| Original research | Peer reviewed |
Cite as: Brown J, Rademacher C, Baker S, et al. Efficacy of a commercial porcine epidemic diarrhea virus vaccine at reducing duration of viral shedding in gilts. J Swine Health Prod. 2019;27(5):256-264. https://doi.org/10.54846/jshap/1127
Also available as a PDF.
SummaryObjective: To evaluate if the use of a commercially available killed porcine epidemic diarrhea virus (PEDV) vaccine shortens the duration of PEDV shedding in replacement gilts. Materials and methods: Four treatment groups composed of 20 females were utilized for this study. Gilts in the CONTROL group had no previous exposure to PEDV, the nonvaccinated (NV) group had been previously exposed, and the PRE and POST groups received two doses of a commercial, killed PEDV vaccine either prior to or following a challenge with PEDV, respectively. Individual fecal samples were collected weekly and tested by real-time reverse transcription-polymerase chain reaction (rRT-PCR) for virus detection. Results: Previous exposure to PEDV was found to shorten the time that virus can be detected in the feces compared to fecal samples of naïve animals (P < .001). Vaccination, either prior to or following the challenge, was not found to shorten the duration of PEDV shedding in feces. Implications: These results showed that vaccination of gilts, either prior to the challenge or afterwards, with a killed commerical PEDV vaccine did not shorten the period that virus was detectable in the feces by rRT-PCR suggesting that viral shedding in feces was not influenced by administration of a killed commercial vaccine. While previous infection with virulent PEDV did not prevent re-infection, it did have a significant effect on the amount of time virus was detected following a subsequent exposure. | ResumenObjetivo: Evaluar si el uso de una vacuna inactivada del virus de la diarrea epidémica porcina (PEDV, por sus siglas en inglés) disponible comercialmente acorta la duración de la excreción del PEDV en cerdas primerizas. Materiales y métodos: Se utilizaron cuatro grupos de tratamiento formados por 20 hembras para este estudio. Las primerizas en el grupo CONTROL no tenían exposición previa al PEDV, el grupo no vacunado (NV) había sido expuesto previamente, y los grupos PRE y POST recibieron dos dosis de una vacuna inactivada comercial del PEDV antes o después del reto con el PEDV, respectivamente. Las muestras fecales individuales se recolectaron semanalmente y se analizaron mediante reacción en cadena de la polimerasa de transcripción inversa en tiempo real (rRT-PCR) para la detección de virus. Resultados: Se observó que la exposición previa al PEDV acorta el tiempo en que el virus se puede detectar en las heces en comparación con las muestras fecales de animales no expuestos (P < .001). No se encontró que la vacunación, ya sea antes o después del desafío, acorte la duración de la eliminación del PEDV en las heces. Implicaciones: Estos resultados mostraron que la vacunación de primerizas, ya sea antes o después de la exposición, con una vacuna inactivada comercial del PEDV no acorta el período en que el virus se detecta en las heces mediante la rRT-PCR, lo que sugiere que la excreción viral en las heces no fue influida por la administración de una vacuna inactivada comercial. Mientras que la infección previa con el PEDV virulento no previno la reinfección, si tuvo un efecto significativo en el tiempo en que se detectó el virus después de una exposición posterior. | ResuméObjectif: Évaluer si l’utilisation d’un vaccin tué commercialement disponible contre le virus de la diarrhée épidémique porcine (VDEP) raccourci la durée d’excrétion du VDEP chez des cochettes de remplacement. Matériels et méthodes: Quatre groupes de traitement composés de 20 femelles furent utilisés pour cette étude. Les cochettes du groupe TÉMOIN n’avaient pas eu d’exposition préalable au VDEP, le groupe non-vacciné (NV) avait préalablement été exposé, et les groupes PRE et POST reçurent deux doses d’un vaccin commercial de VDEP tué soit avant ou à la suite d’une infection défi avec le VDEP, respectivement. Des échantillons fécaux individuels furent obtenus à chaque semaine et testés pour détecter le virus par réaction en temps réel d’amplification en chaîne avec la polymérase reverse (rRT-PCR). Résultats: On nota qu’une exposition préalable au VDEP raccourcissait le temps que le virus pouvait être détecté dans les fèces comparativement aux échantillons fécaux des animaux naïfs (P < .001). La vaccination, soit avant ou après l’infection défi, n’a pas permis de réduire la durée d’excrétion du VDEP dans les fèces. Implications: Ces résultats démontrent que la vaccination des cochettes, soit avant ou après une infection défi, avec un vaccin tué commercial contre le VDEP n’a pas raccourci la période que le virus était détectable dans les fèces par rRT-PCR, ce qui suggère que l’excrétion virale dans les fèces n’était pas influencée par l’administration d’un vaccin tué commercial. Bien qu’une infection préalable avec un VDEP virulent n’ait pas empêché une réinfection, elle avait un effet significatif sur la durée pendant laquelle le virus était détecté suite à une exposition subséquente. |
Keywords: swine, porcine epidemic diarrhea virus, vaccine, acclimatization, shedding, PEDV
Search the AASV web site
for pages with similar keywords.
Received: December 4, 2018
Accepted: April 4, 2019
During May 2013, porcine epidemic diarrhea virus (PEDV) was diagnosed in an acute outbreak of diarrhea and vomiting affecting most sows and nearly 100% of piglets on a commercial breeding farm in the United States.1 Nearly 100% of affected piglets died due to extreme dehydration secondary to the disease during the first 4 weeks of the outbreak. This was the first time PEDV was detected in the United States. Breeding farms have recovered after intentional herd exposure to the virus, allowing herd immunity2 to develop, in addition to the use of sanitation protocols to control the virus.
For breeding herds previously infected with PEDV, some producers have chosen to acclimate their replacement gilts off-site prior to introduction to the herd. If gilts are introduced to a breeding herd too soon after intentional PEDV exposure, there is a risk that the animals will be actively shedding the virus. Exposed gilts could serve as a vector for PEDV and re-infect the resident sow and piglet populations, leading to clinical disease.
Commercially available PEDV vaccines effectively increase antibody levels developed from natural exposure.3-5 However, killed vaccines have not shown to produce protective immunity against clinical disease in PEDV-naïve animals.5,6 Acclimating replacement gilts with PEDV and allowing them the proper period to cease shedding has anecdotally been reported as a successful strategy for introducing replacement females into previously infected herds. Bjustrom-Kraft et al7 examined the duration of shedding in commercial wean-to-finish pigs and found positive fecal swab and oral fluid samples collected at the pen level at 69 days post PEDV exposure. This information could be extrapolated to suggest that gilts should be isolated for a minimum of 10 weeks before introduction to the herd, but direct measurement of replacement gilts would be preferable. Given that gilt acclimation is time dependent and has associated costs, the opportunity for a commercially available vaccine to reduce the duration of PEDV shedding, thereby reducing the time needed for acclimation, would be a valuable resource to producers.
Our hypothesis was that gilts vaccinated with a killed PEDV vaccine would shed virus in feces for a shorter duration than unvaccinated gilts. Therefore, the objective of this study was to evaluate if the use of a commercially available killed PEDV vaccine (Porcine Epidemic Diarrhea Vaccine, Zoetis, Inc, Florham Park, New Jersey) influences the duration of PEDV shedding in replacement gilts, which would subsequently shorten the time that intentionally infected replacement gilts must be isolated before introduction into a breeding herd.
Materials and Methods
All procedures were approved by the Iowa State University Animal Care and Use Committee.
This study utilized 4 treatment groups (Table 1), each composed of 20, commercial crossbred, PEDV naïve gilts. Sixty gilts were conveniently selected from a commercial producer located in central Iowa that had no clinical or diagnostic history of PEDV infection. The 60 gilts were evenly split into 3 groups each composed of 20 females. Twenty naïve gilts, (CONTROL), were moved to an isolated research facility while the remaining 40 stayed at the farm of origin. At the research site, an ear tag (Integra Hog, Allflex, Dallas, Texas) was placed in the right ear of each gilt for individual identification and 12 mL of blood was collected via jugular venipuncture utilizing a 16 gauge, 1.5-inch needle and syringe. Serum samples were tested with a whole virus enzyme-linked immunosorbent assay (ELISA) developed at the Iowa State University Veterinary Diagnostic Laboratory (VDL) to confirm PEDV naïve status prior to the challenge. Following a 4-day acclimation period, each gilt was challenged orally with PEDV. A tissue homogenate of PEDV was obtained from a confirmed, clinical outbreak of PEDV, which had been collected on farm and frozen at -80° C. Ten milliliters of the homogenate were mixed with 590 mL of phosphate buffered saline and 30 mL were administered oronasally to each gilt. The final diluted inoculum was confirmed to be PEDV positive by real-time, reverse transcriptase-polymerase chain reaction (rRT-PCR) with a cycle threshold (Ct) value of 19.6 and identified as the prototype strain of PEDV by virus isolation and sequencing performed at the VDL. Following the challenge, individual fecal samples were collected from each gilt utilizing a fecal loop (VETONE, Boise, Idaho) every 7 days and submitted to the VDL for PEDV rRT-PCR testing. Individual fecal samples were collected every 7 days until a gilt had 3 consecutive negative rRT-PCR results. A cutoff Ct value ≥ 36 was used to assign negative PEDV rRT-PCR status. Pens were observed daily for evidence of diarrhea and a fecal score, adapted from Thomas et al8 (Table 2), was assigned to the pen.
Table 1: Definition of study treatment groups
| Group | Definition |
|---|---|
| CONTROL | No vaccine and no previous PEDV exposure |
| NV | No vaccine with previous PEDV exposure |
| PRE | No previous PEDV exposure Vaccinated at 5 and 2 weeks prior to PEDV challenge |
| POST | No previous PEDV exposure Vaccinated at 1 and 3 weeks following PEDV challenge |
Table 2: Fecal consistency scoring definition*
| Score | Fecal consistency |
|---|---|
| 1 | Normal, no diarrhea |
| 2 | Mild diarrhea, soft (cowpie) |
| 3 | Moderate diarrhea, liquid with some solid content |
| 4 | Watery diarrhea, liquid with no solid content |
Following the 9-week duration of testing for the CONTROL group, 18 of the 20 CONTROL gilts subsequently became the nonvaccinated (NV) group (Tables 1 and 3). Two gilts were removed from the study for reasons unrelated to the study. The 40 remaining conspecifics were moved from the source farm to the same research site. Twenty of these gilts each received a dose (2 mL administered intramuscularly in the neck) of a commercial PEDV vaccine at 5 and 2 weeks before being moved to the research site and were designated the PRE group. The remaining 20 gilts served as the POST group and each received a dose (2 mL administered intramuscularly in the neck) of a commercial PEDV vaccine at 1 and 3 weeks following the PEDV challenge. Upon arrival to the research site, an ear tag was placed in the right ear of each gilt for individual identification and a blood sample was collected via jugular venipuncture for ELISA testing to confirm naïve or immunized status prior to the challenge. Blood sampling and ELISA testing was repeated for the 18 NV animals to confirm PEDV exposure following their previous enrollment as the CONTROL group. Following a 3-day acclimation period, all 58 of the gilts were individually challenged with PEDV, using the same procedures and homogenate described for the CONTROL group. The final inoculum for the NV, PRE, and POST groups was confirmed to be PEDV positive using rRT-PCR with a Ct value of 21.1. Following the challenge, individual fecal samples were collected from each gilt every 7 days by utilizing a fecal loop and submitted to the VDL for rRT-PCR to assess PEDV shedding. Individual fecal samples were collected until a gilt had 3 consecutive negative rRT-PCR results. A cutoff Ct value ≥ 36 was used to assign negative PEDV rRT-PCR status. Pens were observed daily for diarrhea and assigned a fecal score.
Table 3: Timeline of events by treatment group
| Day | Treatment group* | |||
|---|---|---|---|---|
| CONTROL | NV | PRE | POST | |
| -4 | 9-week-old gilts arrive at facility | |||
| 0 | Challenge | |||
| 7, 14, 21 | Individual fecal sampling | |||
| 25 | 1st vaccine dose | |||
| 28, 35, 42 | Individual fecal sampling | |||
| 44 | 2nd vaccine dose | |||
| 49, 56 | Individual fecal sampling | |||
| 60 | Individual fecal sampling | 19-week-old gilts arrive at facility | 19-week-old gilts arrive at facility | |
| 63 | Individual fecal sampling | CONTROL transition to NV, Challenge | Challenge | Challenge |
| 70 | Individual fecal sampling | Individual fecal sampling | 1st vaccine dose Individual fecal sampling |
|
| 77 | Individual fecal sampling | Individual fecal sampling | Individual fecal sampling | |
| 84 | Individual fecal sampling | Individual fecal sampling | 2nd vaccine dose Individual fecal sampling |
|
| 91, 98, 105, 112, 119, 126, 133 | Individual fecal sampling | Individual fecal sampling | Individual fecal sampling | |
Mean Ct values were calculated weekly following the challenge for each treatment group (Table 4). Data analysis for this study was completed using SAS software, Version 9.3 (SAS Institute Inc, Cary, North Carolina). A survival analysis and Cox proportional hazard regression model determined if there were significant differences in the time to negative status, defined in this study as 3 consecutive negative tests, among the treatment groups (CONTROL, NV, PRE, and POST).
Table 4: Mean fecal rRT-PCR Ct by week post-PEDV challenge
| Treatment group* | Week | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
| Control | 25.65 | 25.36 | 27.85 | 28.80 | 28.37 | 25.75 | † | † | 33.00 | ¶ |
| NV | 29.32 | 34.30 | 33.90 | † | † | † | ‡ | ‡ | ‡ | ‡ |
| Post | 19.82 | 28.71 | 30.59 | † | 28.40 | 28.10 | † | † | † | ‡ |
| Pre | 20.30 | 27.45 | 27.99 | 28.50 | 29.75 | 30.00 | 26.60 | † | † | † |
Results
A sample to positive ratio (S:P) value ≥ 0.8 was utilized to determine positive serological status by ELISA. Mean S:P ratios were calculated for each treatment group and are shown in Figure 1. Fecal consistency across all treatment groups was scored as 2 or 3 for 7 days following the challenge, after which the fecal consistency then returned to baseline. Mean fecal rRT-PCR Ct by week is shown in Table 4 and individual animal fecal rRT-PCR results are presented in Figure 2. Virus was not detected in the feces of a majority of the gilts in the CONTROL group by week 6 post challenge. Nor was PEDV detected in any of the 20 gilts on weeks 7 and 8 as indicated by Ct values ≥ 36. The percent of animals that tested positive for PEDV by rRT-PCR by week is presented in Figure 3. One gilt that had 2 previous negative fecal PEDV rRT-PCR tests had a positive result on fecal rRT-PCR on week 9 (Figure 2A). All NV gilts were found to be no longer shedding PEDV in their feces by week 4 post challenge (Figure 2B). Both the POST group (Figure 2C) and PRE group (Figure 2D) were found to be shedding PEDV through week 6. Virus was no longer detected via fecal rRT-PCRs for the POST group beginning on week 7 and the PRE group on week 8.
Figure 1: Mean S:P ratio by treatment group immediately prior to PEDV challenge. The bold horizontal line indicates the cutoff S:P value ≥ 0.8 for determining positive serological status by whole virus ELISA. Treatment groups are described in Table 1. S:P = sample to positive; PEDV = porcine epidemic diarrhea virus; ELISA = enzyme-linked immunosorbent assay.

Figure 2: Individual animal fecal rRT-PCR results by treatment group: A) Control, B) NV, C) POST, and D) PRE. Treatment groups are described in Table 1. Green indicates positive test result with Ct value; red indicates negative test result; X indicates removal from study; and gray represents no testing. rRT-PCR = real-time reverse transcription-polymerase chain reaction, Ct = cycle threshold.
| A | CONTROL pig | Week post challenge | B | NV pig | Week post challenge | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 1 | 2 | 3 | 4 | 5 | 6 | |||||
| 1 | 19.5 | 1 | |||||||||||||||||
| 2 | 28.4 | 23.6 | 2 | ||||||||||||||||
| 3 | 34.9 | 27.2 | 22.1 | 3 | |||||||||||||||
| 4 | 27.0 | X | X | 5 | |||||||||||||||
| 5 | 6 | 23.2 | |||||||||||||||||
| 6 | 27.8 | 7 | 33.9 | 33.9 | |||||||||||||||
| 7 | 34.1 | 23.7 | 8 | 33.9 | |||||||||||||||
| 8 | 32.5 | 26.3 | 28.1 | 27.4 | 9 | ||||||||||||||
| 9 | 19.5 | 10 | 27 | ||||||||||||||||
| 10 | 25.5 | 21.5 | 30.8 | 28.8 | 24.1 | 33.0 | 11 | 28 | |||||||||||
| 11 | 30.2 | 30.8 | 12 | ||||||||||||||||
| 12 | 19.4 | 13 | 34.3 | ||||||||||||||||
| 13 | 20.1 | 14 | |||||||||||||||||
| 14 | 18.1 | 15 | 29.9 | ||||||||||||||||
| 15 | 27.1 | 20.0 | 33.8 | 34.9 | 16 | ||||||||||||||
| 16 | 20.3 | 30.5 | 17 | ||||||||||||||||
| 17 | 32.6 | 19 | |||||||||||||||||
| 18 | 18.0 | X | X | X | X | X | X | X | 20 | ||||||||||
| 19 | 30.3 | 28.5 | 21.2 | ||||||||||||||||
| 20 | 22.8 | ||||||||||||||||||
| C | POST pig | Week post challenge | D | PRE pig | Week post challenge | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |||||
| 21 | 23.5 | 41 | 16.8 | 29.4 | |||||||||||||||||||
| 22 | 25.8 | 35.0 | 33.4 | 42 | 24.2 | 22.2 | |||||||||||||||||
| 23 | 17.2 | 43 | 23.5 | 26.9 | 25.1 | ||||||||||||||||||
| 24 | 14.0 | 31.5 | 44 | 19.9 | 17.3 | ||||||||||||||||||
| 25 | 20.3 | 31.1 | 29.9 | 28.1 | 45 | 18.5 | |||||||||||||||||
| 26 | 13.3 | 29.8 | 46 | 22.6 | 24.6 | 28.5 | |||||||||||||||||
| 27 | 13.2 | 28.2 | 32.2 | 47 | 15.7 | 27.9 | |||||||||||||||||
| 28 | 22.7 | 27.7 | 28.4 | 48 | 21.3 | 31.6 | |||||||||||||||||
| 29 | 14.0 | 26.4 | 49 | 15.8 | 32.5 | ||||||||||||||||||
| 30 | 24.4 | 24.9 | 50 | 22.9 | 27.4 | ||||||||||||||||||
| 31 | 15.9 | 32.3 | 51 | 21.2 | 29.2 | 25.7 | |||||||||||||||||
| 32 | 15.8 | 35.0 | 29.7 | 52 | 21.1 | 24.5 | |||||||||||||||||
| 33 | 23.8 | 32.6 | 53 | 19.8 | 29.1 | 34.6 | |||||||||||||||||
| 34 | 16.0 | 24.2 | 54 | 13.7 | 30.2 | 31.8 | |||||||||||||||||
| 35 | 22.7 | 25.4 | 31.6 | 28.4 | 55 | 17.2 | 32.0 | ||||||||||||||||
| 36 | 26.9 | 30.4 | 56 | 21.4 | 22.2 | ||||||||||||||||||
| 37 | 21.2 | 21.1 | 57 | 25.6 | 28.6 | 25.6 | 30.0 | ||||||||||||||||
| 38 | 15.6 | 33.3 | 58 | 18.2 | 20.7 | 28.7 | |||||||||||||||||
| 39 | 29.6 | 28.7 | 27.0 | 59 | 23.5 | 33.1 | 34.6 | ||||||||||||||||
| 40 | 20.4 | 23.1 | 30.5 | 60 | 23.1 | 26.9 | 27.4 | 27.7 | 26.6 | ||||||||||||||
Figure 3: Positive fecal rRT-PCRs by treatment group by week. Treatment groups are described in Table 1. rRT-PCR = real-time reverse transcription-polymerase chain reaction.

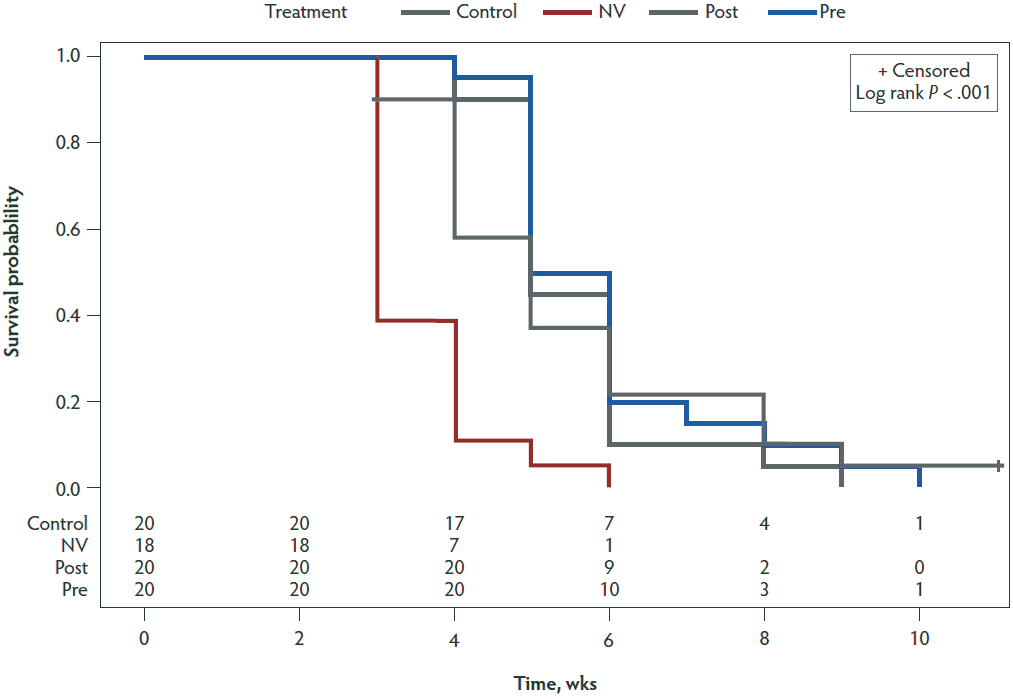
Hazard ratios (Table 5) were calculated for the NV, PRE, and POST groups compared to the CONTROL group by performing Cox proportional hazards regression modeling. A statistically significant difference (P < .001) between the CONTROL and NV groups was found with a hazard ratio of 4.022. Figure 4 shows a Kaplan-Meier plot for the time-to-negative PEDV status for each of the 4 treatment groups.
Table 5: Cox proportional hazards regression model analysis
| Treatment group* comparison | P value | Hazard ratio | 95% Confidence Limits | |
|---|---|---|---|---|
| Control | NV | < .001 | 4.022 | 1.995, 8.11 |
| Control | Post | .95 | 0.979 | 0.513, 1.869 |
| Control | Pre | .64 | 0.858 | 0.452, 1.627 |
Figure 4: Kaplan-Meier plot showing time-to-negative PEDV status for the four treatment groups. Time is displayed on the x-axis in weeks. The y-axis shows the probability that individuals within a treatment group will have a positive status by fecal rRT-PCR by the following week. Treatment groups are described in Table 1. PEDV = porcine epidemic diarrhea virus; rRT-PCR = real-time reverse transcription-polymerase chain reaction.
Discussion
Serological analysis showed an antibody response in both the NV and PRE treatment groups prior to the challenge while the CONTROL and POST groups did not show an antibody response. The CONTROL group had a negative antibody response because the gilts were naïve to PEDV. Similarly, the POST group was naïve and had not been vaccinated prior to the challenge and, therefore, did not show an antibody response. The NV group was positive, as expected, because they had previously been challenged with wildtype virus as the CONTROL group. The PRE group was positive because they had received the PEDV vaccine at 5 and 2 weeks before the challenge. Although numerical differences in the S:P ratio were noted between the treatment groups, there was no observable difference in clinical signs following the challenge. A limitation of this study was that neutralizing antibody levels were not measured for the treatment groups. Further research should be conducted to determine vaccination influence on the development of neutralizing antibodies for PEDV. In this study, vaccination before or after the challenge with a commercially available killed PEDV vaccine was not observed to affect the amount of time that PEDV was shed in the feces of challenged gilts. Prior research has shown that parenteral administration of a killed PEDV vaccine to previously unexposed sows elicited an immune response but did not develop a neutralizing antibody response in milk and only weakly in colostrum.9 Another study found that sows vaccinated with 2 doses of a killed vaccine, compared to 2 doses of a live vaccine or live vaccine followed by killed vaccine, showed the highest neutralizing antibody response in colostrum, 1:1600, compared to sera, 1:800.10 While this study did not evaluate the amount of virus shed in the feces, a previous study found that vaccinated animals shed less virus and the duration of viral shedding was shortened.11 The animals in the study were younger, being vaccinated at 3 and 5 weeks of age compared to 13 and 16 weeks of age (PRE) and 19 and 21 weeks of age (POST) in the present study, and were challenged with a homologous PEDV genotype 2b isolate to a commercial vaccine. Samples were also collected daily for 13 days post challenge, whereas in this study samples were collected weekly for 9 weeks post challenge.
This study also demonstrated that previous infection with PEDV does shorten the amount of time virus is detected in the feces following a second exposure. Gilts that were previously exposed were 4.022 times as likely to become negative compared to naïve and vaccinated individuals. However, previous PEDV infection does not completely prevent shedding of virus in feces. This is likely due to the protection induced by the primary exposure with a homologous strain of PEDV, and similar results have been demonstrated previously.12,13 Gerber et al12 described seeing no clinical signs or lesions following homologous challenge with PEDV and that shedding was observed in less than 10% of the challenged pigs. In the current study, we did observe mild clinical signs in the NV group that had been previously challenged as the CONTROL group, and observed 7 of 18 gilts (38.9%) to shed PEDV following homologous challenge.
This study observed that PEDV can be detected in the feces via rRT-PCR for up to 9 weeks post inoculation when exposed oronasally with the US prototype PEDV strain. Due to time constraints of the study, gilt 10 in the control group was not followed out for 3 consecutive negative rRT-PCR tests prior to re-challenging the group with PEDV. These findings are in congruence with results published by Bjustrom-Kraft et al7 where individual rectal swabs were positive by rRT-PCR for 10 weeks post exposure. This study demonstrates that PEDV may be detected intermittently from individual pigs. Intermittent detection may indicate intermittent shedding which has been reported in previous studies.14,15 A limitation of this study is that we did not determine if shedding was truly intermittent or if the amount of virus present was below the detection threshold of rRT-PCR.
These results show that vaccination of gilts, either prior to challenge or afterwards, with a killed commerical PEDV vaccine does not shorten the period of time that virus is detectable in the feces by rRT-PCR suggesting that viral shedding in feces is not influenced by administration of a killed commercial vaccine. This information does not contradict the vaccine’s label for protection against diarrheal disease in neonatal pigs caused by PEDV. Previous infection with virulent PEDV did have a significant effect on the amount of time virus was detected following a subsequent exposure.
Implications
- Vaccinating gilts prior or post challenge with a killed, commercial PEDV vaccine did not shorten the time that virus was detectable in feces by rRT-PCR.
- Prior PEDV infection significantly decreased the time virus was detected in feces following a subsequent exposure.
- Prior infection with PEDV did not prevent shedding in all animals following a homolgous challenge.
Acknowledgments
This work was supported by Zoetis Inc. We thank Dr Ryan Saltzman, Mike Wilgenbusch, and Vet Resources, Inc for their assistance with the study.
Conflict of interest
None reported.
Disclaimer
Scientific manuscripts published in the Journal of Swine Health and Production are peer reviewed. However, information on medications, feed, and management techniques may be specific to the research or commercial situation presented in the manuscript. It is the responsibility of the reader to use information responsibly and in accordance with the rules and regulations governing research or the practice of veterinary medicine in their country or region.
References
1. Stevenson G, Hoang H, Schwartz K, Burrough E, Sun D, Madson D, Cooper V, Pillatzki A, Gauger P, Schmitt B, Koster L, Killian M, Yoon K. Emergence of porcine epidemic diarrhea virus in the United States: clinical signs, lesions, and viral genomic sequences. J Vet Diagn Invest. 2013; 25(5):649-654. doi:10.1177/1040638713501675
*2. Schwartz K, Henry S, Tokach L, Potter M, Davidson D, Egnor C. Exposing sows to PEDV to build herd immunity. National Hog Farmer. http://nationalhogfarmer.com/business/exposing-sows-pedv-build-herd-immunity. Published March 13, 2014. Accessed October 2018.
*3. Frederickson D, Bandrick M, Taylor LP, Coleman DW, Pfeiffer A, Locke CR, Huether MJ, Zhang J, Verhelle R, Hildebrandt TK, Hardham JM, Rapp-Gabrielson VJ. Safety and antibody response of pigs to an experimental porcine epidemic diarrhea virus (PEDV) vaccine, killed virus. North Am PRRS Symp. Chicago, Illinois. 2014:69.
*4. Rapp-Gabrielson VJ, Frederickson DF, Bandrick M, Taylor LP, Marx J, Ricker T, Coleman D, Pfieffer A, Thompson JR, Zhang J, Zager S, Huether M, Hardham JM, Sornsen S. Field efficacy of an experimental porcine epidemic diarrhea (PED) vaccine administered to pregnant sows. North Am PRRS Symp. Chicago, Illinois. 2014:80.
*5. Greiner L, Connor J, Graham A, Mellor J, Lowe J. Evaluation of a PED vaccine on piglet mortality and sow immunity. Proc AASV. Orlando, Florida. 2015:361.
*6. Schwartz TJ, Rademacher CJ, Giménez-Lirola G, Sun Y, Zimmerman J. Evaluation of the effects of PEDV vaccine on PEDV naïve and previously PEDV exposed sows in a challenge model comparing immune response and preweaning mortality. Proc AASV. New Orleans, Louisiana. 2016:363-366.
7. Bjustrom-Kraft J, Woodard K, Giménez-Lirola L, Rotolo M, Wang C, Sun Y, Lasley P, Zhang J, Baum D, Gauger P, Main R, Zimmerman J. Porcine epidemic diarrhea virus (PEDV) detection and antibody response in commercial growing pigs. BMC Vet Res. 2016;12(1):99. doi:10.1186/s12917-016-0725-5
8. Thomas J, Chen Q, Gauger P, Giménez-Lirola L, Sinha A, Harmon K, Madson D, Burrough E, Magstadt D, Zalzbrenner H, Welch M, Yoon K, Zimmerman J. Effect of porcine epidemic diarrhea virus infectious doses on infection outcomes in naïve conventional neonatal and weaned pigs. PLoS One. 2015;10(10):e0139266. doi:10.1371/journal.pone.0139266
9. Gillespie T, Song Q, Inskeep M, Stone S, Murtaugh M. Effect of booster vaccination with inactivated porcine epidemic diarrhea virus on neutralizing antibody response in mammary secretions. Viral Immunol. 2018;31(1):62-68. doi:10.1089/vim.2017.0023
10. Paudel S, Park JE, Jang H, Hyun BH, Yang DG, Shin HJ. Evaluation of antibody response of killed and live vaccines against porcine epidemic diarrhea virus in a field study. Vet Q. 2014;34(4):194-200. doi:10.1080/01652176.2014.973999
11. Opriessnig T, Gerber PF, Shen H, de Castro AMMG, Zhang J, Chen Q, Halbur P. Evaluation of the efficacy of a commercial inactivated genogroup 2b-based porcine epidemic diarrhea virus (PEDV) vaccine and experimental live genogroup 1b exposure against 2b challenge. Vet Res. 2017;48(1):69. doi:10.1186/s13567-017-0472-z
12. Gerber PF, Xiao CT, Lager K, Crawford K, Kulshreshtha V, Cao D, Meng XJ, Opriessnig T. Increased frequency of porcine epidemic diarrhea virus shedding and lesions in suckling pigs compared to nursery pigs and protective immunity in nursery pigs after homologous re-challenge. Vet Res. 2016;47(1):118. doi:10.1186/s13567-016-0402-5
13. Srijangwad A, Stott C, Temeeyasen G, Senasuthum R, Chongcharoen W, Tantituvanont A, Nilubol D. Immune response of gilts to single and double infection with porcine epidemic diarrhea virus. Arch Virol. 2017;162(7):2029-2034. doi:10.1007/s00705-017-3307-3
14. Bertasio C, Giacomini E, Lazzaro M, Perulli S, Papetti A, Lavazza A, Lelli D, Alborali G, Boniotti M. Porcine epidemic diarrhea virus shedding and antibody response in swine farms: a longitudinal study. Front Microbiol. 2016;7:2009. doi:10.3389/fmicb.2016.02009
15. Gallien S, Moro A, Lediguerher G, Catinot V, Paboeuf F, Bigault L, Berri M, Gauger PC, Pozzi N, Authié E, Rose N, Grasland B. Evidence of porcine epidemic diarrhea virus (PEDV) shedding in semen from infected specific pathogen-free boars. Vet Res. 2018;49(1):7. doi:10.1186/s13567-018-0505-2
* Non-refereed references.