| Original research | Peer reviewed |
Cite as: Moura CAA, Totton SC, Sargeant JM, et al. Evidence of improved reporting of swine vaccination trials in the post-REFLECT statement publication period. J Swine Health Prod. 2019;27(5):265-277. https://doi.org/10.54846/jshap/1125
Also available as a PDF.
SummaryObjectives: Describe and compare the proportion of studies reporting the method used to assign study units to treatment groups, reporting a random allocation approach, reporting 18 REFLECT items, and the proportion of studies having a low risk-of-bias assessment in swine vaccination trial studies published after the REFLECT statement, compared to studies published before. Materials and Methods: The study population was 61 studies that evaluated vaccines targeted at pathogens affecting swine health or pork safety. Two reviewers assessed the reporting of 18 of 22 REFLECT items and 5 risk-of-bias domains. Results: Authors reported the method used to allocate experimental units in 33 of 42 (79%) and 14 of 19 (74%) studies published prior to and following REFLECT, respectively. There has been a substantial shift in the reporting of allocation approaches. Before 2011, only 2 of 25 (8%) studies that reported using random allocation provided supporting evidence. This increased in studies published between 2011-2017 (4 of 6; 66%). Before 2011, 8 of 33 (24%) studies reported using systematic allocation, which increased to 43% (6 of 14 studies) between 2011-2017. There has also been an increase in the prevalence of reporting for 14 of the 18 REFLECT items. There was an increase in the number of studies reporting evidence to support true randomization to group and data that suggests few baseline imbalances. Implications: Data from this study suggests swine vaccination trial reporting improved, which may be due to researchers having more access to better quality information. | ResumenObjetivos: Describir y comparar la proporción de estudios que describen el método utilizado para asignar unidades de estudio a grupos de tratamiento, que reportan un enfoque de asignación aleatoria, reportando 18 ítems REFLECT y la proporción de estudios que tienen una evaluación de bajo riesgo de parcialidad en estudios de vacunación porcina publicados después de la declaración REFLECT, comparados con estudios publicados anteriormente. Materiales y métodos: La población del estudio fue de 61 estudios que evaluaron vacunas contra patógenos que afectan la salud de los cerdos o la seguridad de la carne. Dos revisores evaluaron el informe de 18 de los 22 elementos REFLECT y 5 áreas de riesgo de parcialidad. Resultados: Los autores reportaron el método utilizado para asignar unidades experimentales en 33 de 42 (79%) y 14 de 19 (74%) estudios publicados antes y después de REFLECT, respectivamente. Ha habido un cambio importante en el reporte de los enfoques de asignación. Antes de 2011, solo 2 de 25 (8%) estudios que informaron el uso de una asignación aleatoria proporcionaron evidencia de apoyo. Esto aumentó en los estudios publicados entre 2011-2017 (4 de 6; 66%). Antes de 2011, 8 de 33 (24%) estudios informaron el uso sistemático, que aumentó a 43% (6 de 14 estudios) entre 2011-2017. También ha habido un aumento en la prevalencia de reporte de 14 de los 18 ítems REFLECT. Hubo un aumento en el número de estudios que informaron evidencia para respaldar la asignación al azar real al grupo y los datos que sugieren pocos desequilibrios de base. Implicaciones: Los datos de este estudio sugieren que los reportes de los estudios de vacunación porcina mejoraron, lo que puede deberse a que los investigadores tienen más acceso a información de mejor calidad. | ResuméObjectifs: Décrire et comparer la proportion d’études rapportant : la méthode utilisée pour attribuer les unités à l’étude aux groupes de traitement, une approche d’attribution aléatoire, 18 items REFLECT, et la proportion d’études ayant un risque faible de biais d’évaluation dans les essais de vaccination de porcs publiés après l’énoncé REFLECT, comparativement aux études publiées avant. Matériels et méthodes: La population étudiée consistait en 61 études qui ont évalué des vaccins ciblant des agents pathogènes affectant la santé porcine ou la salubrité de la viande porcine. Deux réviseurs ont évalué la publication de 18 des 22 items REFLECT et cinq domaines de risque de biais. Résultats: Les auteurs rapportaient la méthode pour distribuer les unités expérimentales dans 33 des 42 (79%) et 14 des 19 (74%) études publiées préalablement et après REFLECT, respectivement. Il y eu un changement notoire dans la publication des approches d’attribution. Avant 2011, seulement 2 des 25 (8%) des études qui rapportaient utiliser une attribution aléatoire fournissaient des preuves à cet effet. Ceci augmenta dans les études publiées entre 2011-2017 (4 de 6; 66%). Avant 2011, 8 des 33 (24%) études rapportaient utiliser une attribution aléatoire, proportion qui augmenta à 43% (6 de 14 études) entre 2011-2017. Il y eu également une augmentation de la prévalence à rapporter pour 14 des 18 items REFLECT. Il y avait une augmentation dans le nombre d’études qui rapportaient des preuves pour supporter une réelle randomisation pour regrouper et des données qui suggèrent peu de débalancements au départ. Implications: Les données de la présente étude suggère que les rapports d’essais de vaccination chez le porc se sont améliorés, ce qui pourrait être dû au fait que les chercheurs ont accès à des information de meilleure qualité. |
Keywords: swine, REFLECT, vaccine, risk-of-bias, randomization
Search the AASV web site
for pages with similar keywords.
Received: November 12, 2018
Accepted: May 7, 2019
Infectious diseases of swine and infectious causes of foodborne illness impact the sustainability of the food supply. Diseases such as African swine fever, porcine reproductive and respiratory syndrome, and swine influenza can lead to reduced pork supply,1 while outbreaks of foodborne pathogens associated with pork, such as Salmonella, lead to reduced demand and risk of public health-related problems.2-4 Therefore, it is critical that swine veterinarians have access to comprehensive reports of vaccine efficacy, allowing them to make science-driven decisions on the best immunization process to control or eradicate diseases in the herd. Unfortunately, scientific reporting of intervention studies in swine production often lacks critical information that enables assessment of biases, and there is an apparent need to improve reporting.5
In 2010, the Reporting Guidelines for Randomized Controlled Trials for Livestock and Food Safety (REFLECT) statement and the companion Explanation and Elaboration document were published.6-11 The REFLECT statement has a 22-item checklist developed by an international group to help investigators improve the reporting of livestock trials that have a production, health, or food-safety outcome. The long-term goal of reporting checklists such as the REFLECT statement and similar reporting guidelines, such as the CONSORT statement,12 the ARRIVE statement for biomedical experiments,13-17 and STROBE-Vet,18-22 is to reduce research wastage and maximize research utility for decision-making through improved reporting. Therefore, it is critical to periodically evaluate reporting and determine if progress toward improved reporting is occurring. In 2018, a study was performed to assess the reporting characteristics of bovine respiratory disease clinical trials published before and following the publication of the REFLECT statement. The authors reported positive trends toward improved reporting after 2010.23 However, to our knowledge, there are no studies in swine production assessing if reporting has improved in recent years coinciding with efforts such as the REFLECT statement and Meridian Network (https://meridian.cvm.iastate.edu), a website that acts as a clearinghouse for reporting guidelines related to animals used in research.
Reporting guidelines are designed to improve reporting with an underlying hope that once reporting is improved, end-users will be able to identify well-executed studies and clearly extract the results. It is also hoped that in reality the vast majority of studies are well executed, and that comprehensive reporting will enable this fact to be more obvious. Currently, it is often not possible to differentiate well-executed studies from poorly executed studies. If reporting is noncomprehensive then it is difficult, if not impossible, to differentiate between well-executed studies with a low risk-of-bias from poorly executed studies with a high risk-of-bias. For example, if 2 studies exist and one randomized properly and the other did not and neither reported randomization, then these differential risks-of-bias cannot be determined. However, not all aspects of reporting relate to risk-of-bias; some items are included to help end-users understand the generalizability of the results while other aspects are designed to help end-users properly comprehend the efficacy of the interventions. The lack of detail in reporting means that many studies with interventions of interest cannot be properly assessed by veterinarians, thus reducing the impact and utility of these studies. These aspects are still relevant as they ensure maximized utility of resources, including animals, involved in animal studies.
The objective of this study was to assess whether reporting and risk-of-bias standards have changed for swine vaccination trials in the publication period from 2011 to 2017 (post-REFLECT) compared to the publication period before 2011 (pre-REFLECT). Aim 1 described the proportion of studies reporting the allocation of study units to treatment group in studies published after the REFLECT statement compared to studies published before. Our hypothesis was that the proportion of articles reporting the allocation methods would have increased in recent years, as awareness of the impact of poor reporting has increased. Aim 2 described the proportion of studies reporting a random allocation approach in studies published after the REFLECT statement compared to studies published before. Our hypothesis was that the proportion of articles reporting a random allocation approach have increased in the last years; however, prior evidence suggests that there is some misunderstanding in the veterinary sciences of the difference between truly random and pseudo-random allocation approaches.23 Aim 3 sought to describe the reporting prevalence of 18 REFLECT items in studies published after the REFLECT statement compared to studies published before. Our hypothesis was that the proportion of articles reporting the REFLECT items have increased over the years. Aim 4 sought to describe the proportion of studies having a low risk-of-bias assessment in studies published after the REFLECT statement compared to studies published before. Our hypothesis was that the proportion of articles having a low risk-of-bias assessment have increased over the years.
Materials and methods
Study protocol
A study protocol was developed and registered with the Open Science Framework.24 For all aspects of the project (title and abstract screening, full-text screening, and risk-of-bias assessment), 2 reviewers independently completed forms in DistillerSR (Evidence Partners, Ottawa, Canada). Conflicts between reviewers were resolved by discussion or, when consensus could not be reached, by consulting a third reviewer. The authorship on the title page of each article was redacted before evaluation; however, because of the small community of researchers in this subject area, it was not possible to ensure that blinding occurred. Additionally, the reviewers could not be blinded to publication dates because the date on which the study was conducted was usually reported in the Methods section and was part of the comprehensive reporting assessment (Items 3 and 14). The screening form, the reporting assessment form, and the risk-of-bias form were pretested on 20, 2, and 4 studies respectively. All forms are provided in the online supplementary materials (https://doi.org/10.25380/iastate.7946732.v1).
Study population
For this cross-sectional observational survey, the population of interest was controlled trials where at least one study group received a vaccine targeting pathogens associated with swine health or food safety in pork. Further, the study had to be published in 1 of the 5 journals that published the REFLECT statement: Preventive Veterinary Medicine, Journal of Food Protection, Journal of Veterinary Internal Medicine, Journal of Swine Health and Production, and Zoonoses and Public Health. These journals were selected because they recommend authors to use the REFLECT statement. The outcome reported by the investigators did not impact eligibility. Controlled trials were defined as having a concurrent/parallel comparison arm with either artificial challenge or natural infection. The publication periods were defined as pre-REFLECT, which included 2010 and earlier, and post-REFLECT, which was 2011 and later. As REFLECT was published in 2010, we considered studies published in 2010 as being written before REFLECT.
Screening assessment
The literature search was conducted in Web of Science (Clarivate Analytics, United States) using the Centre for Agriculture and Bioscience International database using the search strategy presented in Table 1. Two levels of screening were used to identify eligible manuscripts: title and abstract followed by the full text.
| Search No. | Search string | No. of hits |
|---|---|---|
| 1 | Topic = (swine OR pig* OR piglet* OR gilt* OR boar* OR sow* OR weaner* OR hog* OR porcine OR pork* OR Sus scrofa OR Sus domesticus) | 645,575 |
| 2 | Topic = (Vaccin* OR immuniz*) | 149,140 |
| 3 | Journals = (Preventive Veterinary Medicine OR Journal of Food Protection OR Journal of Veterinary Internal Medicine OR Swine Health and Production OR Journal of Swine Health and Production OR Zoonoses and Public Health) | 17,169 |
| 4 | #1 AND #2 AND #3 | 239 |
* Search was conducted September 28, 2017.
CABI = Centre for Agriculture and Bioscience International.
Comprehensive reporting assessment
The reporting assessment form was based on a form developed for a bovine respiratory disease study,23 which was in turn based on the REFLECT Statement6-11 and was modified for use in swine. We assessed reporting 18 of the 22 REFLECT items (items 1 and 3-19). Items 2, 20, 21, and 22 were considered too subjective for a consistent and valid assessment. Signaling questions and notes that guided the consistent assessment of the items are included with the forms in the online supplementary materials (https://doi.org/10.25380/iastate.7946732.v1).
Risk-of-bias assessment
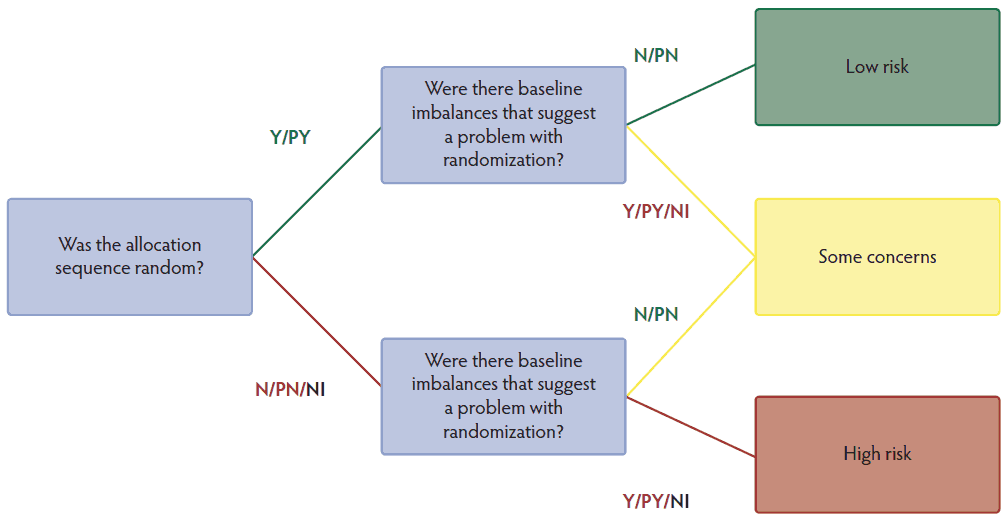
We used the Cochrane risk-of-bias 2.0 algorithm25 to assess the risk-of-bias that arose from deviations from intended interventions, from missing outcome data, from measurement of the outcome, and from selection of the reported results. However, for assessing the risk-of-bias due to randomization process, we modified the algorithm so that it followed the schema in Figure 1. The risk-of-bias algorithm we used did not consider failure to report allocation concealment to be critical to assessing bias in swine vaccine trials, as is suggested by the Cochrane risk-of-bias algorithm. We would propose that the Cochrane risk-of-bias algorithm authors consider the allocation concealment important in human health because the knowledge of potential intervention might cause some recruiters to modify the allocation schedule. For example, Kahan et al,26 described the following:
Figure 1: Risk-of-bias algorithm arising from the allocation process used for 61 extracted swine vaccine studies published pre- or post-REFLECT publication. Y = yes; PY = probably yes; N = no; PN = probably no; NI = no information.
If a recruiter believes the next allocation will be the intervention, they may wait to enroll a very sick patient, as they do not want to ‘waste’ an intervention allocation on a relatively healthy patient who is less likely to need it.
However, in swine vaccine studies, which are the topic of this study, we considered the probability that the recruiter had either differential personal attachment to the pig or a priori knowledge of the pig potential production value to be low.
Therefore, we included the scenario where studies could fail to report allocation concealment and random method of allocation and this would result in a different pathway, with lower risk-of-bias, than the Cochrane Risk of Bias (ROB) 2.0 algorithm. We also considered that providing no information about baseline differences to be more similar in risk to having evidence of baseline imbalances.
Our risk-of-bias assessment algorithm for individual and cluster-randomized trials (which are the trials that conduct the randomization at the group level, instead of at the individual animal level) are the same.
Statistical analysis
We estimated the prevalence ratios for the post-REFLECT publication period (numerator) compared to the pre-REFLECT publication period (denominator) for:
- reporting of any allocation method (Aim 1),
- reporting of a valid random allocation, given an allocation approach was reported (Aim 2),
- reporting 18 of the REFLECT items (Aim 3), and
- a low risk-of-bias assessment for the five bias domains (low versus high/some concerns; Aim 4).
We did not conduct any null hypothesis testing as they have limited value in an observational study of unknown pre-planned power. Additionally, since we sampled all available papers that met our eligibility criteria, we considered the population to be a census. Therefore, we did not calculate any measures of precision (confidence intervals), because we have no uncertainty about the point estimates reported. When we could not calculate the prevalence ratio due to zeros, we reported the results of a Fisher test for binomial proportions. All statistical analyses were done using R 3.4.1 program.
Results
Screening for eligibility and characteristics of included studies
The search retrieved 239 records. One hundred seventy-two records were excluded based on the title or abstract. Six papers were excluded based on the full-text assessment. For the 61 manuscripts assessed, 42 studies27-68 were published before the REFLECT statement (date range: 1982-2010), while 19 studies69-87 were published between 2011 and 2017. Forty-seven trials were published in the Journal of Swine Health and Production (formerly published as Swine Health and Production), 11 in Preventive Veterinary Medicine, 2 in Zoonoses and Public Health, and 1 in Journal of Food Protection. Only the Journal of Swine Health and Production and Preventive Veterinary Medicine had articles published from 2011 to 2017, with 14 and 5 papers, respectively. Fifty-six studies had individual allocation to an intervention group and 5 studies were cluster-randomized trials.
Aim 1: Reporting of an allocation method
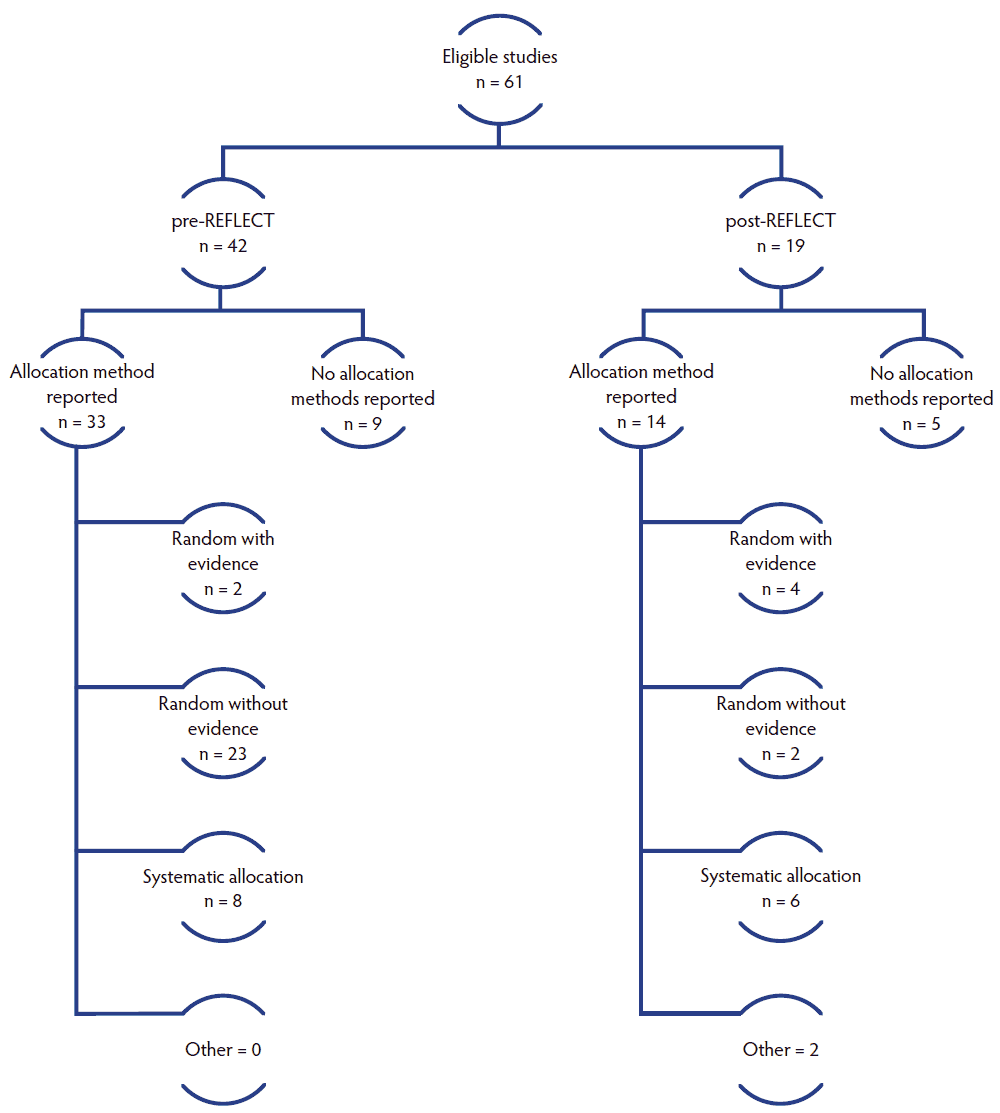
Investigators reported in the title, abstract, or methods section the method used to allocate the experimental units to the interventions in 33 of 42 (79%) and 14 of 19 (74%) studies in the pre-REFLECT and post-REFLECT publication periods, respectively (Figure 2). The prevalence ratio was 0.94.
Figure 2: Distribution of allocation approaches reported in 61 swine vaccine studies published pre- or post-REFLECT publication.
Aim 2: Approach to allocation reported
This outcome was limited to studies that reported an allocation approach in Aim 1. For 25 of 33 (76%) studies published before 2011 and 6 of 14 (43%) studies published between 2011-2017, the approach to allocation was reported as random. Before 2011, 23 of 25 (92%) studies that reported a random allocation approach did not provide any evidence of the randomization process, for example, the method used to generate the random allocation sequence, the method used to implement the random allocation sequence, or who conducted the randomization process. Yet, in the period from 2011-2017, this number had decreased to 2 of 6 (33%) studies (Figure 2). Before 2011, only 2 of 25 (8%) studies that reported a random allocation approach provided evidence of the randomization process, and this increased in studies published between 2011 and 2017 to 4 of 6 (67%). Of the studies that did report information about allocation, 8 of the 33 studies (24%) published before 2011 and 6 of 14 (43%) studies published between 2011 and 2017 reported using a systematic allocation method. In systematic random allocation approaches, the researcher picks the first individual at random and keeps selecting the other subjects by alternation. Two studies published post-REFLECT reported another allocation method (non-random and arbitrary selection).
Aim 3: Reporting of REFLECT checklist items
The reporting characteristics for the REFLECT checklist items are reported in Table 2 and Figure 3. After REFLECT publication, the prevalence of reporting the following REFLECT items had improved: randomization in the title and abstract (item 1), eligibility criteria for owner/managers and study units and the description of settings (item 3), details of the interventions (item 4), primary outcome (item 6), how the sample size was calculated (item 7), method used to generate the random allocation sequence (item 8), whether or not blinding was done (item 11), whether statistical methods were used (item 12), flow of study units through the study (item 13), baseline demographic and clinical characteristics of each group (item 15), number of study units used in analysis (item 16), summary of results for each group – estimated effect size and its precision (item 17), multiplicity (item 18), and adverse events or side effects (item 19). After REFLECT publication, the prevalence of reporting the objective and hypothesis (item 5) and dates defining the periods of recruitment and follow-up (item 14) decreased. Concealment of the allocation sequence (item 9) as well as who generated the allocation sequence/who enrolled study units/who assigned study units to their groups (item 10) were not reported for any of the 61 studies reviewed. Data about reporting characteristics of the challenge models (REFLECT item 4B) are not shown in Figure 3, as it could not be dichotomized, and these results are instead reported in Table 3. The percentage of challenge model studies was higher in studies published before 2011 (21 of 42 studies; 50%) than between 2011 and 2017 (6 of 19 studies; 32%).
Table 2: Reporting characteristics of 18 REFLECT statement items from 61 extracted swine vaccine studies published pre- or post-REFLECT publication
REFLECT reporting items | Published studies reporting, No. (%) | Prevalence ratio | |
|---|---|---|---|
Pre-REFLECT studies |
Post-REFLECT studies |
||
Item 1: In the Title or Abstract, did the investigators report that the study units were randomly allocated to the interventions? (eg, “random allocation”, “randomized”, or “randomly assigned”) |
11/42 (26) |
6/19 (32) |
1.2 |
Item 3: In the Methods, did the investigators report eligibility criteria for owner/managers and study units at each level of the organizational structure, and did they describe the settings and locations where the data were collected? |
2/42 (5) |
4/19 (21) |
4.4 |
Item 4: In the Methods, did the investigators give precise details of the interventions intended for each group, the level at which the intervention was allocated, and how and when interventions were administered? |
28/42 (67) |
15/19 (79) |
1.2 |
Item 5: Did the investigators report the specific objectives and hypotheses of the study? |
6/42 (14) |
2/19 (11) |
0.7 |
Item 6: Did the investigators give clearly defined primary outcome measures and the levels at which they were measured, and, when applicable, any methods used to enhance the quality of the measurements? |
6/42 (14) |
8/19 (42) |
2.9 |
Item 7: Did the investigators report how the sample size was determined and, when applicable, explain any interim analyses and stopping rules? |
7/42 (17) |
7/19 (37) |
2.2 |
Item 8: If the authors described an approach to allocation anywhere in the manuscript then did the investigators report the method used to generate the random allocation sequence at the relevant level of the organizational structure, including details of any restrictions (eg, blocking, stratification)? |
2/25 (08) |
4/6 (67) |
8.3 |
Item 9: Did the investigators report the method used to implement the random allocation sequence at the relevant level of the organizational structure, (eg, numbered containers), clarifying whether the sequence was concealed until interventions were assigned? |
0/25 (0) |
0/6 (0) |
* |
Item 10: Did the investigators report who generated the allocation sequence, who enrolled study units, and who assigned study units to their groups at the relevant level of the organizational structure? |
0/25 (0) |
0/6 (0) |
* |
Item 11: Did the investigators report whether those administering the interventions, caregivers, and those assessing the outcomes were blinded to group assignment? |
15/42 (36) |
12/19 (63) |
1.8 |
Item 12: Were statistical methods used to compare groups for all outcome(s)? Did the investigators clearly state the level of statistical analysis and methods used to account for the organizational structure (where applicable)? Were the methods for additional analyses, such as subgroup analyses and adjusted analyses reported? |
34/42 (81) |
17/19 (89) |
1.1 |
| Item 13: In the Results, did the investigators report the flow of study units through each stage for each level of the organization structure of the study (a diagram is strongly recommended)? | 29/42 (69) | 15/19 (79) | 1.1 |
| Item 14: Did the investigators report dates defining the periods of recruitment and follow-up? | 12/42 (29) | 5/19 (26) | 0.9 |
| Item 15: Did the investigators report the baseline demographic and clinical characteristics of each group, explicitly providing information for each relevant level of the organizational structure? | 11/42 (26) | 8/19 (42) | 1.6 |
| Item 16: Did the investigators report the number of study units (denominator) in each group included in each analysis? | 25/42 (60) | 15/19 (79) | 1.3 |
| Item 17: Did the investigators report a summary of results for each group, accounting for each relevant level of the organizational structure, and the estimated effect size and its precision? | 1/42 (2) | 3/19 (16) | 6.6 |
| Item 18: For the studies with 2 or more arms, did the investigators address multiplicity by reporting any other analyses performed, including subgroup analyses and adjusted analyses, indicating those pre-specified and those exploratory? | 8/28 (29) | 6/12 (50) | 1.75 |
| Item 19: Did the investigators report all important adverse events or side effects in each intervention group? | 4/42 (10) | 2/19 (11) | 1.1 |
Figure 3: The prevalence comparison plot of 18 REFLECT items reported in 42 studies published before 2011 and 19 studies published between 2011 and 2017. Item 4B had multiple categories and is not included. Items 2 and 20 to 23 were considered too subjective for assessment and were not included.
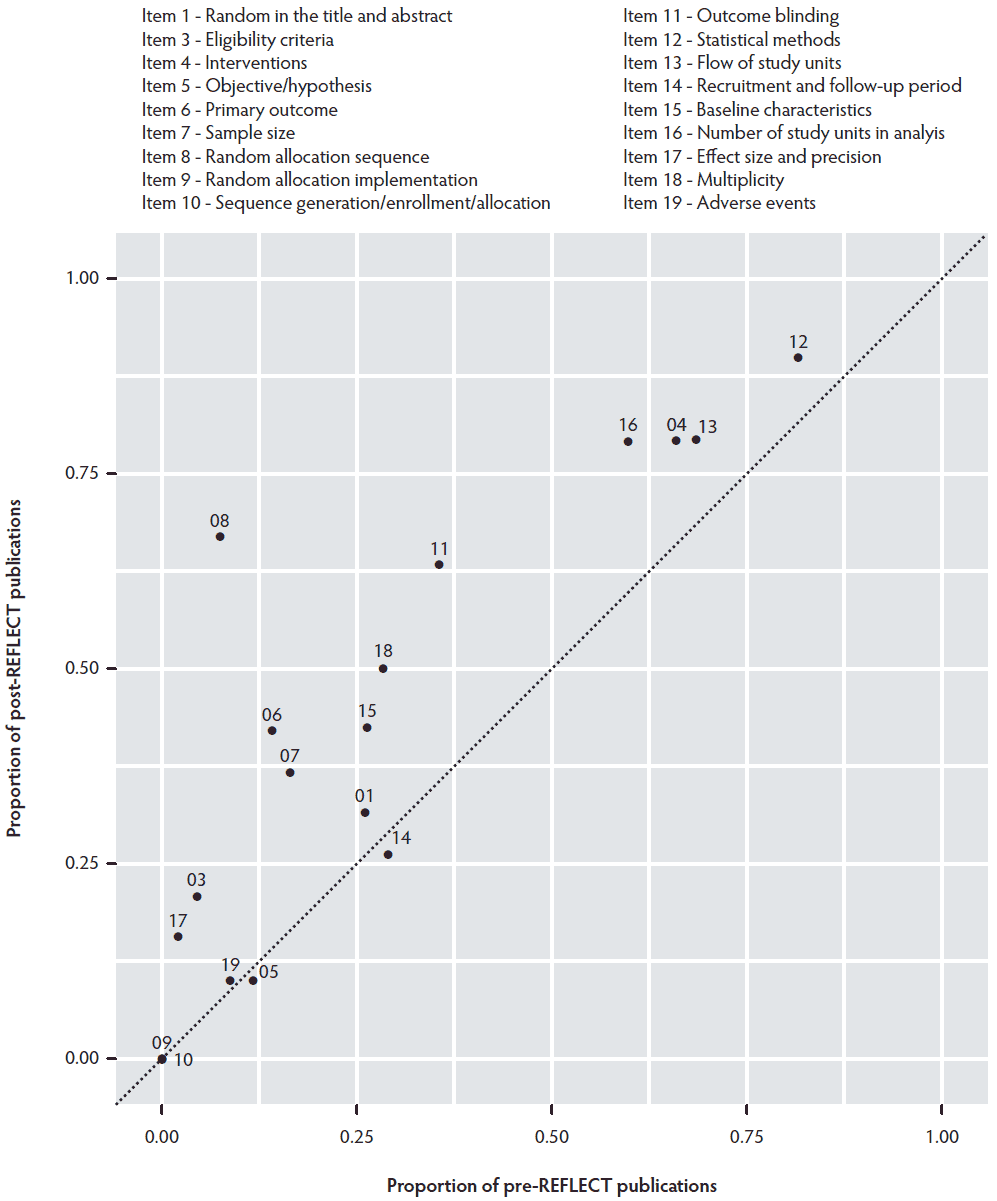
Table 3: Reporting characteristics of swine vaccine challenge studies (item 4B of REFLECT statement) published pre- or post-REFLECT publication.
| Publication period | No. of challenge studies/ Total No. of studies (%) | No. of studies reporting item/ No. of challenge studies (%) |
|---|---|---|
| Pre-REFLECT studies | 21/42 (50) | 5/21 (24) – complete description: organism growth details, route of administration and dose of the organism |
| 13/21 (62) – partial description: route of administration and dose of the organism | ||
| 1/21 (5) – partial description: route of administration | ||
| 2/21 (10) – partial description: seeder pigs | ||
| Post-REFLECT studies | 6/19 (32) | 2/19 (11) – complete description: description of organism growth details, route of administration and dose of the organism |
| 4/19 (21) – route of administration and dose of the organism only |
Aim 4: Risk-of-bias assessment
Of the 61 manuscripts assessed, 5 were cluster-randomized and published before 2011, so there were no cluster-randomized trials identified in the post-REFLECT period. The reporting characteristics of the 61 extracted studies for the risk-of-bias assessments are shown in Table 4. There was an increase in the prevalence of low risk-of-bias studies, based on the randomization process domain, between the post- and pre-REFLECT studies. All the other risk-of-bias domains appeared to be unchanged.
Table 4: The risk-of-bias assessment of the 61 extracted swine vaccine studies published pre- or post-REFLECT publication
| Bias arising from …. | Published pre-REFLECT, No. (n = 42) | Published post-REFLECT, No. (n = 19) | Prevalence ratio* | ||||
|---|---|---|---|---|---|---|---|
| High | Some concerns | Low | High | Some concerns | Low | ||
| Randomization process | 32 | 9 | 1 | 11 | 6 | 2 | 4.42 |
| Deviations from intended interventions | 0 | 2 | 40 | 0 | 0 | 19 | 1.02 |
| Missing outcome data | 2 | 12 | 28 | 1 | 6 | 12 | 0.95 |
| Measurement of the outcome | 3 | 1 | 38 | 1 | 0 | 18 | 1.05 |
| Selection of the reported results | 2 | 40 | 0 | 1 | 18 | 0 | † |
* Prevalence ratio between low risk compared to some concerns and high risk combined.
† Not calculated.
Discussion
One of the main advantages of randomized controlled trials is their ability to reduce confounding, a significant source of bias in the assessment of interventions.88 It is interesting therefore that the prevalence of reporting an allocation method to study units was virtually unchanged (or decreased) in the two publication periods (79% to 74%). However, although the proportion of studies that reported using a random allocation method has decreased, the proportion of studies that reported using a systematic method has increased. This finding also occurred in other veterinary studies.89 Two hypotheses might explain this finding: 1) that there has been a change in the approach to allocation away from random allocation to systematic allocation, and 2) that there has been a change in the language used to report systematic or haphazard allocation approaches in veterinary sciences. Studies that previously described the allocation method as random have changed the description of the method to reflect the actual approach ie, systematic allocation. The first hypothesis suggests that there was no reporting improvement on studies published after REFLECT. The second hypothesis suggests that reporting is improving, if the studies published before 2011 that used systematic or haphazard methods were misreporting or misrepresenting those approaches as random allocation. This latter hypothesis is supported by the increase in the number of studies that provided evidence for the designation of random allocation from 8% to 66%.
We attempted to identify in veterinary clinical trial texts where the concept of systematic or alternative allocation arose, and we cannot trace its origin. One study we are aware of discusses and recommends the use of alternative approaches as being equivalent to random allocation and practical, ie, every-other-calf or odd-and-even number schemes.90 The authors suggested that valid random allocation is impractical in field settings and alternation could serve as a practical method under field conditions while still controlling confounding bias. We were not able to identify similar advice for swine studies, although apparently this approach is used commonly. What is unknown is if, and under what circumstances, a systematic allocation approach is an adequate replacement for random allocation. We were unable to find empirical evidence for this assumption.90
Another interesting finding is the number of studies that reported using a random allocation approach while providing no support for this statement. Although the percentage of studies reporting a random allocation approach was higher before 2011, most of those studies did not report details of the randomization process (23 of 25). The majority of studies (4 of 6) reporting a random allocation approach between 2011 and 2017 provided some information to support the randomization process. This finding suggests improved reporting.
For Aim 3, the results show an increase in the prevalence of reporting most of the REFLECT items and suggest that the overall reporting of swine intervention trials has improved. It is less clear whether improved reporting has translated into lower risk-of-bias. Although the risk-of-bias due to the randomization process appears to have decreased, the other risk-of-bias domains were unchanged. Even for the randomization process ROB domain, the evidence is poor because the low risk-of-bias was based on 2 studies published between 2011 and 2017 and 1 study before 2011.
Additional information is needed to determine if the increased reported use of systematic allocation is based on the tendencies of the industry and is, therefore, unlikely to change. It is also necessary to establish the true benefit of randomized over systematic allocation methods to determine if it is essential to use truly random approaches.91 One approach would be to assess if there are differences in effect sizes in systematically allocated versus randomly allocated studies. Arguing against the need for such evidence is the fact that proper randomization to group is the established standard for intervention trials and the basis for inference. It is also not currently feasible to obtain empirical evidence that allocation concealment is associated with bias as there are too few studies that include this component for comparison to be made. Further, it is hard to envision veterinary schools and graduate programs teaching study design approaches that are not acceptable at the federal level for registration of drugs or vaccines, especially as so many livestock veterinarians are employed by the pharmaceutical and biologics industry to conduct trials. However, if evidence were found that some design elements identified by Cochrane ROB 2.0 are not relevant to livestock studies this would not be unprecedented. In human health, some groups have reported that some Cochrane risks-of-bias domains appear not to be related to empirical evidence of bias.92
Although reporting has improved, there remains room for improvement on all REFLECT items, since none of them were reported by all papers. However, it is unclear what would be the best way to make this improvement occur. The journals that published the studies all endorse the use of the REFLECT statement; however, none require a checklist be submitted or require that reviewers use REFLECT to assess the studies. Even if these journals did require that submitting authors include a completed reporting checklist there is no evidence that such an approach would improve reporting.93 We would propose that several next steps are needed. It is essential that increased education efforts in veterinary schools, graduate programs, and groups involved in post-graduation professional development such as the American Association of Swine Veterinarians raise awareness of the value of improved reporting to veterinarians, especially as prior studies have shown that many editors are unaware of reporting guidelines.94 These efforts will ensure that veterinarians are aware that poor reporting is associated with biased results and that veterinarians can recognize poor reporting. Further, more education of researchers about the obligation to provide stakeholders, including funding groups, veterinarians, and producers, with research reports that comprehensively describe the research is required. Providing comprehensive reports ensures that maximum value is obtained from the human and financial capital investment made in research studies. Also, very importantly, if the basic premise of the call for improved reporting is disputed, we would strongly support that such evidence be included in the peer-reviewed literature so that the role of comprehensive research reporting can be properly discussed among scientists and stakeholders.
Implications
- Substantially more studies are reporting the use of systematic allocation methods, and it is unclear if such an approach adequately ensures exchangeable groups.
- The prevalence of reporting a random allocation method decreased between the pre- and post-REFLECT studies; however, the prevalence of evidence to support a claim that valid random allocation was used has increased.
- The prevalence of reporting most REFLECT items increased between the pre- and post-REFLECT publication periods.
- The prevalence of low risk-of-bias due to the allocation approach might have increased between the pre- and post-REFLECT publication periods. Other risk-of-bias domains appear unchanged.
Acknowledgments
Contributions
Dr Moura contributed to the development of the study concept, the risk-of-bias modifications, performed data extraction, risk-of-bias assessment, data analysis, and preparation of manuscript drafts. Dr Totton performed data extraction, risk-of-bias assessment, and provided feedback on drafts of the manuscript. Dr Linhares contributed to the development of the study concept and approved the final report. Dr Sargeant contributed to the development of the study concept, the risk-of-bias modifications, and approved the final report. Dr O’Sullivan contributed to the development of the study concept, the risk-of-bias modifications, and approved the final report. Dr O’Connor contributed to the development of the study concept, the risk-of-bias modifications, data analysis, preparation of manuscript drafts, and approved the final report.
Declarations
Ethical approval for animal use or human subjects was not required for this project. All preplanned results are reported and modifications from the protocol are reported.
Funding
No external sources of funding were used for this project.
Conflict of interest
Drs O’Connor and Sargeant are co-authors of the REFLECT statement. Drs Moura, Linhares, Totton, and O’Sullivan have no conflicts of interest to declare.
Disclaimer
Scientific manuscripts published in the Journal of Swine Health and Production are peer reviewed. However, information on medications, feed, and management techniques may be specific to the research or commercial situation presented in the manuscript. It is the responsibility of the reader to use information responsibly and in accordance with the rules and regulations governing research or the practice of veterinary medicine in their country or region.
References
*1. USDA Risk Management Agency. Study on Swine Catastrophic Disease. https://legacy.rma.usda.gov/pubs/2015/swinedisease.pdf. Published 2015. Accessed July 22, 2019.
2. Schroeder S, Harries M, Prager R, Höfig A, Ahrens B, Hoffmann L, Rabsch W, Mertens E, Rimek D. A prolonged outbreak of Salmonella Infantis associated with pork products in central Germany, April-October 2013. Epidemiol Infect. 2016;144(7):1429-1439.
3. Kuhn KG, Sorensen G, Torpdahl M, Kjeldsen MK, Jensen T, Gubbels S, Bjerager GO, Wingstrand A, Porsbo LJ, Ethelberg S. A long-lasting outbreak of Salmonella Typhimurium U323 associated with several pork products, Denmark, 2010. Epidemiol Infect. 2013;141(2):260-268.
4. Gossner CM, van Cauteren D, Le Hello S, Weill FX, Terrien E, Tessier S, Janin C, Brisabois A, Dusch V, Vaillant V, Jourdan-da Silva N. Nationwide outbreak of Salmonella enterica serotype 4,[5],12:i:-infection associated with consumption of dried pork sausage, France, November to December 2011. Euro Surveill. 2012;17(5):pii:20071.
5. Brace S, Taylor D, O’Connor AM. The quality of reporting and publication status of vaccines trials presented at veterinary conferences from 1988 to 2003. Vaccine. 2010;28(32):5306-5314.
6. O’Connor AM, Sargeant JM, Gardner IA, Dickson JS, Torrence ME, Dewey CE, Dohoo IR, Evans RB, Gray JT, Greiner M, Keefe G, Lefebvre SL, Morley PS, Ramirez A, Sischo W, Smith DR, Snedeker K, Sofos JN, Ward MP, Wills R, Consensus Meeting Participants. The REFLECT statement: methods and processes of creating reporting guidelines for randomized controlled trials for livestock and food safety. J Food Prot. 2010;73(1):132-139.
7. O’Connor AM, Sargeant JM, Gardner IA, Dickson JS, Torrence ME, Dewey CE, Dohoo IR, Evans RB, Gray JT, Greiner M, Keefe G, Lefebvre SL, Morley PS, Ramirez A, Sischo W, Smith DR, Snedeker K, Sofos J, Ward MP, Wills R, Steering Committee. The REFLECT statement: methods and processes of creating reporting guidelines for randomized controlled trials for livestock and food safety. J Vet Intern Med. 2010;24(1):57-64.
8. O’Connor AM, Sargeant JM, Gardner IA, Dickson JS, Torrence ME, Dewey CE, Dohoo IR, Evans RB, Gray JT, Greiner M, Keefe G, Lefebvre SL, Morley PS, Ramirez A, Sischo W, Smith DR, Snedeker K, Sofos J, Ward MP, Wills R. The REFLECT statement: methods and processes of creating reporting guidelines for randomized controlled trials for livestock and food safety. Prev Vet Med. 2010;93(1):11-18.
9. O’Connor AM, Sargeant JM, Gardner IA, Dickson JS, Torrence ME, Consensus Meeting Participants, Dewey CE, Dohoo IR, Evans RB, Gray JT, Greiner M, Keefe G, Lefebvre SL, Morley PS, Ramirez A, Sischo W, Smith DR, Snedeker K, Sofos J, Ward MP, Wills R. The REFLECT statement: methods and processes of creating reporting guidelines for randomized controlled trials for livestock and food safety by modifying the CONSORT statement. Zoonoses Public Health. 2010;57(2):95-104.
10. Sargeant JM, O’Connor AM, Gardner IA, Dickson JS, Torrence ME, Dohoo IR, Lefebvre SL, Morley PS, Ramirez A, Snedeker K. The REFLECT statement: reporting guidelines for randomized controlled trials in livestock and food safety: explanation and elaboration. J Food Prot. 2010;73(3):579-603.
11. Sargeant JM, O’Connor AM, Gardner IA, Dickson JS, Torrence ME, Consensus Meeting Participants. The REFLECT statement: reporting guidelines for randomized controlled trials in livestock and food safety: explanation and elaboration. Zoonoses Public Health. 2010;57(2):105-136.
12. Turner L, Shamseer L, Altman DG, Weeks L, Peters J, Kober T, Dias S, Schulz KF, Plint AC, Moher D. Consolidated standards of reporting trials (CONSORT) and the completeness of reporting of randomised controlled trials (RCTs) published in medical journals. Cochrane Database Syst Rev. 2012;11:MR000030.
13. Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG, NC3Rs Reporting Guidelines Working Group. Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol. 2010;160(7):1577-1579.
14. Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG, NC3Rs Reporting Guidelines Working Group. Animal research: reporting in vivo experiments: the ARRIVE guidelines. J Gene Med. 2010;12(7):561-563.
15. Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG, National Centre for the Replacement Refinement and Reduction of Animals in Research. Animal research: reporting in vivo experiments–the ARRIVE guidelines. J Cereb Blood Flow Metab. 2011;31(4):991-993.
16. NC3Rs Reporting Guidelines Working Group. Animal research: reporting in vivo experiments: the ARRIVE guidelines. J Physiol. 2010;588(Pt 14):2519-2521.
17. NC3Rs Reporting Guidelines Working Group. Animal research: reporting in vivo experiments: the ARRIVE guidelines. Exp Physiol. 2010;95(8):842-844.
18. O’Connor AM, Sargeant JM, Dohoo IR, Erb HN, Cevallos M, Egger M, Ersbøll AK, Martin SW, Nielsen LR, Pearl DL, Pfeiffer DU, Sanchez J, Torrence ME, Vigre H, Waldner C, Ward MP. Explanation and elaboration document for the STROBE-Vet statement: Strengthening the reporting of observational studies in epidemiology-veterinary extension. J Vet Intern Med. 2016;30(6):1896-1928.
19. Sargeant JM, O’Connor AM, Dohoo IR, Erb HN, Cevallos M, Egger M, Ersbøll AK, Martin SW, Nielsen LR, Pearl DL, Pfeiffer DU, Sanchez J, Torrence ME, Vigre H, Waldner C, Ward MP. Methods and processes of developing the strengthening the reporting of observational studies in epidemiology – veterinary (STROBE-Vet) statement. Zoonoses Public Health. 2016;63(8):651-661.
20. Sargeant JM, O’Connor AM, Dohoo IR, Erb HN, Cevallos M, Egger M, Ersbøll AK, Martin SW, Nielsen LR, Pearl DL, Pfeiffer DU, Sanchez J, Torrence ME, Vigre H, Waldner C, Ward MP. Methods and processes of developing the strengthening the reporting of observational studies in epidemiology – veterinary (STROBE-Vet) statement. J Vet Intern Med. 2016;30(6):1887-1895.
21. Sargeant JM, O’Connor AM, Dohoo IR, Erb HN, Cevallos M, Egger M, Ersbøll AK, Martin SW, Nielsen LR, Pearl DL, Pfeiffer DU, Sanchez J, Torrence ME, Vigre H, Waldner C, Ward MP. Methods and processes of developing the strengthening the reporting of observational studies in epidemiology – veterinary (STROBE-Vet) statement. Prev Vet Med. 2016;134:188-196.
22. Sargeant JM, O’Connor AM, Dohoo IR, Erb HN, Cevallos M, Egger M, Ersbøll AK, Martin SW, Nielsen LR, Pearl DL, Pfeiffer DU, Sanchez J, Torrence ME, Vigre H, Waldner C, Ward MP. Methods and processes of developing the strengthening the reporting of observational studies in epidemiology-veterinary (STROBE-Vet) statement. J Food Prot. 2016;79(12):2211-2219.
23. Totton SC, Cullen JN, Sargeant JM, O’Connor AM. The reporting characteristics of bovine respiratory disease clinical intervention trials published prior to and following publication of the REFLECT statement. Prev Vet Med. 2018;150:117-125.
*24. O’Connor A, Moura C, Totton S, O’Sullivan T, Linhares D, Sargeant J. The reporting characteristics of swine intervention trials published prior to and following publication of the REFLECT statement. https://osf.io/7qu8h/. Published October 9, 2017. Updated January 28, 2019. Accessed July 22, 2019.
25. Higgins J, Sterne JA, Savović J, Page MJ, Hróbjartsson A, Boutron I, Reeves B, Eldridge S. A revised tool for assessing risk of bias in randomized trials. In: Chandler J, Clarke M, McKenzie J, Boutron I, Welch V, eds. Cochrane Methods. Cochrane Database of Systematic Reviews. 2016;10 (Suppl 1).:29-31. doi:10.1002/14651858.CD201601
26. Kahan BC, Rehal S, Cro S. Risk of selection bias in randomised trials. Trials. 2015;16:405.
27. Campbell TA, Garcia MR, Miller LA, Ramirez MA, Long DB, Marchand J-B, Hill F. Immunocontraception in male feral swine treated with a recombinant gonadotropin-releasing hormone vaccine. J Swine Health Prod. 2010;18(3):118-124.
28. King D, Painter T, Holtkamp D, DuBois P, Wang C. Effect of injection tool on incidence of head and neck abscesses at slaughter. J Swine Health Prod. 2010;18(6):290-293.
29. Husa JA, Edler RA, Walter DH, Holck JT, Saltzman RJ. A comparison of the safety, cross-protection, and serologic response associated with two commercial oral Salmonella vaccines in swine. J Swine Health Prod. 2009;17(1):10-21.
30. Desrosiers R, Clark E, Tremblay D, Tremblay R, Polson D. Use of a one-dose subunit vaccine to prevent losses associated with porcine circovirus type 2. J Swine Health Prod. 2009;17(3):148-154.
31. Schmoll F, Kauffold J, Pfützner A, Baumgartner J, Brock F, Grodzycki M, Andrews S. Growth performance and carcass traits of boars raised in Germany and either surgically castrated or vaccinated against gonadotropin-releasing hormone. J Swine Health Prod. 2009;17(5):250-255.
32. Najdenski H, Golkocheva-Markova E, Kussovski V, Vesselinova A, Garbom S, Wolf-Watz H. Attenuation and preserved immunogenic potential of Yersinia pseudotuberculosis mutant strains evidenced in oral pig model. Zoonoses Public Health. 2009;56(4):157-168.
33. Rapp-Gabrielson V, Hoover T, Sornsen S, Kesl L, Taylor L, Jolie R, Runnels P, Weigel D, Yu S, Opriessnig T, Ruebling-Jass K, Strait E, Halbur PG. Effects of Mycoplasma hyopneumoniae vaccination in pigs co-infected with M hyopneumoniae and porcine circovirus type 2. J Swine Health Prod. 2008;16(1):16-26.
34. Jirawattanapong P, Stockhofe-Zurwieden N, van Leengoed L, Binnendijk G, Wisselink HJ, Taymakers R, Cruijsen T, van der Peet-Schwering C, van Nes A, Nielen M. Efficacy of a subunit vaccine against Actinobacillus pleuropneumoniae in an endemically infected swine herd. J Swine Health Prod. 2008;16(4):193-199.
35. Strait E, Rapp-Gabrielson V, Erickson B, Evans RB, Taylor LP, Yonkers TK, Keich RL, Jolie R, Thacker EL. Efficacy of a Mycoplasma hyopneumoniae bacterin in pigs challenged with two contemporary pathogenic isolates of M hyopneumoniae. J Swine Health Prod. 2008;16(4):200-206.
36. Fangman TJ, Kleiboeker SB, Coleman M. Tonsilar crypt exudate to evaluate shedding and transmission of porcine reproductive and respiratory syndrome virus after inoculation with live field virus or vaccination with modified live virus vaccine. J Swine Health Prod. 2007;15(4):219-223.
37. Thomas PJ, Opriessnig T, Juhan NM, Meng XJ, Halbur PG. Planned exposure to porcine circovirus type 2 by serum injection is not effective at preventing porcine circovirus associated disease. J Swine Health Prod. 2007;15(6):330-338.
38. Allan GM, Caprioli A, McNair I, Lagan-Tregaskis P, Ellis J, Krakowka S, McKillen J, Ostanello F, McNeilly F. Porcine circovirus 2 replication in colostrum-deprived piglets following experimental infection and immune stimulation using a modified live vaccine against porcine respiratory and reproductive syndrome virus. Zoonoses Public Health. 2007;54(5):214-222.
39. Hoogland MJ, Opriessnig T, Halbur PG. Effects of adjuvants on porcine circovirus type 2-associated lesions. J Swine Health Prod. 2006;14(3):133-139.
40. Holyoake PK, Callinan APL. How effective is Mycoplasma hyopneumoniae vaccination in pigs less than three weeks of age? J Swine Health Prod. 2006;14(4):189-195.
41. Jones GF, Rapp-Gabrielson V, Wilke R, Thacker EL, Thacker BJ, Gergen L, Sweeney D, Wasmoen T. Intradermal vaccination for Mycoplasma hyopneumoniae. J Swine Health Prod. 2005;13(1):19-27.
42. Opriessnig T, Pallarés FJ, Nilubol D, Vincent AL, Thacker EL, Vaughn EM, Roof M, Halbur PG. Genomic homology of ORF 5 gene sequence between modified live vaccine virus and porcine reproductive and respiratory syndrome virus challenge isolates is not predictive of vaccine efficacy. J Swine Health Prod. 2005;13(5):246-253.
43. Loynachan AT, Nugent JM, Erdman MM, Harris DL. Acute infection of swine by various Salmonella serovars. J Food Prot. 2004;67(7):1484-1488.
44. Hodgins DC, Shewen PE, Dewey CE. Influence of age and maternal antibodies on antibody responses of neonatal piglets vaccinated against Mycoplasma hyopneumoniae. J Swine Health Prod. 2004;12(1):10-16.
45. Chernysheva L, Friendship R, Dewey C, Gyles C. The effect of dietary chicken egg-yolk antibodies on the clinical response in weaned pigs challenged with a K88+ Escherichia coli isolate. J Swine Health Prod. 2004;12(3):119-122.
46. Oliveira S, Pijoan C, Morrison R. Evaluation of Haemophilus parasuis control in the nursery using vaccination and controlled exposure. J Swine Health Prod. 2004;12(3):123-128.
47. Dewulf J, Laevens H, Koenen F, Mintiens K, de Kruif A. Efficacy of E2-sub-unit marker and C-strain vaccines in reducing horizontal transmission of classical swine fever virus in weaner pigs. Prev Vet Med. 2004;65(3/4):121-133.
48. Dewey CE, Wilson S, Buck P, Leyenaar JAK. Effects of porcine reproductive and respiratory syndrome vaccination in breeding-age animals. Prev Vet Med. 2004;62(4):299-307.
49. Martens M, Rosales C, Morilla A. Evaluation of the use of a subunit classical swine fever marker vaccine under field conditions in Mexico. J Swine Health Prod. 2003;11(2):81-85.
50. Ruiz AR, Utrera V, Pijoan C. Effect of Mycoplasma hyopneumoniae sow vaccination on piglet colonization at weaning. J Swine Health Prod. 2003;11(3):131-135.
51. Liao C, Chiou H, Yeh K, Chen J, Weng C. Oral immunization using formalin-inactivated Actinobacillus pleuropneumoniae antigens entrapped in microspheres with aqueous dispersion polymers prepared using a co-spray drying process. Prev Vet Med. 2003;61(1):1-15.
52. Boettcher T, Thacker B, Halbur P, Waters W, Nutsch R, Thacker E. Vaccine efficacy and immune response to Mycoplasma hyopneumoniae challenge in pigs vaccinated against porcine reproductive and respiratory syndrome virus and M hyopneumoniae. J Swine Health Prod. 2002;10(6):259-264.
53. Charles SD, Abraham AS, Trigo ET, Jones GF, Settje TL. Reduced shedding and clinical signs of Salmonella Typhimurium in nursery pigs vaccinated with a Salmonella Choleraesuis vaccine. Swine Health Prod. 2000;8(3):107-112.
54. Benson JE, Yaeger MJ, Lager KM. Effect of porcine reproductive and respiratory syndrome virus (PRRSV) exposure dose on fetal infection in vaccinated and nonvaccinated swine. Swine Health Prod. 2000;8(4):155-160.
55. Amass SF, Stevenson GW, Vyverberg BD, Huxford TW, Knox KE, Grote LA. Administration of a homologous bacterin to sows prefarrowing provided partial protection against streptococcosis in their weaned pigs. Swine Health Prod. 2000;8(5):217-219.
56. Wongnarkpet S, Pfeiffer DU, Morris RS, Fenwick SG. An on-farm study of the epidemiology of Actinobacillus pleuropneumoniae infection in pigs as part of a vaccine efficacy trial. Prev Vet Med. 1999;39(1):1-11.
57. Wongnarkpet S, Morris RS, Pfeiffer DU. Field efficacy of a combined use of Mycoplasma hyopneumoniae and Actinobacillus pleuropneumoniae vaccines in growing pigs. Prev Vet Med. 1999;39(1):13-24.
58. Diekman MA, Scheidt AB, Grant AL, Kelly DT, Sutton AL, Martin TG, Cline TR. Effect of vaccination against Mycoplasma hyopneumoniae on health, growth, and pubertal status of gilts exposed to moderate ammonia concentrations in all-in-all-out versus continuous-flow systems. Swine Health Prod. 1999;7(2):55-61.
59. Sornsen SA, Zimmerman JJ, Polson DD, Roof MB. Effect of PRRS vaccination on average daily gain: a comparison of intranasal and intranasal-intramuscular administration. Swine Health Prod. 1998;6(1):13-19.
60. Thacker EL, Thacker BJ, Boettcher TB, Jayappa H. Comparison of antibody production, lymphocyte stimulation, and protection induced by four commercial Mycoplasma hyopneumoniae bacterins. Swine Health Prod. 1998;6(3):107-112.
61. Drum SD, Walker RD, Marsh WE, Mellencamp MM, King VL. Growth performance of segregated early-weaned versus conventionally weaned pigs through finishing. Swine Health Prod. 1998;6(5):203-210.
62. Torremorell M, Pijoan C, Trigo E. Vaccination against Streptococcus suis: effect on nursery mortality. Swine Health Prod. 1997;5(4):139-143.
63. Papatsas IC, Kyriakis SC, Papadopoulos O, Sarris KJ, Lekkas S. Intradermal vaccination against pseudorabies virus and swine influenza in growing/finishing pigs. Swine Health Prod. 1996;4(6):279-285.
64. Schinckel AP, Clark LK, Stevenson G, Knox KE, Nielsen J, Grant AL, Hancock DL, Turek J. Effects of antigenic challenge on growth and composition of segregated early-weaned pigs. Swine Health Prod. 1995;3(6):228-234.
65. Swenson SL, Hill HT, Zimmerman JJ, Evans LE, Wills RW, Yoon K-J, Schwartz KJ, Althouse GC, McGinley MJ, Brevik AK. Preliminary assessment of an inactivated PRRS virus vaccine on the excretion of virus in semen. Swine Health Prod. 1995;3(6):244-247.
66. Scheidt AB, Mayrose VB, van Alstine WG, Clark LK, Cline TR, Einstein ME. The effects of vaccinating pigs for mycoplasmal pneumonia in a swine herd affected by enzootic pneumonia. Swine Health Prod. 1994;2(1):7-11.
67. Morrow WEM, Iglesias G, Stanislaw C, Stephenson A, Erickson G. Effect of a mycoplasma vaccine on average daily weight gain in swine. Swine Health Prod. 1994;2(6):13-18.
68. Nabuurs MJA, Bokhout BA, van der Heijden PJ. Intraperitoneal injection of an adjuvant for the prevention of post-weaning diarrhea and oedema disease in piglets: a field study. Prev Vet Med. 1982;1(1):65-76.
69. Nielsen GB, Nielsen JP, Haugegaard J, Denwood MJ, Houe H. Effect of vaccination against sub-clinical porcine circovirus type 2 infection in a high-health finishing pig herd: a randomised clinical field trial. Prev Vet Med. 2017;141:14-21.
70. Kang I, Kang H, Jeong J, Park C, Choi K, Park S-J, Sung HJ, Park EK, Oh B, Kim S-H, Chae C. Comparison of growth performance under field conditions in growing pigs each vaccinated with one of two commercial modified-live porcine reproductive and respiratory syndrome vaccines. J Swine Health Prod. 2017;25(1):24-28.
71. Jeong J, Kang H, Park C, Seo HW, Kang I, Choi K, Chae C. Comparative efficacy of concurrent administration of a porcine circovirus type 2 (PCV2) vaccine plus a porcine reproductive and respiratory syndrome virus (PRRSV) vaccine from two commercial sources in pigs challenged with both viruses. J Swine Health Prod. 2016;24(3):130-141.
72. O’Sullivan TL, Johnson R, Poljak Z, Gu Y, DeLay J, Friendship RM. An experimental study with a vaccine strain of porcine reproductive and respiratory syndrome virus to determine effects on viremia assessed by reverse transcriptase-polymerase chain reaction in pigs fed rations medicated with tilmicosin or non-medicated. J Swine Health Prod. 2016;24(2):81-92.
73. Young MG, Cunningham GL, Sanford SE. Circovirus vaccination in pigs with subclinical porcine circovirus type 2 infection complicated by ileitis. J Swine Health Prod. 2011;19(3):175-180.
74. Scherba G, Bromfield CR, Jarrell VL, Shipley CF. Evaluation of responses to both oral and parenteral immunization modalities for porcine epidemic diarrhea virus in production units. J Swine Health Prod. 2016;24(1):29-35.
75. Palzer A, Eddicks M, Zoels S, Stark J, Reese S, Strutzberg-Minder K, Fiebig K, Ritzmann M. Field evaluation of the efficacy, compatibility and serologic profiling of a combined vaccine against porcine reproductive and respiratory syndrome and Haemophilus parasuis in nursery pigs. Prev Vet Med. 2015;119(3/4):134-140.
76. Fraile L, Segalés J, Ticó G, López-Soria S, Valero O, Nofrarias M, Huerta E, Llorens A, López-Jiménez R, Pérez D, Sibila M. Virological and serological characterization of vaccinated and non-vaccinated piglet subpopulations coming from vaccinated and non-vaccinated sows. Prev Vet Med. 2015;119(3-4):153-161.
77. Seo H, Lee J, Park C, Kim HJ, Kwak T-K, Kim S-H, Chae C. Comparison of commercial one-dose and two-dose baculovirus-expressed porcine circovirus type 2 subunit vaccines. J Swine Health Prod. 2014;22(6):291-295.
78. Linhares DCL, Cano JP, Torremorell M, Morrison RB. Comparison of time to PRRSv-stability and production losses between two exposure programs to control PRRSv in sow herds. Prev Vet Med. 2014;116(1-2):111-119.
79. Scheid IR, Oliveira FTT Jr, Borges AC, Braga TF, Soncini RA, Mathur S, Allison JR, Hennessy DP. A single dose of a commercial anti-gonadotropin releasing factor vaccine has no effect on testicular development, libido, or sperm characteristics in young boars. J Swine Health Prod. 2014;22(4):185-192.
80. Hillen S, von Berg S, Kohler K, Reinacher M, Willems H, Reiner G. Occurrence and severity of lung lesions in slaughter pigs vaccinated against Mycoplasma hyopneumoniae with different strategies. Prev Vet Med. 2014;113(4):580-588.
81. Beckler DC, Segal MU, Weiss DL, Nimmo RD, Guggenbiller DJ. Virginiamycin: lack of interference with Lawsonia intracellularis immunization. J Swine Health Prod. 2013;21(5):253-260.
82. Baker SR, Mondaca E, Polson D, Dee SA. Evaluation of a needle-free injection device to prevent hematogenous transmission of porcine reproductive and respiratory syndrome virus. J Swine Health Prod. 2012;20(3):123-128.
83. Shen HG, Loiacono CM, Halbur PG, Opriessnig T. Age-dependent susceptibility to porcine circovirus type 2 infections is likely associated with declining levels of maternal antibodies. J Swine Health Prod. 2012;20(1):17-24.
84. Potter ML, Tokach LM, Dritz SS, Henry SC, DeRouchey JM, Tokach MD, Goodband RD, Nelssen JL, Rowland RRR, Hesse RA, Oberst R, Anderson J, Hays M. Genetic line influences pig growth rate responses to vaccination for porcine circovirus type 2. J Swine Health Prod. 2012;20(1):34-43.
85. Venegas-Vargas MC, Bates R, Morrison R, Villani D, Straw B. Effect of porcine circovirus type 2 vaccine on postweaning performance and carcass composition. J Swine Health Prod. 2011;19(4):233-237.
86. Jacela JY, Dritz SS, DeRouchey JM, Tokach MD, Goodband RD, Nelssen JL. Field evaluation of the effects of a porcine circovirus type 2 vaccine on finishing pig growth performance, carcass characteristics, and mortality rate in a herd with a history of porcine circovirus-associated disease. J Swine Health Prod. 2011;19(1):10-18.
87. Fangman TJ, Johnson AK, Okones J, Edler RA. Willingness-to-approach behavior of weaned pigs after injection with Mycoplasma hyopneumoniae vaccines. J Swine Health Prod. 2011;19(1):19-25.
88. Dohoo IR. The design of randomized controlled trials of veterinary vaccines. Anim Health Res Rev. 2004;5(2):235-238.
89. Yuan C, Krull A, Wang C, Erdman M, Fedorka-Cray PJ, Logue CM, O’Connor AM. Changes in the prevalence of Salmonella serovars associated swine production and correlations of avian, bovine and swine-associated serovars with human-associated serovars in the United States (1997-2015). Zoonoses Public Health. 2018;65(6):648-661.
90. Perino LJ, Apley MD. Clinical trial design in feedlots. Vet Clin North Am Food Anim Pract. 1998;14(2):343-365.
91. Mansournia MA, Higgins JP, Sterne JA, Hernan MA. Biases in randomized trials: A conversation between trialists and epidemiologists. Epidemiology. 2017;28(1):54-59.
92. Bolvig J, Juhl CB, Boutron I, Tugwell P, Ghogomu EAT, Pardo JP, Rader T, Wells GA, Mayhew A, Maxwell L, Lund H, Bliddal H, Christensen R, Editorial Board of the Cochrane Musculoskeletal Group. Some Cochrane risk-of-bias items are not important in osteoarthritis trials: a meta-epidemiological study based on Cochrane reviews. J Clin Epidemiol. 2018;95:128-136.
93. Hair K, Macleod MR, Sena ES. A randomised controlled trial of an intervention to improve compliance with the ARRIVE guidelines (IICARus). Res Integr Peer Rev. 2019;4:12.
94. Grindlay DJ, Dean RS, Christopher MM, Brennan ML. A survey of the awareness, knowledge, policies and views of veterinary journal Editors-in-Chief on reporting guidelines for publication of research. BMC Vet Res. 2014;10:10.
* Non-refereed references.