| Practice Tip | Peer reviewed |
Cite as: Robbins RC, Betlach AM, Mondragon-Evans MR, et al. Development of a herd-specific lung homogenate for exposure to Mycoplasma hyopneumoniae under field conditions. J Swine Health Prod. 2019;27(4):221–227. https://doi.org/10.54846/jshap/1123
Also available as a PDF.
SummaryThe swine industry is known for holding high standards of disease control and elimination. However, partial disease control for Mycoplasma hyopneumoniae at the farm level has been evident and has driven initiatives for unconventional health management strategies. Several approaches focused on gilt exposure for M hyopneumoniae using a herd-specific lung homogenate have been performed in the field. Nevertheless, variations in efficacy are apparent and a publicly available protocol for producing M hyopneumoniae lung homogenate under field conditions is not available. In this practice tip, a protocol is described for developing a herd-specific lung homogenate for M hyopneumoniae exposure intended for use in veterinary-supervised elimination or control programs. A herd-specific lung homogenate inoculum, free of secondary respiratory pathogens for the herd of intended use and with an adequate M hyopneumoniae concentration, was obtained through extensive diagnostic testing and evaluation of M hyopneumoniae localization within the lung. Molecular methods were applied to characterize the M hyopneumoniae present in the lung and to evaluate the genomic stability of the bacterium during the exposure process. In doing so, a herd-specific M hyopneumoniae lung homogenate for gilt acclimation was obtained under field conditions. | ResumenLa industria porcina es conocida por mantener altos estándares de control y eliminación de enfermedades. Sin embargo, el control parcial de la enfermedad causada por Mycoplasma hyopneumoniae a nivel de granja ha sido evidente y ha impulsado iniciativas para desarrollar estrategias de control de salud no convencionales. En el campo, se han desarrollado varios enfoques centrados en la exposición a la hembra primeriza contra M hyopneumoniae con un homogeneizado de pulmón hato-específico. Sin embargo, la variación en la eficacia es evidente y no se dispone de un protocolo publicado para producir el homogeneizado pulmonar con M hyopneumoniae en condiciones de campo. En este consejo práctico, se describe un protocolo para la preparación de un homogeneizado de pulmón hato-específico para la exposición de M hyopneumoniae destinado a ser utilizado en programas de eliminación o control supervisados por veterinarios. A través de extensas pruebas diagnósticas y la evaluación de la localización de M hyopneumoniae dentro del pulmón, se obtuvo un inóculo hato-específico de un homogeneizado de pulmón, libre de patógenos respiratorios secundarios para ser utilizado en el hato previsto y con una concentración adecuada de M hyopneumoniae. Se utilizaron métodos moleculares para caracterizar al M hyopneumoniae presente en el pulmón y para evaluar la estabilidad genómica de la bacteria durante el proceso de exposición. Al hacerlo, se obtuvo un homogeneizado de pulmón de M hyopneumoniae específico para la aclimatación de hembras primerizas en condiciones de campo. | ResuméL’industrie porcine est reconnue pour le maintien de standards élevés en ce qui a trait à la maitrise et à l’élimination des maladies. Toutefois, à la ferme la maitrise partielle de l’infection par Mycoplasma hyopneumoniae est évidente et a entrainé des initiatives pour des stratégies non-conventionnelles de gestion de la santé. Plusieurs approches ont misé sur l’exposition de cochettes à M hyopneumoniae en utilisant un homogénat de poumon spécifique au troupeau ont été réalisées sur le terrain. Cependant, des variations dans l’efficacité sont apparentes et un protocole disponible à tous pour produire en condition de terrain un homogénat pulmonaire contenant M hyopneumoniae n’est pas disponible. Dans la présente astuce de pratique, un protocole est décrit pour développer et utiliser, sous supervision vétérinaire, un homogénat pulmonaire spécifique de troupeau contenant M hyopneumoniae dans le cadre de programmes de maitrise ou d’élimination. Un inoculum d’homogénat de poumon spécifique de troupeau, exempt d’agents pathogènes respiratoires secondaires pour le troupeau sélectionné et avec une concentration adéquate de M hyopneumoniae, fut obtenu à la suite d’épreuves diagnostiques nombreuses et à l’évaluation de la localisation de M hyopneumoniae dans le tissu pulmonaire. Des méthodes moléculaires furent utilisées afin de caractériser les M hyopneumoniae présents dans le poumon et pour évaluer la stabilité génomique de la bactérie durant le processus d’exposition. Ainsi, un homogénat de poumon spécifique de troupeau contenant M hyopneumoniae pour l’acclimatation des cochettes fut obtenu dans des condition de terrain. |
Keywords: swine, Mycoplasma hyopneumoniae, gilt acclimation, lung homogenate, disease control and elimination
Search the AASV web site
for pages with similar keywords.
Received: October 28, 2018
Accepted: March 5, 2019
Veterinarians are responsible for applying their knowledge to improve animal health and welfare. The swine industry aims for high herd health to rear healthy pigs and safe pork. To do so, veterinarians, producers, industry professionals, and scientists attempt to implement practical and science-driven solutions that can be applied in the field. The herd veterinarian is tasked with recommending solutions based on professional judgement, scientific literature, experience, field research, and consultation with colleagues and experts. Historically, herd management practices have evolved in response to issues faced in the field and are adopted as ethically and scientifically substantiated solutions. In the case of disease control, the swine industry has been keen to develop and apply strategies towards disease management and elimination, including the use of biosecurity and the modification of production practices to decrease the detrimental effect of disease transmission (eg, early weaning1 and all-in/all-out production2). In cases where ideal disease control cannot be achieved with the available tools, novel solutions are generated.
The administration of a herd-specific infectious product for disease control has been used in veterinary medicine to confer complete and strain-specific protection when other measures have proven inadequate to contain the disease process. In some instances, administration of a herd-specific tissue homogenate is the best option for a controlled exposure to indigenous pathogens when the exposure is intended to protect the larger population. Use of herd-specific tissue homogenate for controlled exposure requires veterinary oversight and must adhere to any applicable regulations ensuring that it does not adversely affect the health and performance of the individual animal exposed. For example, the control of viruses (ie, porcine parvovirus and porcine enterovirus) known to cause stillbirths, mummification, embryonic deaths, and infertility has been achieved by exposing dams to infectious feedback material composed of feces or tissues from contaminated litters.3,4 This exposure serves to homogenize herd immunity and acclimatize incoming gilts to prevent herd disequilibrium. Immunity to porcine epidemic diarrhea virus (PEDV) and porcine rotavirus has been accomplished by using pre-farrow oral controlled exposure of dams with infectious feedback material5,6 resulting in protection of piglets through the development of humoral and cell-mediated immunity.
Mycoplasma hyopneumoniae causes a chronic respiratory condition in pigs known as enzootic pneumonia (EP), which affects herds worldwide.7,8 Control measures for EP include the use of immunization, antimicrobial medication, increased biosecurity practices, parity segregation, all-in/all-out movement, and elimination strategies.9 However, in certain situations such as gilt acclimation, partial control can be obtained with the use of these measures, even if they are employed in combination. Thus, veterinary professionals have proposed the use of alternative measures to control M hyopneumoniae infections in the field, which are tailored to be herd-specific and include pathogen exposure using lung homogenate.
Statement of the problem
Replacement gilts play an important role in the dynamics of a sow farm, as approximately half of the herd is replaced with young females every year for genetic improvement and maintenance of parity structure.10 However, every new batch of replacement females needs to be evaluated for their potential to cause disturbance of the sow farm dynamics, especially as it pertains to infectious agents. Incoming gilts may introduce new pathogens not currently prevalent in the herd or be naïve to existing pathogens on the recipient sow farm. Gilt health status is closely surveilled before and after transportation and during introduction to the recipient herd. Assurance from suppliers regarding freedom from economically important swine pathogens (ie, porcine reproductive and respiratory syndrome virus [PRRSV], PEDV, and M hyopneumoniae) may or may not be required by the buyer. Although freedom of infectious agents and disease is a desirable attribute in replacement animals, it is hypothesized that in certain circumstances the health conditions of the recipient farm may be more severely affected by the introduction of naïve pigs. This is the case for M hyopneumoniae infections, which are considered endemically prevalent in a significant proportion of swine farms.11 Introduction of naïve gilts into M hyopneumoniae-positive farms is hypothesized to be a risk factor for sow herd disequilibrium and results in difficulty to control disease presentation in downstream flows.12-14
Various options can be pursued to address the issue of naïve gilt introductions into M hyopneumoniae endemically infected farms. Disease elimination is most favorable for any swine production unit, and recently efforts for M hyopneumoniae eradication have increased in the United States.15 One of the most commonly utilized strategies for M hyopneumoniae elimination, which is herd closure and medication, implies uniform exposure of the entire herd at the same time prior to the start of closure.16 A protocol directed at exposure with M hyopneumoniae is needed when pursuing disease elimination. To achieve and maintain the elimination of M hyopneumoniae, farm geographical location, area prevalence, facility design, production system flow, and constant and continuous supply of negative gilts should be accounted for. However, these factors often cannot be modified to achieve successful elimination. Therefore, disease control is viewed as one of the oldest and most cost-effective strategy to deal with M hyopneumoniae on endemically infected farms, keeping in mind the necessity to maintain the health of incoming and resident dam populations.
One common question in the industry is whether control can be achieved with commercial products directed at treating or controlling M hyopneumoniae infections. The species-specific vaccines and antimicrobial drugs with activity towards mycoplasmas play an important role in decreasing the negative outcomes of EP. However, it is widely known that partial protection is conferred by M hyopneumoniae bacterins17 and vaccinated pigs can become colonized after contact with shedding pigs.18,19 In addition, elimination of the bacterium from the respiratory tract of pigs has not been achieved with antimicrobial treatment alone, even during the chronic phase of infection.20 Therefore, a need exists for a practical protocol for herd exposure to M hyopneumoniae. In this practice tip, we describe a procedure to develop a herd-specific lung homogenate for M hyopneumoniae exposure under field conditions to potentially stimulate immunity and decrease the proportion of susceptible animals in the population. This practice tip is intended to be used as a resource for swine veterinarians who are designing gilt acclimatization strategies that involve the procurement of a herd-specific lung homogenate.
Definitions
For the purpose of providing clarity to this practice tip, the following definitions are proposed:
Gilt acclimation: The process of adapting gilts to a new environment or exposure to an infectious agent prior to introduction into a recipient breeding herd.13,21
Lung homogenate: Lung tissue made uniform through a blending process that is used for exposure.
Animal care
All animals were under veterinary oversight and care with a veterinarian-client-patient relationship and Pork Quality Assurance Plus certification in place. Feed and water were available ad libitum in stainless steel feeders and through water nipples, respectively. Pigs and their environment were monitored daily by caretakers. All feed rations were formulated to meet or exceed nutritional recommendations for swine.22 Gilts were raised in standard indoor production facilities with fully slatted floors, fed a diet to meet or exceed their nutritional needs, and received immunizations against porcine circovirus type 2 (PCV2), PRRSV, and M hyopneumoniae as a growing pig, followed by a booster immunization for M hyopneumoniae, PCV2, and PRRSV at selection (26 weeks of age). All injections were performed with a needleless device using commercially available products.
Procuring a herd-specific M hyopneumoniae lung homogenate
Under experimental conditions, viable culture and tissue homogenate have been administered to stimulate M hyopneumoniae exposure.23-25 However due to the fastidious growth of this microorganism, the procurement of a herd-specific lung homogenate was proposed. To obtain a herd-specific lung homogenate, a procedure focusing on lung homogenate preparation from tissue donor gilts was developed for use in field scenarios (Figure 1). Several factors including farm history and health status, clinical observations, and diagnostic testing were taken into consideration by the herd veterinarian during the selection of donor gilts and lung tissue. With diagnostic aid, the concentration of M hyopneumoniae and presence of secondary agents were evaluated to ensure adequate lung homogenate quality. It was up to the herd veterinarian to consider the herd’s indigenous organisms when developing parameters for homogenate quality. In addition, the infectivity and genomic stability of the M hyopneumoniae lung homogenate were assessed under field conditions.
Figure 1: Procedure to obtain a Mycoplasma hyopneumoniae lung homogenate. Mhp = Mycoplasma hyopneumoniae; PCR = polymerase chain reaction; Ct = cycle threshold; CCU = color changing units; Mhr = Mycoplasma hyorhinis; PRRSV = porcine reproductive and respiratory syndrome virus; IAV = influenza A virus; PCV2 = porcine circovirus type 2; H parasuis = Haemophilus parasuis.
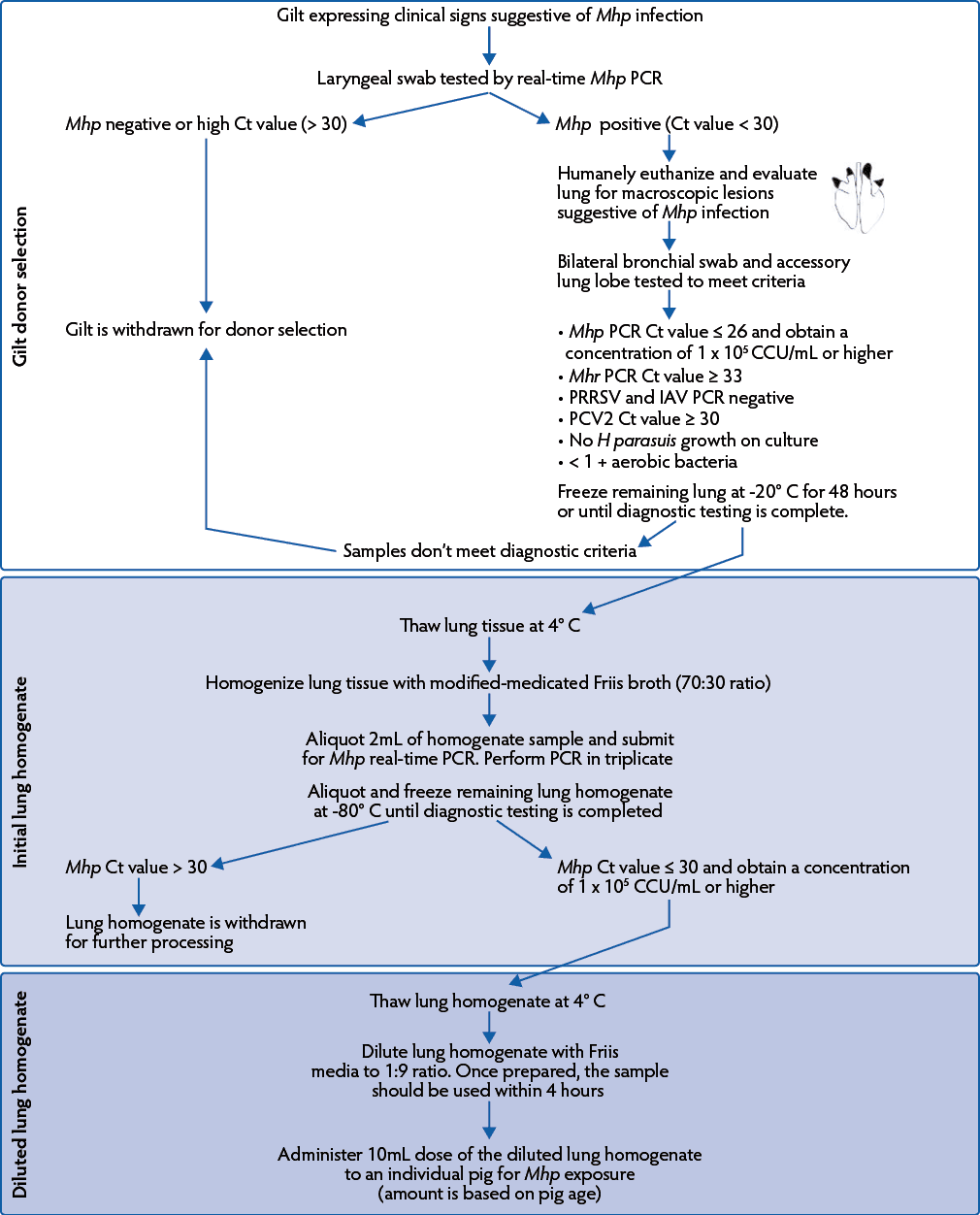
Donor gilt selection
Initial tissue donor gilt
The initial tissue donor gilt was from a PRRSV, influenza A virus (IAV), PCV2, and Mycoplasma species positive farm and was selected at 31 weeks of age when she exhibited clinical signs (ie, dyspnea and loss of body condition) suggestive of M hyopneumoniae infection.26 Alternatively, an initial donor may be chosen through testing of ante-mortem samples (eg, laryngeal swabs)27 using sterile swabs (BBL CultureSwab, Sparks, Maryland) and tested for M hyopneumoniae by species-specific real-time polymerase chain reaction (real-time PCR) to confirm infection.28 The donor was humanely euthanized and lung tissue harvested if macroscopic lesions (ie, consolidation of apical and cardiac lung lobes) consistent with M hyopneumoniae infection were observed26 and no lesions of secondary bacterial infection (eg, polyserositis) were evident. A bronchial swab was obtained by inserting a sterile swab into bilateral bronchioles of affected lung tissue and submitted to the University of Minnesota Veterinary Diagnostic Laboratory (UMN VDL), along with a portion of the affected lung lobe for diagnostic testing. Remaining lung tissue was stored at -20° C for a minimum of 48 hours and until diagnostic testing was completed to ensure a high recovery of M hyopneumoniae.
Diagnostic criteria were established for the initial donor to ensure adequate exposure following M hyopneumoniae infection and to minimize the risk of introducing and spreading secondary respiratory pathogens (Figure 1). The criteria for initial lung selection were: 1) observation of macroscopic lesions (ie, consolidation of apical and cardiac lung lobes) suggestive of M hyopneumoniae infection; 2) M hyopneumoniae real-time PCR cycle threshold (Ct) value ≤ 26; 3) Mycoplasma hyorhinis real-time PCR Ct value ≥ 33; 4) PRRSV and IAV negative real-time PCR result; 5) PCV2 real-time PCR Ct value ≥ 30; 6) no Haemophilus parasuis growth on culture; and 7) identification of < 1+ bacteria on aerobic culture.
The diagnostic parameters were designed to prevent the introduction, amplification, or spread of secondary respiratory pathogens, including but not limited to PRRSV, IAV, PCV2 and H parasuis, which could cause unintended infection and compromise gilt health. Mycoplasma hyorhinis is a commensal microorganism in swine; however, clinical disease associated with polyserositis is often evident at high bacterial concentrations.29 Therefore, an M hyorhinis Ct value ≥ 33 was chosen as the cut-off parameter while considering the ubiquitous nature of this microorganism in swine herds and the clinical history of the herd. A PCV2 Ct value ≥ 30 was chosen as the cut-off parameter due to the endemic nature of this microorganism in swine herds.30 If additional respiratory pathogens were detected, continuation of lung homogenate development protocol was at the discretion of the veterinarian.
The M hyopneumoniae Ct value of ≤ 26 was selected by fitting a standard curve with known concentrations of bacterial infectivity (color changing units/mL [CCU/mL]) to the real-time PCR assay and obtaining a Ct value equivalent to 1 × 103 CCU/mL. A concentration of 1 × 105 CCU/mL of M hyopneumoniae has been suggested as the minimum required infectious dose for successful colonization of a pig’s lung in experimental conditions.31 Differences in virulence across M hyopneumoniae strains have been observed,32 therefore, a potentially lower infectious dose equivalent of 1 × 103 CCU/mL was chosen by the veterinarian. In addition, within-sample variation was assumed based on the nature of the sample, therefore, the infectious dose may potentially vary. Lungs fulfilling the diagnostic criteria, with the intent to inoculate M hyopneumoniae-negative gilts, were used to make enough homogenate for the herd-specific gilt acclimation program recommended by the veterinarian.
Donor gilts for amplification and lung homogenate procurement
To amplify and procure lung homogenate for M hyopneumoniae exposure for replacement gilts to a 65,000-sow herd, 3- to 5-week old PRRSV, IAV, and M hyopneumoniae-negative gilts (n = 38) were intra-tracheally inoculated with 10mL of the diluted lung homogenate.
Four weeks post inoculation, laryngeal swabs were collected and tested for M hyopneumoniae by species-specific real-time PCR to confirm infection. If swabs were positive, lungs were harvested at 5 weeks post inoculation and diagnostic testing was performed as previously described for the initial donor (Figure 1). The accessory lung lobe was submitted for diagnostic testing to evaluate the presence of viruses and secondary bacteria while preserving the remaining lung sections for subsequent lung homogenate development. Sample collection and tissue harvest took place 5 weeks post inoculation because peak M hyopneumoniae shedding has been shown to occur at 4 weeks post inoculation under experimental conditions33 and to account for the lower M hyopneumoniae infectious dose (1 × 103 CCU/mL). Lungs that fulfilled the diagnostic criteria were processed into lung homogenate and used to expose larger gilt populations as part of the herd-specific acclimation program.
Lung homogenate preparation
Selection of lung tissue
To identify the maximum amount of lung tissue meeting the diagnostic criteria for homogenate production, a pilot study was performed. Localization of M hyopneumoniae was evaluated by determining the relative bacterial load within different anatomical lung sections using lung homogenate samples of two gilts, which were evaluated individually (Figure 2). The lung homogenates were obtained 5 weeks post inoculation and tested for M hyopneumoniae using real-time PCR. Each lung homogenate was run in triplicate, in which the genetic material from 3 sample subsets was extracted and tested individually to account for possible diagnostic variation due to sample consistency. Of the 2 gilts sampled, M hyopneumoniae bacterial loads were numerically higher in the proximal lung sections (median Ct values = 21.4 and 19.8) compared to the distal lung sections (median Ct values = 36.3 and 23.3) and the caudal diaphragmatic lobe (median Ct values = 21.7 and 30.4). However, the amount of viable M hyopneumoniae based on anatomical lung sections was not assessed because of the difficulty to obtain an M hyopneumoniae culture, especially under field conditions. In addition, the proportion of affected lung within each anatomical lung section was not evaluated.
Figure 2: Mycoplasma hyopneumoniae bacterial load (Ct value) based on anatomical lung section. ● =Distal sections of apical, cardiac, and diaphragmatic lobes; ● =Proximal sections of apical, cardiac, and diaphragmatic lobes; ● = Caudal diaphragmatic lobe. Each dot represents one sample tested by real-time PCR. Ct = cycle threshold; PCR = polymerase chain reaction.
For the detection of this microorganism, within-homogenate variance was observed for each anatomical lung region, but to a greater extent in distal and proximal lung sections compared to the caudal diaphragmatic lobe (Figure 2). The degree of within-homogenate variance could have resulted from the anatomic nature of the tissue as the homogenate includes cartilaginous airways, pleura, and lung tissue with the specific localization of the microorganism. In this investigation, a small sample size was evaluated, however, insight regarding the relative bacterial load based on anatomical lung section was gained at the individual pig level. Further research involving a larger sample size and evaluating the impact of different M hyopneumoniae infection lengths and lung lesion scores on the relative bacterial load within each anatomical lung section is needed.
Since this microorganism was localized across the three different anatomical lung sections, the relative bacterial load of M hyopneumoniae within different lung homogenate preparations was evaluated in 38 gilts at 5 weeks post inoculation. Mycoplasma hyopneumoniae Ct values were compared in bronchial swabs and 2 types of lung homogenate samples prepared from either whole lung tissue or from lesioned apical, cardiac, and diaphragmatic lobes that contained adjacent apparently non-affected tissue (Table 1). All bronchial swabs were collected from affected apical and cardiac lung lobes and the lung tissue was homogenized using 70% lung tissue and 30% modified, medicated Friis broth.34 Samples were submitted for M hyopneumoniae testing using real-time PCR, in which the homogenate samples were run in triplicate and the median Ct value was used for data analysis. For statistical analysis, a two-sample t-test assuming equal variances was performed using R (v3.5.1; R Core Team, 2018) to compare lung homogenate Ct values based on preparation type. Differences were considered significant at P < .05. Based on the conditions of this study, the M hyopneumoniae lung homogenate derived from lesioned apical, cardiac, and diaphragmatic lung lobes showed significantly lower Ct values compared to whole lung tissue Ct values (P = .003; Table 1). In both lung homogenates, the mean M hyopneumoniae Ct values were 20.9 and 24.9, suggesting a high bacterial presence of the microorganism regardless of tissue preparation method (Table 1). In addition, tissue preparation using whole lung provided a larger volume of lung homogenate, resulting in the use of fewer donor gilts. Since the whole lung homogenate preparation met or exceeded the veterinarian’s homogenate quality criteria, the lung homogenate was prepared by incorporating the whole lung tissue.
Table 1: Detection of Mycoplasma hyopneumoniae (Ct values) in bronchial swabs and lung homogenate samples based on tissue preparation. Different superscript letters represent significant difference (P < .05) based on a two-sample t-test. Ct = cycle threshold.
| Lung section | No. of samples | Bronchial swabs, Ct value (SD) | Lung homogenate, Ct value (SD) |
|---|---|---|---|
| Lesioned apical, cardiac, and diaphragmatic lobes | 14 | 22.6 (4.5) | 20.9 (3.6)a |
| Whole lung | 24 | 22.9 (2.7) | 24.9 (3.9)b |
Initial lung homogenate
Frozen whole lung tissue was homogenized using a ratio of 70% tissue and 30% modified medicated Friis broth34 using a Ninja Professional blender. This ratio was chosen based on the sampling procedure used for viral isolation by the UMN VDL and the feasibility to handle and process the material considering its viscosity. The blending process was repeated until lung tissue reached a slurry consistency. Friis medium was used to support M hyopneumoniae viability during the preparation and inoculation of the lung homogenate because this medium is commonly used for the culture and isolation of this microorganism.34 Lung tissue was processed, aliquoted, and stored at -80° C. Currently, there is minimal information regarding the freeze-thaw effect on M hyopneumoniae viability. It is hypothesized that thawing frozen lung tissue aids in the detachment of this microorganism from the targeted tissue leading to a higher bacterial recovery. However, further information on this topic is necessary to assess the viability and storage of frozen M hyopneumoniae clinical samples. Previous literature suggests that freezing a Mycoplasma organism culture at -70o C and -30o C for up to 2 years may result in up to 1 and 2 log10 reduction in bacterial titers, respectively.35 Prior to freezing, 2 mL of the lung homogenate was submitted for M hyopneumoniae real-time PCR and tested in triplicate, resulting in an average 25.5 Ct value.
Lung homogenate dilution
Thawed lung homogenate was diluted in a 1:9 ratio with Friis base media (Teknova, Hollister, California) in a clean laboratory, while technicians wore personal protective equipment. Since M hyopneumoniae adheres to ciliated epithelium within the respiratory airways, the diluted lung homogenate was not filtered to potentially increase the likelihood of infectivity. Ten milliliters of the diluted lung homogenate were delivered intra-tracheally to the 3- to 5-week old donor pigs as previously described.36 The M hyopneumoniae concentration was not evaluated at the time of exposure.
Evaluating lung homogenate infectivity and genomic stability
Lung homogenate infectivity
Diagnostic monitoring post inoculation was performed to evaluate the diluted lung homogenate infectivity. The veterinarian considered the lung homogenate to be infectious if an M hyopneumoniae infection was observed or detected post inoculation. Laryngeal swabs were collected 4 weeks post inoculation for M hyopneumoniae detection using real-time PCR. All the pigs sampled (n = 38) were M hyopneumoniae positive, evidencing sample infectivity. Post inoculation, clinical signs and mortality were closely monitored. If clinical signs suggestive of secondary bacterial infections (eg, unthriftiness, cough, thumping, or increased respiratory effort) were observed, antimicrobials without activity towards mycoplasmas (eg, Ceftiofur) were administered according to label directions.
Genomic stability
Multiple locus variable number tandem repeat analysis (MLVA)37 was employed to identify M hyopneumoniae types in the lung homogenate and to evaluate for potential genomic mutations that could have occurred during the tissue processing and inoculation. The molecular characterization method was performed from M hyopneumoniae-positive bronchial swabs that were collected from the initial and subsequent donor gilts’ lung tissue. All samples showed an MLVA type 11-15. This suggests a lack of detectable genomic change in the targeted amplicon during the initial lung homogenate preparation and throughout the subsequent exposure-harvest processes. This finding is supportive of other research that describes M hyopneumoniae in vitro and in vivo genomic stability.37,38
Conclusion
In this practice tip, a procedure for the development of a herd-specific lung homogenate for M hyopneumoniae exposure under field conditions is described. This practice tip details a step-by-step process focusing on lung homogenate preparation. In doing so, gilt acclimatization practices that encompass herd-specific pathogen exposure methods may be achieved to provide adequate M hyopneumoniae exposure and immunization.
Acknowledgments
The authors would like to thank Dr Albert Rovira and Casey Thomlinson for their valuable contributions to the diagnostics phase and support in the application of this protocol.
Conflict of interest
None reported.
Disclaimer
Scientific manuscripts published in the Journal of Swine Health and Production are peer reviewed. However, information on medications, feed, and management techniques may be specific to the research or commercial situation presented in the manuscript. It is the responsibility of the reader to use information responsibly and in accordance with the rules and regulations governing research or the practice of veterinary medicine in their country or region.
References
1. Alexander TJL, Thornton K, Boon G, Lysons RJ, Gush AF. Medicated early weaning to obtain pigs free from pathogens endemic in the herd of origin. Vet Rec. 1980;106:114-119.
2. Clark LK, Scheidt AB, Armstrong CH, Knox KE, Mayrose VB. The effect of all-in/all-out management on pigs from a herd with enzootic pneumonia. Vet Med. 1991;86(9):948-951.
3. Truyen U, Streck AE. Porcine Parvovirus. In: Zimmerman JJ, Karriker L, Ramirez A, Schwartz K, Stevenson G, eds. Diseases of Swine. 10th ed. Ames, IA: Wiley-Blackwell; 2012:447-455.
4. Alexandersen S, Knowles NJ, Dekker A, Belsham GJ, Zhang Z, Koenen F. Picornaviruses. In: Zimmerman JJ, Karriker L, Ramirez A, Schwartz K, Stevenson G, eds. Diseases of Swine. 10th ed. Ames, IA: Wiley-Blackwell; 2012:587-620.
*5. Schwartz KJ. The science of feedback. Proc Annu Iowa State University Swine Dis Conf Swine Pract. Ames, IA. 2014;51-66.
*6. Pittman JS. Field experiences with interventions for Rotavirus control. Proc Annu Iowa State University Swine Dis Conf Swine Pract. Ames, IA. 2016;30-36.
7. Goodwin RFW, Pomeroy AP, Whittlestone P. Production of enzootic pneumonia in pigs with a mycoplasma. Vet Rec. 1965;77:1247-1249.
8. Mare C, Switzer WP. New species: Mycoplasma hyopneumoniae; a causative agent of virus pig pneumonia. Vet Med Small Anim Clin. 1965;60,841-846.
9. Maes D, Sibila M, Kuhnert P, Segalés J, Haesebrouck F, Pieters M. Update on Mycoplasma hyopneumoniae infections in pigs: Knowledge gaps for improved disease control. Transbound Emerg Dis. 2017;65:110-124.
10. Stalder K, D’Allaire S, Drolet R, Abell C. Longevity in breeding animals. In: Zimmerman JJ, Karriker L, Ramirez A, Schwartz K, Stevenson G, eds. Diseases of Swine. 10th ed. Ames, IA: Wiley-Blackwell; 2012:50-59.
*11. United States Department of Agriculture. Part II: Reference of Swine Health and Health Management in the United States, 2012. In: National Animal Health Monitoring System Swine 2012. Fort Collins, Colorado: Center for Epidemiology and Animal Health, Animal and Plant Health Inspection Service, US Dept of Agriculture; 2015. Publication #676.0216.
*12. Lowe L. Mycoplasma hyopneumoniae: Gilts, are they the problem? Proc Allen D. Leman Conf. Saint Paul, MN. 2012;(39):83-85.
13. Nathues H, Chang YM, Wieland B, Rechter G, Spergser J, Rosengarten R, Kreinebrock L, Grosse Beilage E. Herd-level risk factors for the seropositivity to Mycoplasma hyopneumoniae and the occurrence of enzootic pneumonia among fattening pigs in areas of endemic infection and high pig density. Transbound Emerg Dis. 2014;61(4):316-328.
14. Pieters M, Fano E. Mycoplasma hyopneumoniae management in gilts. Vet Rec. 2016;178(5):122-123.
*15. Yeske PE. Economic impact of M. hyopneumoniae eliminations. Proc IPVS. Cancun, Mexico. 2012;246.
16. Holst S, Yeske P, Pieters M. Elimination of Mycoplasma hyopneumoniae from breed-to-wean farms: A review of current protocols with emphasis on herd closure and medication. J Swine Health Prod. 2015;23:321-330.
17. Haesebrouck F, Pasmans F, Chiers K, Maes D, Ducatelle R, Decostere A. Efficacy of vaccines against bacterial disease in swine: what can we expect? Vet Microbiol. 2004; 100(3-4):255-268.
18. Villarreal I, Meyns T, Dewulf J, Vranchx K, Calus D, Pasmans F, Haesebrouck F, Maes D. The effect of vaccination on the transmission of Mycoplasma hyopneumoniae in pigs under field conditions. Vet J. 2011;188(1):48-52.
19. Pieters M, Fano E, Pijoan C, Dee S. An experimental model to evaluate Mycoplasma hyopneumoniae transmission from asymptomatic carriers to unvaccinated and vaccinated sentinel pigs. Can J Vet Res. 2010;74(2):157-160.
*20. Painter T, Kuhn M, Wolff T, Pieters M. Efficacy and duration of infection study for Respisure® and Draxxin® against a Mycoplasma hyopneumoniae challenge in swine. Proc Allen D. Leman Swine Conf. Saint Paul, MN. 2012;39(12):225.
21. Kraeling RR, Webel SK. Current strategies for reproductive management of gilts and sows in North America. J Anim Sci Biotechnol. 2015;6:3. doi:10.1186/2049-1891-6-3
22. National Research Council. Nutrient Requirements of Swine. 11th ed. Washington, DC: National Academy Press. 2012.
23. Kobisch M, Ross RF. Experimental infections of swine. In: Tully JG, Razin S, eds. Molecular and Diagnostic Procedures in Mycoplasmology. Vol II. San Diego, CA: Academic Press. 1996;371-376.
24. Ross RF, Zimmermann-Erickson BJ, Young TF. Characteristics of protective activity of Mycoplasma hyopneumoniae vaccine. Am J Vet Res. 1984;45(10):1899-1905.
25. Thacker EL, Halbur PG, Ross RF, Thanawongnuwech R, Thacker BJ. Mycoplasma hyopneumoniae potentiation of porcine reproductive and respiratory syndrome virus-induced pneumonia. J Clin Microbiol.1999;37(3):620-627.
26. Thacker E, Minion FC. Mycoplasmosis. In: Zimmerman JJ, Karriker L, Ramirez A, Schwartz K, Stevenson G, eds. Diseases of Swine. 10th ed. Ames, IA: Wiley-Blackwell; 2012:779-797.
27. Pieters M, Daniels J, Rovira A. Comparison of sample types and diagnostic methods for in vivo detection of Mycoplasma hyopneumoniae during early stages of infection. Vet Microbiol. 2017;203,103-109.
28. Strait EL, Madsen ML, Minion FC, Christopher-Hennings J, Dammen M, Jones KR, Thacker EL. Real-time PCR assays to address genetic diversity among strains of Mycoplasma hyopneumoniae. J Clin Microbiol. 2008;46(8):2491-2498.
29. Switzer WP. Studies on infectious atrophic rhinitis. IV. Characterization of a pleuropneumonia-like organism isolated from the nasal cavities of swine. Am J Vet Res. 1955;16:540-544.
30. Zhao K, Han F, Zou Y, Zhu L, Li C, Xu Y, Zhang C, Tan F, Wang J, Tao S, He X, Zhou Z, Tang X. Rapid detection of porcine circovirus type 2 using a TaqMan-based real-time PCR. Virol J. 2010;7:374. doi:10.1186/1743-422X-7-374
31. Marois C, Dory D, Fablet C, Madec F, Kobisch M. Development of a quantitative real-time TaqMan PCR assay for determination of the minimal dose of Mycoplasma hyopneumoniae strain 116 required to induce pneumonia in SPF pigs. J Appl Microbiol. 2010;108(5):1523-1533.
32. Vicca J, Stakenborg T, Maes D, Butaye P, Peeters J, de Kruif A, Haesebrouck F. Evaluation of virulence of Mycoplasma hyopneumoniae field isolates. Vet Microbiol. 2003;97:177-190.
33. Roos LR, Fano E, Homwong N, Payne B, Pieters M. A model to investigate the optimal seeder-to-naïve ratio for successful natural Mycoplasma hyopneumoniae gilt exposure prior to entering the breeding herd. Vet Microbiol. 2016;184:51-58.
34. Friis NF. Some recommendations concerning primary isolation of Mycoplasma suipneumoniae and Mycoplasma flocculare a survey. Nord Vet Med. 1975;27:337-339.
35. Addey JP, Taylor-Robinson D, Dimic M. Viability of mycoplasmas after storage in frozen or lyophilized states. J Med Microbiol. 1970;3:137-145.
36. Pieters M, Pijoan C, Fano E, Dee S. An assessment of the duration of Mycoplasma hyopneumoniae infection in an experimentally infected population of pigs. Vet Microbiol. 2009;134:261-266.
37. Dos Santos LF, Sreevatsan S, Torremorell M, Moreira MA, Sibila M, Pieters M. Genotype distribution of Mycoplasma hyopneumoniae in swine herds from different geographical regions. Vet Microbiol. 2015;175:374-381.
*38. Betlach A, Fano E, Sponheim A, Dalquist L, Pieters M. Minimal Mycoplasma hyopneumoniae genetic variability within production flows. Proc AASV. San Diego, CA. 2018; 342-343.
* Non-refereed references.