected in pigs with respiratory syndrome at 12 to 16 weeks of age (Figure 4). Although providing images of different quality, ultrasonograms obtained using the linear multifrequency ultrasound transducer provided similar ultrasonographic pattern at the same anatomical site when compared with the 3.5 MHz sector transducer.
Discussion
In modern and intensive pig farming, it is always important to assess the impact (ie, the prevalence and severity) of pneumonia, as well as monitor the effectiveness of strategies implemented to treat and prevent such disease conditions.5 The Madec’s grid is the most common method used to score lung lesions and is usually performed on slaughtered pigs.6 However, EP lesions can be missed when the animals recover, this being more probable in heavy pigs slaughtered at 9 to 10 months of age. Accordingly, some studies indicate that losses are associated with a clinical history of pneumonia rather than with lung lesions at slaughter. This is not surprising when the impact of complex and multifactorial disorders is investigated.7,8
Clinical assessment and laboratory tests (eg, serological test) can be complementary to scoring lesions at slaughter providing useful information about the timing of infection and the prevalence and severity of the disease to plan effective control strategies.1,7 Investigative methods on live animals should be easy, fast, reliable, and cost-effective, yet such requisites often remain disregarded. As an example, systems for cough recording could be of value for early EP detection, but they are still unavailable in practice.7 Likewise, radiography is a highly informative tool, but not feasible under field conditions.9
In human10,11 and veterinary medicine, thoracic ultrasonography has been increasingly used as a diagnostic tool, as well as to monitor clinical outcomes on individual patients. In particular, a number of scientific papers have been published regarding the application of thoracic ultrasonography in pets, ruminants, and horses.12-17 To the best of our knowledge, limited data are currently available about thoracic ultrasonography in pigs under field conditions, with only a few reports dealing with experimental investigations.18-20 Overall, the present study indicates that ultrasonography is effective to discriminate between healthy and diseased lungs with EP-like lesions showing an easily recognizable appearance. In our opinion, the main strengths of thoracic ultrasonography include:
- Ultrasonography is considered safe both for investigators and for pigs.
- It is inexpensive, as ultrasound transducers are commonly available on pig farms where they are routinely used to detect pregnancy. When the same ultrasound equipment is used at different herd sites, it is essential to comply with biosecurity measures to avoid the spread of pathogens.
- Thoracic ultrasonography could be useful to evaluate the main features of lung lesions (ie, extent, involvement of cranio-ventral vs dorsal-caudal areas, unilateral vs bilateral distribution, absence vs presence of pleuritis), which are needed to better address the diagnostic approach and a rational and effective treatment.
The main weaknesses of thoracic ultrasonography that should be considered include:
- Accurate interpretation of the ultra- sonographic patterns requires targeted professional training even though image acquisition is relatively easy. For example, severe pleuritis and pericarditis, as observed in Glasser’s disease affected pigs, can be very easily identified by ultrasonography, while the detection of virus-induced interstitial pneumonia, although possible, requires more focused skills.
- Because of the reverberation artefacts, thoracic ultrasonography can only detect disease conditions reaching the pleural surface, thus overlooking deeper lesions (eg, abscesses fully embedded within the lung parenchyma).
- Thoracic ultrasonography could be time-consuming in large pig farms where a high number of animals should be investigated to have a suitable sample size. As a consequence, this tool may not be routinely used to determine EP prevalence.
- Thoracic ultrasonography often provides a presumptive diagnosis, which should be confirmed by further laboratory investigations. This is crucial for proper management of PRDC through vaccination strategy or to select the most effective antimicrobial.
- Thoracic ultrasonography is less accurate and should not replace post-mortem investigations, even under the best conditions. Moreover, post-mortem changes (eg, reduction of pulmonary air content) make the interpretation of ultrasonograms more challenging and could provide false positive results, especially when ultrasonography occurs several hours after death.
To conclude, thoracic ultrasonography could be a useful tool for the clinical appraisal of pig herds to assess the kinetics of respiratory infections/diseases, as well as the severity and the main features of pneumonia. Data resulting from thoracic ultrasonography should integrate with, not replace, other diagnostic tools, including necropsy, with the aim to manage PRDC in a more precise and rational manner.
Acknowledgments
The present study has been carried out in the framework of the Project Demetra (Dipartimenti di Eccellenza 2018-2022, CUP_C46C18000530001) funded by the Italian Ministry for Education, University, and Research.
Conflict of interest
None reported.
Disclaimer
Scientific manuscripts published in the Journal of Swine Health and Production are peer reviewed. However, information on medications, feed, and management techniques may be specific to the research or commercial situation presented in the manuscript. It is the responsibility of the reader to use information responsibly and in accordance with the rules and regulations governing research or the practice of veterinary medicine in their country or region.
References
1. Yaeger MJ, Van Alstine WG. Respiratory system. In: Zimmerman JJ, Karriker LA, Ramirez A, Schwartz KJ, Stevenson GW, eds. Diseases of Swine. 11th ed. Ames, Iowa: Wiley-Blackwell; 2019:393-407.
2. Carr J, Chen SP, Connor JF, Kirkwood R, Segales J. Respiratory disorders. In: Carr J, Chen SP, Connor JF, Kirkwood R, Segales J, eds. Pig Health. Boca Raton, Florida: CRC Press; 2018:103-152.
3. Pieters MG, Maes D. Mycoplasmosis. In: Zimmerman JJ, Karriker LA, Ramirez A, Schwartz KJ, Stevenson GW, eds. Diseases of Swine. 11th ed. Ames, Iowa: Wiley-Blackwell; 2019:863-883.
4. Maes D, Sibila M, Kuhnert P, Segales J, Haesebrouck F, Pieters M. Update on Mycoplasma hyopneumoniae infections in pigs: Knowledge gaps for improved disease control. Transbound Emerg Dis. 2018;65(1):110-124.
5. Luppi A, Merialdi G. Lesioni al Macello. In: Martelli P, ed. Le Patologie del Maiale. Milano, Italy: Point Veterinaire Italie; 2013:199-218.
6. Madec F, Kobish M. Bilan lesionnel des puiomons de porcs charcutiers a l’abattoir. Journ Rech Porc Fr. 1982;14:405-412.
7. Morris CR, Gardner IA, Hietala SK, Carpenter TE. Enzootic pneumonia: comparison of cough and lung lesions as predictors of weight gain in swine. Can J Vet Res. 1995;59:197-204.
8. Noyes EP, Feeney DA, Pijoan C. Comparison of the effect of pneumonia detected during lifetime with pneumonia detected at slaughter on growth in swine. JAVMA. 1990;197:1025-1029.
9. Brauer C, Hennig-Pauka I, Hoeltig D, Buettner FR, Beyerbach M, Gasse H, Gerlach GF, Waldmann KH. Experimental Actinobacillus pleuropneumoniae challenge in swine: Comparison of computed tomographic and radiographic findings during disease. BMC Vet Res. 2012;8:47. doi:10.1186/1746-6148-8-47
10. Smargiassi A, Inchingolo R, Soldati G, Copetti R, Marchetti G, Zanforlin A, Giannuzzi R, Testa A, Nardini S, Valente S. The role of chest ultrasonography in the management of respiratory diseases: document II. Multidiscip Respir Med. 2013;8:55. doi:10.1186/2049-6958-8-55
11. Zanforlin A, Giannuzzi R, Nardini S, Testa A, Soldati G, Copetti R, Marchetti G, Valente S, Inchingolo R, Smargiassi A. The role of chest ultrasonography in the management of respiratory diseases: document I. Multidiscip Respir Med. 2013;8:54. doi:10.1186/2049-6958-8-54
12. Flöck M. Diagnostic ultrasonography in cattle with thoracic disease. Vet J. 2004;167:272–280.
13. Hussein AH, Binici C, Staufenbiel R. Comparative evaluation of ultrasonography with clinical respiratory score in diagnosis and prognosis of respiratory diseases in weaned dairy buffalo and cattle calves. J Anim Sci Technol. 2018;60:29. doi:10.1186/s40781-018-0187-3
14. Reef VB, Boy MG, Reid CF, Elser A. Comparison between diagnostic ultrasonography and radiography in the evaluation of horses and cattle with thoracic disease: 56 cases (1984–1985). JAVMA. 1991;198:2112–2118.
15. Tidwell AS. Ultrasonography of the thorax (excluding the heart). Vet Clin North Am Small Anim Pract. 1998;4:993-1015.
16. Tharwat M, Al-Sobayil F. Ultrasonographic findings in goats with contagious caprine pleuropneumonia caused by Mycoplasma capricolum subsp. capripneumoniae. BMC Vet Res. 2017;13:263. doi:10.1186/s12917-017-1167-4
17. Ollivett TL, Caswell JL, Nydam DV, Duffield T, Leslie KE, Hewson J, Kelton D. Thoracic ultrasonography and bronchoalveolar lavage fluid analysis in Holstein calves with subclinical lung lesions. J Vet Intern Med. 2015;29:1728-1734.
18. Oveland NP, Sloth E, Andersen G, Lossius HM. A porcine pneumothorax model for teaching ultrasound diagnostics. Acad Emerg Med. 2012;19:586-592.
19. Zachary JF, O’Brien Jr WD. Lung lesions induced by continuous- and pulsed-wave (diagnostic) ultrasound in mice, rabbits, and pigs. Vet Pathol. 1995;32:43-54.
20. Wojtczak J, Wood RW. High-frequency ultrasound in ex vivo animal lungs in pulmonary edema. J Anesthesiol Clin Sci. 2013;2:21. doi:10.7243/2049-9752-2-21
 PDF version
PDF version RIS
citation
RIS
citation