| Original research | Peer reviewed |
Cite as: Bjustrom-Kraft J, Christopher-Hennings J, Daly R, et al. The use of oral fluid diagnostics in swine medicine. J Swine Health Prod. 2018;26(5):262-269. https://doi.org/10.54846/jshap/1091
Also available as a PDF.
SummarySwine veterinarians in North America have applied oral fluid-based testing methodologies for an increasing number of systemic, respiratory, and enteric disease diagnostic applications. Since the first report of oral fluid testing in 2008, nucleic acid and antibody assays have been described in the peer-reviewed literature for many pathogens affecting swine. As evidence of the US swine industry’s growing utility of oral fluids as a diagnostic tool, the cumulative number of swine oral fluid diagnostic tests conducted at three veterinary diagnostic laboratories in the upper Midwest (Iowa State University, South Dakota State University, and University of Minnesota) has increased from approximately 21,000 tests in 2010 to nearly 370,000 tests in 2016. The objective of this review is to describe the developments in oral fluid diagnostics that have led to its widespread use and to highlight areas of concern as this technology is increasingly implemented by producers and veterinarians. | ResumenEn Norteamérica, los veterinarios especialistas en cerdos han utilizado metodologías de testeo basadas en fluidos orales para diferentes aplicaciones diagnósticas en un creciente número de enfermedades sistémicas, respiratorias y entéricas. Desde el primer reporte de testeo con fluido oral en 2008, en la literatura editada, se han descrito diferentes ensayos para ácido nucleico y anticuerpos, para muchos patógenos que afectan a los cerdos. Como evidencia del creciente uso en la industria porcina de los Estados Unidos de los fluidos orales como herramienta de diagnóstico, el número acumulado de pruebas de diagnóstico de fluido oral porcino conducidas en tres laboratorios de diagnóstico veterinario en la parte superior del Medio Oeste (Universidad del Estado de Iowa, Universidad del Estado de Dakota del Sur, y Universidad de Minnesota) se han incrementado de aproximadamente 21,000 pruebas en 2010 a cerca de 370,000 pruebas en 2016. El objetivo de esta revisión es describir los desarrollos en el diagnóstico de fluido oral que han llevado a su uso generalizado y resaltar las áreas de preocupación conforme esta tecnología es implementada, cada vez más, por productores y veterinarios. | ResuméEn Amérique du Nord les vétérinaires en médecine porcine ont appliqué des méthodologies utilisant les fluides oraux dans un nombre croissant d’applications diagnostiques pour des maladies systémiques, respiratoires et entériques. Depuis le premier rapport en 2008 de test utilisant du fluide oral, des épreuves pour détecter des acides nucléiques et des anticorps ont été décrites dans la littérature jugées par les pairs pour plusieurs agents pathogènes affectant les porcs. À titre de preuve de l’utilité grandissante dans l’industrie porcine américaine des fluides oraux comme outil diagnostique, le nombre cumulatif d’épreuves diagnostiques effectuées sur du fluide oral dans trois laboratoires de diagnostic vétérinaires dans le Midwest (Iowa State University, South Dakota State University, and University of Minnesota) a augmenté d’environ 21,000 test en 2010 à environ 370,000 tests en 2016. L’objectif de la présente revue est de décrire les développements dans le diagnostic utilisant les fluides oraux qui ont mené à cet usage répandu et de faire ressortir les inquiétudes étant donné que l’application de cette technologie est en augmentation par les producteurs et les vétérinaires. |
Keywords: swine, review, oral fluids, diagnostics
Search the AASV web site
for pages with similar keywords.
Received: January 5, 2018
Accepted: April 6, 2018
The first technical report on swine oral fluid diagnostics was presented at the 2005 International PRRS Symposium when Simer et al1 reported 20 of 24 pen-based oral fluid samples (83.3%) and 17 of 24 serum samples (70.8%) were porcine reproductive and respiratory syndrome virus (PRRSV) reverse transcription polymerase chain reaction (RT-PCR) positive in finishing pigs. The purpose of this review is to provide an update on the development and implementation of oral fluid diagnostics in swine medicine subsequent to this initial report.
Collection of oral fluid samples has been described at length by Prickett et al.2 In brief, cotton ropes are hung in the pen at pig shoulder height. Pigs chew on the rope, saturating the rope with oral fluids. After 20 to 30 minutes, the ropes are placed in a single-use plastic bag, the fluid is wrung from the rope, and then decanted into a tube for submission to the diagnostic laboratory. Pigs with prior experience respond immediately to the presence of the rope. In experienced groups, a 20- to 30-minute sampling period is sufficient to allow adequate participation of pigs in the pen. In pigs without prior rope sampling experience, 60 minutes is recommended to allow pigs to learn the new “game” and achieve an adequate level of participation.3
Oral fluids are most commonly collected from pens of pigs, but oral fluid samples can also be obtained from individual pigs.3 Oral fluids can be successfully collected at all production stages, ie, growing pigs3,4 and in the breeding herd for individually- or group-housed sows and boars.5,6 Samples can be collected from suckling piglets as they approach weaning age,7,8 but family sampling, ie, placing the rope so that both sows and their litters have access, has been shown to be more successful than collecting solely from the piglets. Thus, Almeida et al8 reported an approximate 73% success rate when collecting family oral fluid samples versus 44% success when collecting only from litters. From a collection of 72 family oral fluid samples and matching sera from 718 piglets, 84.4% (27 of 32 litters) were PRRSV RT-PCR positive while 24.2% (174 of 718 piglets) of serum samples were positive for PRRSV nucleic acid.
At the present time, the detection of nucleic acid or antibodies in oral fluids has been documented for most of the major swine pathogens including: Actinobacillus pleuropneumoniae (APP),9,10 African swine fever virus,11,12 classical swine fever virus,13 foot-and-mouth disease virus,14,15 influenza A virus (IAV),16-18 Lawsonia intracellularis,19 Mycoplasma spp.,20-22 porcine circovirus type 2 (PCV2),2,23 porcine epidemic diarrhea virus (PEDV),24 PRRSV,2,6,25-27 Senecavirus A (SVA),28 and others. Field applications or research on the use of oral fluid diagnostics have been described in Australia,15 Belgium,29 Canada,30 France,31 Germany,13 Italy,32 Japan,33 Malaysia,34 Poland,35 Spain,36 the United Kingdom,37,38 the United States,2 Vietnam,39 and others. Many of the assays reported in the literature have only been described under research conditions, but it is reasonable to expect their future commercialization and adoption for routine use in diagnostic laboratories.
Oral fluid testing
In the United States, veterinary diagnostic laboratories with a major swine focus began offering oral fluid-based tests to clientele in 2010. The data provided in Figure 1 and Tables 1, 2, 3, and 4 describe the number of oral fluid tests performed at Iowa State University, South Dakota State University, and University of Minnesota. The following is a review of pathogens for which testing is commonly performed and for which peer-reviewed literature is available.
Figure 1: Total number of oral fluid tests conducted at Iowa State University, South Dakota State University, and the University of Minnesota from 2010 to 2016. PCR = polymerase chain reaction; ELISA = enzyme-linked immunosorbant assay.
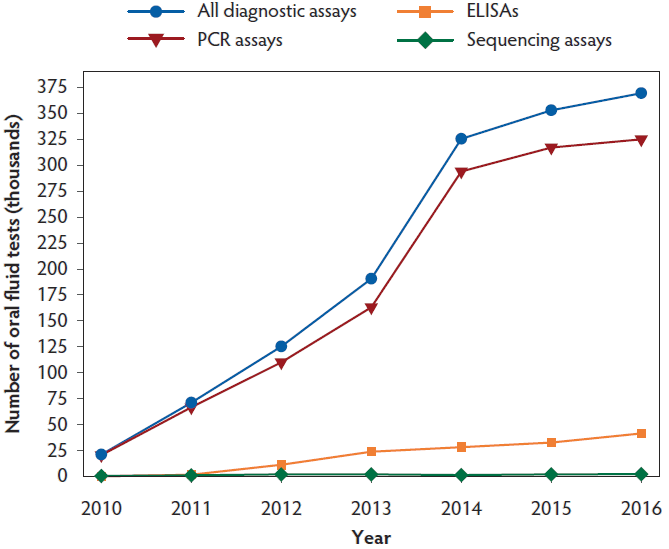
Table 1: Total number of tests on oral fluid specimens by pathogen in 3 US veterinary diagnostic laboratories from 2010 to 2016 *
| Pathogen | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 |
|---|---|---|---|---|---|---|---|
| PRRSV | 14,603 | 46,239 | 77,756 | 109,868 | 126,165 | 144,773 | 148,526 |
| IAV | 4785 | 16,495 | 34,297 | 46,940 | 48,688 | 48,895 | 47,454 |
| MHP | 760 | 4514 | 7079 | 10,286 | 11,203 | 11,741 | 13,178 |
| PCV2 | 751 | 2047 | 4147 | 2149 | 5676 | 4807 | 3176 |
| APP | 0 | 37 | 4 | 93 | 14 | 287 | 3306 |
| TGEV | 0 | 34 | 0 | 4651 | 32,848 | 12,497 | 12,996 |
| PEDV | 0 | 0 | 0 | 14,361 | 75,965 | 76,063 | 73,494 |
| LI | 0 | 0 | 0 | 454 | 1519 | 3290 | 2443 |
| PDCoV | 0 | 0 | 0 | 0 | 21,393 | 46,366 | 58,513 |
| SVA | 0 | 0 | 0 | 0 | 0 | 1597 | 3598 |
| Other | 64 | 1630 | 1919 | 1804 | 2010 | 2595 | 2755 |
| Total | 20,963 | 70,996 | 125,202 | 190,606 | 325,481 | 352,911 | 369,439 |
Table 2: Number of nucleic acid (PCR) tests on oral fluid specimens in 3 US veterinary diagnostic laboratories from 2010 to 2016*
| Pathogen | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 |
|---|---|---|---|---|---|---|---|
| PRRSV | 14,251 | 43,464 | 64,984 | 84,835 | 96,715 | 110,650 | 116,671 |
| IAV | 4581 | 14,898 | 31,806 | 44,410 | 46,738 | 47,304 | 42,261 |
| PCV2 | 751 | 2047 | 4147 | 2142 | 5669 | 4773 | 3168 |
| MHP | 750 | 4514 | 7056 | 10,271 | 11,201 | 11,708 | 13,169 |
| TGEV | 0 | 34 | 0 | 4651 | 32,848 | 12,497 | 12,996 |
| PEDV | 0 | 0 | 0 | 14,361 | 75,931 | 76,048 | 69,324 |
| LI | 0 | 0 | 0 | 454 | 1519 | 3290 | 2443 |
| PDCoV | 0 | 0 | 0 | 0 | 21,393 | 46,365 | 58,513 |
| SVA | 0 | 0 | 0 | 0 | 0 | 1597 | 3533 |
| Other | 64 | 1584 | 1923 | 1881 | 2024 | 2863 | 2886 |
| Total | 20,397 | 66,541 | 109,916 | 163,005 | 294,038 | 317,095 | 324,964 |
Table 3: Number of antibody (ELISA) tests on oral fluid specimens in 3 US veterinary diagnostic laboratories from 2010 to 2016*
| Pathogen | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 |
|---|---|---|---|---|---|---|---|
| PRRSV | 43 | 1575 | 11,224 | 23,785 | 28,107 | 32,564 | 30,051 |
| MHP | 10 | 0 | 0 | 4 | 1 | 33 | 8 |
| IAV | 0 | 0 | 5 | 0 | 0 | 2 | 3960 |
| PEDV | 0 | 0 | 0 | 0 | 0 | 4 | 4168 |
| APP | 0 | 0 | 0 | 0 | 0 | 0 | 3176 |
| SVA | 0 | 0 | 0 | 0 | 0 | 0 | 60 |
| Total | 53 | 1575 | 11,229 | 23,789 | 28,108 | 32,603 | 41,423 |
Table 4: Number of oral fluid specimens submitted for nucleic acid sequencing in 3 US veterinary diagnostic laboratories from 2010 to 2016*
| Pathogen | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 |
|---|---|---|---|---|---|---|---|
| PRRSV | 300 | 919 | 1444 | 1223 | 893 | 1524 | 1718 |
| IAV | 37 | 110 | 522 | 650 | 327 | 433 | 465 |
| PCV2 | 0 | 0 | 6 | 7 | 7 | 34 | 8 |
| PEDV | 0 | 0 | 0 | 0 | 34 | 3 | 2 |
| Other | 0 | 0 | 23 | 27 | 1 | 4 | 10 |
| Total | 337 | 1029 | 1995 | 1907 | 1262 | 1998 | 2203 |
Porcine reproductive and respiratory syndrome virus
Porcine reproductive and respiratory syndrome virus was the first virus detected by RT-PCR in oral fluid samples.2 Porcine reproductive and respiratory syndrome virus oral fluid enzyme-linked immunosorbent assays (ELISA) for antibody detection have been routinely offered since 2010. In 2016, RNA detection was performed for 116,671 of the 148,526 PRRSV tests (Tables 1 and 2).
Nucleic acid detection
Prickett et al2 first reported the detection of PRRSV by quantitative RT-PCR (qRT-PCR) in oral fluids collected in the field from 8-week-old pigs. Oral fluid qRT-PCR-positive results were coincident with RT-PCR-positive serum samples, ie, showed 77% agreement. Under experimental conditions, Prickett et al25 reported that PRRSV RNA was detected in oral fluid samples from 3 to 35 days post inoculation (DPI), with sporadic positives thereafter. Similar results were obtained from individual boars inoculated with modified-live virus, type 1 PRRSV, or type 2 PRRSV.6 On the first DPI, virus was detected in 10% of the boars sampled (7 of 69); by 3 DPI, virus was detected in 100% of boars sampled (67 of 67).6 Cumulatively, the literature indicates that PRRSV RNA can be detected for at least 36 DPI in oral fluid samples.5,25,26,33,35,40-44
Sequencing
Successful PRRSV sequencing from oral fluids has been described.35,45,46 Kittawornrat et al45 obtained PRRSV open reading frame-5 sequences from 2 of 6 RT-PCR-positive oral fluid samples from pre-weaned pigs. Zhang et al46 reported successful full-genome sequencing from oral fluid samples with RT-PCR cycle threshold (Ct) values between 18.7 and 20.6, whereas no full-length sequences were obtained from oral fluids with Ct values between 22.9 and 35.4.
Antibody detection
Porcine reproductive and respiratory syndrome virus IgG antibody is detected in oral fluids by ELISA between 7 and 14 days after inoculation or vaccination.5,25-27,40,47 Kittawornrat et al,27 working with oral fluid samples from individually housed boars and a serum ELISA adapted to oral fluids, reported that IgM was detectable at 3 DPI, IgA at 7 DPI, and IgG at 8 DPI. Antibody responses in oral fluids mirrored antibody responses in serum. Maternal PRRSV IgG is readily detected in pigs from PRRSV-positive sow herds and may be detected for up to 60 days post-weaning; however, a PRRSV IgM-IgA (dual isotype) ELISA was shown to detect pig-specific IgM and IgA, even in the presence of maternal IgG.48 Porcine reproductive and respiratory syndrome virus antibody ELISA testing has been well documented in the literature and may provide a cost-effective approach to PRRSV monitoring and surveillance.
Influenza A virus
As shown in Tables 1, 2, 3, and 4, IAV oral fluid testing has been offered for routine testing since 2010. Nucleic acid detection was performed for 42,261 of the 47,454 IAV tests in 2016 (Table 1 and 2).
Nucleic acid detection
Detmer et al49 first reported the detection of IAV nucleic acid in oral fluid samples from both experimentally and naturally infected pigs. Under experimental conditions, IAV RNA was detected in oral fluids from 3 to 21 DPI; whereas, no IAV RT-PCR-positive nasal swabs were detected past 7 DPI.50 Ramirez et al43 reported highly variable detection patterns for IAV infection in 10 wean-to-finish barns. Cumulatively, the literature indicates that IAV RNA can be detected in oral fluids, but widely variable detection patterns have been noted in the literature.35,37,50-54
Sequencing
Influenza A virus sequencing has been described in the literature.49,51,53 Detmer et al49 obtained hemagglutinin (HA) sequences from 2 of 4 positive oral fluid samples submitted for analysis. Panyasing et al53 reported unsuccessful attempts to sequence HA and neuraminidase genes, but successfully sequenced M genes for all 18 IAV qRT-PCR-positive oral fluid samples collected from neonatal pigs. In oral fluid field samples submitted for routine analysis, HA sequences were obtained from 34 of 61 (55.7%) samples with Ct values < 25; 5 of 34 (14.7%) samples with Ct values between 25 and 29.9; and 0 of 39 (0%) samples with Ct values > 30 (Jianqiang Zhang, Personal Communication).
Virus isolation
Isolation of IAV from oral fluids in pigs is difficult and reports of both success and failure may be found in the literature. Detmer et al49 and Allerson et al51 were not able to isolate and sequence IAV from oral fluid samples. However, Romagosa et al54 reported 51.4% (19 of 37) of RT-PCR-positive oral fluid samples were also positive by virus isolation. Similarly, Goodell et al16 reported successful IAV virus isolation, but isolation was significantly less likely in oral fluids when compared to nasal swabs, particularly in vaccinated animals. Virus isolation was successful in 82 of 180 (45.6%) oral fluid samples with Ct values < 25; 62 of 346 (17.9%) samples with Ct values between 25 and 29.9; and 21 of 407 (5.2%) samples with Ct values between 30 and 35 (Jianqiang Zhang, Personal Communication). Additional research is needed to determine the best time to collect samples and the optimum laboratory protocol for successful IAV virus isolation.16,49 As reviewed by Baron et al55 in the context of human immunodeficiency virus, the extreme hypotonicity of oral fluids (one-seventh the tonicity of interstitial fluid) severely reduces virus infectivity. This is a factor that should be considered for future research because, like humans, swine oral fluids are hypotonic and may have an impact on the isolation of IAV and other viral agents from porcine oral fluids.
Antibody detection
Panyasing et al18 first reported detection of IAV-specific antibodies in oral fluid samples using a blocking ELISA based on the viral nucleoprotein (NP). Using a NP indirect ELISA, IAV IgM antibody responses peaked at 8 DPI and declined quickly thereafter while IgA and IgG were detected around 6 DPI and lasted through the conclusion of the study (42 DPI).17 In this same study, Panyasing et al17 showed a rapid anamnestic oral fluid antibody response in vaccinated animals. Cumulatively, the literature agrees that IAV antibodies can be detected in oral fluids as early as 6 DPI.17,39,52,56,57
Porcine coronaviruses
The majority of research on the porcine coronaviruses has focused on PEDV. Cumulatively, the research strongly supports the use of oral fluids for PEDV detection. Similar assumptions have been made for other porcine coronaviruses, ie, transmissible gastroenteritis virus and porcine deltacoronavirus (PDCoV), on the strength of the PEDV research.
Porcine epidemic diarrhea virus
Porcine epidemic diarrhea virus RT-PCR testing for oral fluids was implemented in 2013 and was used extensively thereafter, as reflected in the test numbers reported in Tables 1, 2, 3, and 4. Oral fluid antibody testing for PEDV became available in 2016 (Table 3). Reverse transcription PCR testing was performed for 69,324 of the 73,494 PEDV tests conducted in 2016 (Tables 1 and 2).
Nucleic acid detection. Bjustrom-Kraft et al24 published the first peer-reviewed study on the detection of PEDV in oral fluid samples by RT-PCR. Detectable levels of PEDV were found in fecal swabs, oral fluids, and pen fecal samples collected in the field following a natural planned exposure to PEDV. Significant differences were detected between individual fecal swabs and pen-based oral fluid; oral fluids had lower Ct values indicating higher virus concentrations. PEDV was detected in oral fluids for approximately 69 days post exposure (DPE). Under experimental conditions, Bower et al58 reported detection of PEDV by RT-PCR in fecal swabs and oral fluids from 1 to 35 DPI in both sample types.
Antibody detection. Bjustrom-Kraft et al24 reported the detection of PEDV IgG and IgA in oral fluid samples collected 13 days after natural planned exposure. Porcine epidemic diarrhea virus IgA sample to positive ratio (S/P) responses in oral fluid increased until 97 DPE whereas oral fluid IgG responses peaked at 13 DPE and declined thereafter.
Porcine deltacoronavirus
Under experimental conditions, Zhang59 reported detection of PDCoV in oral fluids from 3-week-old pigs. Individual fecal swabs, pen-based feces, and oral fluids were collected and PDCoV RNA was detected from 7 to 28 DPI, 7 to 14 DPI, and 7 to 35 DPI, respectively. Homwong et al60 evaluated PDCoV RT-PCR testing results from routine submissions (n = 602) to the University of Minnesota Veterinary Diagnostic Laboratory and found that oral fluid samples were more likely to test positive for PDCoV than feces.
Less commonly used oral fluid tests in the United States
Tests are offered at the diagnostic laboratories for several pathogens for which little peer-reviewed literature is available.
Porcine circovirus type 2
As shown in Tables 1, 2, and 4, routine PCV2 oral fluid testing began in 2010. Relatively few tests have been performed in recent years, which suggests that the current PCV2 vaccines are effective.61 Porcine circovirus type 2 was detected in oral fluids from each of the three sites with at least 1 to 2 positive samples in oral fluids by quantitative polymerase chain reaction (qPCR) in 2008.2 Similar results were reported in PCV2-inoculated 11-week-old pigs where PCV2 was detected by qPCR from 2 DPI until the conclusion of the study (98 DPI).23 Ramirez et al43 reported 508 of 600 (84.7%) oral fluid samples were PCV2 positive by qPCR in 10 wean-to-finish barns. Van Cuong et al39 reported a slightly lower PCV2 detection rate (61%) in 68 farms throughout Vietnam. Under experimental conditions, PCV2 antibody (IgG, IgA, and IgM) was first reported in 2011.23 All PCV2-inoculated pigs seroconverted between 14 and 21 DPI (IgG, IgA, and IgM), and antibody responses remained detectable through the conclusion of the study (98 DPI).
Senecavirus A
For SVA, 3,598 oral fluid-based tests have been conducted (Tables 1, 2, and 3). Senecavirus A detection in oral fluids has been documented under field conditions.28 Although no clinical signs were observed, SVA was detected by RT-PCR in oral fluid samples at day zero in one of the sites. The fact that 9 of 10 serum samples were SVA positive on the same farm supported the validity of the oral fluid results. Little peer-reviewed research is available on SVA, but this initial report suggests oral fluids may be a useful sample for monitoring and surveillance of SVA.
Bacterial pathogens
Little research has been done on the detection of bacterial pathogens in oral fluid samples. Regardless, peer-reviewed publications reporting detection by polymerase chain reaction under experimental or field conditions have included the following bacterial agents: APP,9,62 Brachyspira spp.,63 Erysipelothrix rhusiopathiae,64 Haemophilus parasuis,62 L intracellularis,19 Mycoplasma spp.,20,21,62 Pasteurella multocida,62 Salmonella,19 and Streptococcus suis.62
Bacterial pathogens for which antibodies are reportedly detected in oral fluids include: APP,9,10 E rhusiopathiae,64 and Mycoplasma spp.22
General conclusions
Pig production changed dramatically over the last several decades from smaller single-site farms to larger multisite production systems.65 These changes made it possible for producers and veterinarians to achieve higher production efficiencies, but also facilitated the appearance of production diseases, ie, multifactorial diseases and the appearance of new, high-impact pathogens, such as PRRSV and PEDV.66-69
Diagnostic medicine needs to respond to new disease challenges with new methods capable of providing timely, accurate, informative results. Individual pig samples, such as serum or swabs, have historically served this purpose, but individual animal sampling is not compatible with efficient surveillance in contemporary swine production systems. As an alternative to individual animal samples, Prickett et al2 described the use of pen-based oral fluid samples (rope testing) for the detection of PRRSV and PCV2 in growing pigs. Since this initial report, oral fluid-adapted nucleic acid and antibody tests have been reported for many of the major swine pathogens and oral fluid-based surveillance has been widely adopted by swine veterinarians and swine producers. This process will continue as more and better tests are adapted to the oral fluid matrix.
Nevertheless, there are good reasons to exercise caution. In particular, the peer-reviewed literature has shown that nucleic acid and antibody assays can be adapted to oral fluids, but the literature has also consistently shown that the procedures need to be carefully modified for optimum performance with the oral fluid matrix.70,71 Chittick et al70 and Gibert et al36 working with PRRSV and Goodell et al71 working with IAV found significant differences in test performance among RT-PCR protocols offered in veterinary diagnostic laboratories. Once optimum protocols are identified, they should be broadly implemented to achieve reproducibility among diagnostic laboratories. Overall, the development of oral fluid-based testing has changed the way we monitor disease in swine populations. However, careful work on the part of researchers and critical thinking on the part of producers and veterinarians will be needed to fully develop a reliable and robust oral fluid diagnostics system capable of meeting the current and future needs of the swine industry.
Acknowledgements
The authors would like to acknowledge and thank Dr Stephanie Rossow and Michele Leiferman from the University of Minnesota, Travis Clement from South Dakota State University, and Dr Luis Giménez-Lirola and Wendy Stensland from Iowa State University for their significant contributions to oral fluid test development and to this manuscript.
Conflict of interest
None reported.
Disclaimer
Scientific manuscripts published in the Journal of Swine Health and Production are peer reviewed. However, information on medications, feed, and management techniques may be specific to the research or commercial situation presented in the manuscript. It is the responsibility of the reader to use information responsibly and in accordance with the rules and regulations governing research or the practice of veterinary medicine in their country or region.
References
*1. Simer R, Prickett J, Zhou E-M, Zimmerman J. An improved method for PRRS virus surveillance and monitoring. Proc International PRRS Symposium. St. Louis, Missouri. 2005.
2. Prickett J, Kim W, Simer R, Yoon KJ, Kim WI, Zimmerman J. Oral-fluid samples for surveillance of commercial growing pigs for porcine reproductive and respiratory syndrome virus and porcine circovirus type 2 infections. J Swine Health Prod. 2008;16:86-91.
3. White D, Rotolo M, Olsen C, Wang C, Prickett J, Kittawornrat A, Panyasing Y, Main R, Rademacher C, Hoogland M, Zimmerman JJ. Recommendations for pen-based oral-fluid collection in growing pigs. J Swine Health Prod. 2014;22(3):138-141.
4. Seddon YM, Guy JH, Edwards SA. Optimising oral fluid collection from groups of pigs: effect of housing system and provision of ropes. Vet J. 2012;193(1):180-184.
5. Pepin BJ, Kittawornrat A, Liu F, Gauger PC, Harmon K, Abate S, Main R, Garton C, Hargrove J, Rademacher C, Ramirez A, Zimmerman J. Comparison of specimens for detection of porcine reproductive and respiratory syndrome virus infection in boar studs. Transbound Emerg Dis. 2015;62(3):295-304.
6. Kittawornrat A, Prickett J, Chittick W, Wang C, Engle M, Johnson J, Patnayak D, Schwartz T, Whitney D, Olsen C, Schwartz K, Zimmerman J. Porcine reproductive and respiratory syndrome virus (PRRSV) in serum and oral fluid samples from individual boars: Will oral fluid replace serum for PRRSV surveillance? Virus Res. 2010;154:170-176.
*7. Yeske-Livermore L, O’Neil K, Main R, Zimmerman J. Improved pre-weaning surveillance using oral fluids: A pilot study. Proc AASV. Dallas, Texas. 2014:317-318.
*8. Almeida MN, Zimmerman JJ, Holtkamp D, Rademacher C, Linhares DCL. Maximizing herd sensitivity to detect PRRSV in due-to-wean pigs using family oral fluids sampling. Proc James D. McKean Swine Dis Conf. Ames, Iowa. 2017:66-68.
9. Costa G, Oliveira S, Torrison J. Detection of Actinobacillus pleuropneumoniae in oral fluid samples obtained from experimentally infected pigs. J Swine Health Prod. 2012;20(2):78-81.
10. González W, Giménez-Lirola LG, Holmes A, Lizano S, Goodell C, Poonsuk K, Sitthicharoenchai P, Sun Y, Zimmerman J. Detection of Actinobacillus pleuropneumoniae ApxIV toxin antibody in serum and oral fluid specimens from pigs inoculated under experimental conditions. J Vet Res. 2017;61(2):163-171.
11. Giménez-Lirola LG, Mur L, Rivera B, Mogler M, Sun Y, Lizano S, Goodell C, Harris DH, Rowland RR, Gallardo C, Sánchez-Vizcaíno JM, Zimmerman J. Detection of African swine fever virus antibodies in serum and oral fluid specimens using a recombinant protein 30 (p30) dual matrix indirect ELISA. PloS One. 2016;11(9):e0161230. doi:10.1371/journal.pone.016123.
12. Mur L, Gallardo C, Soler A, Zimmerman J, Pelayo V, Nieto R, Sánchez-Vizcaíno JM, Arias M. Potential use of oral fluid samples for serological diagnosis of African swine fever. Vet Microbiol. 2013;165(1):135-139.
13. Dietze K, Tucakov A, Engel T, Wirtz S, Depner K, Globig A, Kammerer R, Mouchantat S. Rope-based oral fluid sampling for early detection of classical swine fever in domestic pigs at group level. BMC Vet Res. 2017;13:5. doi:10.1186/212917-016-0930-2.
14. Senthilkumaran C, Bittner H, Ambagala A, Lung O, Babiuk S, Yang M, Zimmerman J, Giménez-Lirola LG, Nfon C. Use of oral fluids for detection of virus and antibodies in pigs infected with swine vesicular disease virus. Transbound Emerg Dis. 2017;64:1762-1770.
15. Vosloo W, Morris J, Davis A, Giles M, Wang J, Nguyen HT, Kim PV, Quach NV, Le PT, Nguyen PH, Dang H, Tran HX, Vu PP, Hung VV, Le QT, Tran TM, Mai TM, Le QT, Singanallur NB. Collection of oral fluids using cotton ropes as a sampling method to detect foot-and-mouth disease virus infection in pigs. Transbound Emerg Dis. 2015;62(5):e71-e75. doi:10.1111/tbed.12196.
16. Goodell CK, Prickett J, Kittawornrat A, Zhou F, Rauh R, Nelson W, O’Connell C, Burrell A, Wang C, Yoon KJ, Zimmerman JJ. Probability of detecting influenza A virus subtypes H1N1 and H3N2 in individual pig nasal swabs and pen-based oral fluid specimens over time. Vet Microbiol. 2013;166:450-460.
17. Panyasing Y, Goodell C, Giménez-Lirola L, Kittawornrat A, Wang C, Schwartz KJ, Zimmerman J. Kinetics of influenza A virus nucleoprotein antibody (IgM, IgA, IgG) in serum and oral fluid specimens from pigs infected under experimental conditions. Vaccine. 2013;31:6210-6215.
18. Panyasing Y, Goodell CK, Wang C, Kittawornrat A, Prickett JR, Schwartz KJ, Ballagi A, Lizano S, Zimmerman JJ. Detection of influenza A virus nucleoprotein antibodies in oral fluid specimens from pigs infected under experimental conditions using a blocking ELISA. Transbound Emerg Dis. 2014;61(2):177-184.
*19. Frana T, Warneke H, Stensland W, Kinyon J, Bower L, Burrough E. Comparative detection of Lawsonia intracellularis, Salmonella, and Brachyspira from oral fluids and feces. Proc AASV. Dallas, Texas. 2014:67-69.
20. Clavijo MJ, Oliveira S, Zimmerman J, Rendahl A, Rovira A. Field evaluation of a quantitative polymerase chain reaction assay for Mycoplasma hyorhinis. J Vet Diagn Invest. 2014;26(6):755-760.
21. Gomes Neto JC, Bower L, Erickson BZ, Wang C, Raymond M, Strait EL. Quantitative real-time polymerase chain reaction for detecting Mycoplasma hyosynoviae and Mycoplasma hyorhinis in pen-based oral, tonsillar, and nasal fluids. J Vet Sci. 2015;16(2):195-201.
22. Gomes Neto JC, Strait EL, Raymond M, Ramirez A, Minion FC. Antibody responses of swine following infection with Mycoplasma hyopneumoniae, M. hyorhinis, M. hyosynoviae and M. flocculare. Vet Microbiol. 2014;174(1):163-171.
23. Prickett JR, Johnson J, Murtaugh MP, Puvanendiran S, Wang C, Zimmerman JJ, Opriessnig T. Prolonged detection of PCV2 and anti-PCV2 antibody in oral fluids following experimental inoculation. Transbound Emerg Dis. 2011;58(2):121-127.
24. Bjustrom-Kraft J, Woodard K, Giménez-Lirola L, Rotolo M, Wang C, Sun Y, Lasley P, Zhang J, Baum D, Gauger P, Main R, Zimmerman J. Porcine epidemic diarrhea virus (PEDV) detection and antibody response in commercial growing pigs. BMC Vet Res. 2016;12:99. doi:10.1186/s12917-016-0725-5.
25. Prickett J, Simer R, Christopher-Hennings J, Yoon KJ, Evans RB, Zimmerman J. Detection of porcine reproductive and respiratory syndrome virus infection in porcine oral fluid samples: a longitudinal study under experimental conditions. J Vet Diagn Invest. 2008;20:156-163.
26. Kittawornrat A, Prickett J, Wang C, Olsen C, Irwin C, Panyasing Y, Ballagi A, Rice A, Main R, Johnson J, Rademacher C, Hoogland M, Rowland R, Zimmerman J. Detection of porcine reproductive and respiratory syndrome virus (PRRSV) antibodies in oral fluid specimens using a commercial PRRSV serum antibody enzyme-linked immunosorbent assay. J Vet Diagn Invest. 2012;24:262-269.
27. Kittawornrat A, Engle M, Panyasing Y, Olsen C, Schwartz K, Rice A, Lizano S, Wang C, Zimmerman J. Kinetics of the porcine reproductive and respiratory syndrome virus (PRRSV) humoral immune response in swine serum and oral fluids collected from individual boars. BMC Vet Res. 2013;9:61. doi:10.1186/1746-6148-9-61.
28. Tousignant SJ, Bruner L, Schwartz J, Vannucci F, Rossow S, Marthaler DG. Longitudinal study of Senecavirus a shedding in sows and piglets on a single United States farm during an outbreak of vesicular disease. BMC Vet Res. 2017;13(1):277. doi:10.1186/s12917-017-1172-7.
29. De Regge N, Cay B. Comparison of PRRSV nucleic acid and antibody detection in pen-based oral fluid and individual serum samples in three different age categories of post-weaning pigs from endemically infected farms. PloS One. 2016;11(11):e0166300. doi:10.1371/journal.pone.0166300.
30. Senthilkumaran C, Yang M, Bittner H, Ambagala A, Lung O, Zimmerman J, Giménez-Lirola LG, Nfon C. Detection of genome, antigen, and antibodies in oral fluids from pigs infected with foot-and-mouth disease virus. Can J Vet Res. 2017;81(2):82-90.
31. Fablet C, Renson P, Pol F, Dorenlor V, Mahé S, Eono F, Eveno E, Le Dimna M, Liegard-Vanhecke D, Eudier S, Rose N, Bourry O. Oral fluid versus blood sampling in group-housed sows and finishing pigs: Feasibility and performance of antibody detection for porcine reproductive and respiratory syndrome virus (PRRSV). Vet Microbiol. 2017;204:25-34.
32. Petrini S, Pierini I, Giammarioli M, Feliziani F, De Mia GM. Detection of Classical swine fever virus infection by individual oral fluid of pigs following experimental inoculation. J Vet Diagn Invest. 2017;29(2):254-257.
33. Trang NT, Hirai T, Yamamoto T, Matsuda M, Okumura N, Giang NT, Lan NT, Yamaguchi R. Detection of porcine reproductive and respiratory syndrome virus in oral fluid from naturally infected pigs in a breeding herd. J Vet Sci. 2014;15(3):361-367.
34. Kuiek AM, Ooi PT, Yong CK, Ng CF. Comparison of serum and oral fluid antibody responses after vaccination with a modified live (MLV) porcine reproductive and respiratory syndrome virus (PPRSV) vaccine in PRRS endemic farms. Trop Anim Health Prod. 2015;47(7):1337-1342.
35. Biernacka K, Karbowiak P, Wróbel P, Charęza T, Czopowicz M, Balka G, Goodell C, Rauh R, Stadejek T. Detection of porcine reproductive and respiratory syndrome virus (PRRSV) and influenza A virus (IAV) in oral fluid of pigs. Res Vet Sci. 2016;109:74-80.
36. Gibert E, Martín-Valls G, Mateu E. Comparison of protocols for the analysis of type 1 porcine reproductive and respiratory syndrome virus by RT-PCR using oral fluids. J Virol Methods. 2017;243:190-195.
37. Hernandez-Garcia J, Robben N, Magnée D, Eley T, Dennis I, Kayes SM, Thomson JR, Tucker AW. The use of oral fluids to monitor key pathogens in porcine respiratory disease complex. Porcine Health Manag. 2017;3(1):7. doi:10.1186/s40813-017-0055-4.
38. Guinat C, Reis AL, Netherton CL, Goatley L, Pfeiffer DU, Dixon L. Dynamics of African swine fever virus shedding and excretion in domestic pigs infected by intramuscular inoculation and contact transmission. Vet Res. 2014;45(1):93. doi:10.1186/s13567-014-0093-8.
39. Van Cuong N, Carrique-Mas J, Thu HT, Hien ND, Hoa NT, Nguyet LA, Anh PH, Bryant JE. Serological and virological surveillance for porcine reproductive and respiratory syndrome virus, porcine circovirus type 2, and influenza A viruses among smallholder swine farms of the Mekong Delta, Vietnam. J Swine Health Prod. 2014;22(5):224-231.
40. Decorte I, Van Campe W, Mostin L, Cay AB, De Regge N. Diagnosis of the Lelystad strain of porcine reproductive and respiratory syndrome virus infection in individually housed pigs: comparison between serum and oral fluid samples for viral nucleic acid and antibody detection. J Vet Diagn Invest. 2015;27(1):47-54.
41. Linhares DC, Cano JP, Wetzell T, Nerem J, Torremorell M, Dee SA. Effect of modified-live porcine reproductive and respiratory syndrome virus (PRRSv) vaccine on the shedding of wild-type virus from an infected population of growing pigs. Vaccine. 2012;30(2):407-413.
42. Olsen C, Wang C, Christopher-Hennings J, Doolittle K, Harmon KM, Abate S, Kittawornrat A, Lizano S, Main R, Nelson EA, Otterson T, Panyasing Y, Rademacher C, Rauh R, Shah R, Zimmerman J. Probability of detecting porcine reproductive and respiratory syndrome virus infection using pen-based swine oral fluid specimens as a function of within-pen prevalence. J Vet Diagn Invest. 2013;25(3):328-335.
43. Ramirez A, Wang C, Prickett JR, Pogranichniy R, Yoon KJ, Main R, Johnson JK, Rademacher C, Hoogland M, Hoffmann P, Kurtz A, Kurtz E, Zimmerman J. Efficient surveillance of pig populations using oral fluids. Prev Vet Med. 2012;104(3):292-300.
44. Rotolo ML, Sun Y, Wang C, Giménez-Lirola L, Baum DH, Gauger PC, Harmon KM, Hoogland M, Main R, Zimmerman JJ. Sampling guidelines for oral fluid-based surveys of group-housed animals. Vet Microbiol. 2017;209:20-29.
45. Kittawornrat A, Panyasing Y, Goodell C, Wang C, Gauger P, Harmon K, Rauh R, Desfresne L, Levis I, Zimmerman J. Porcine reproductive and respiratory syndrome virus (PRRSV) surveillance using pre-weaning oral fluid samples detects circulation of wild-type PRRSV. Vet Microbiol. 2013;168(2):331-339.
46. Zhang J, Zheng Y, Xia XQ, Chen Q, Bade SA, Yoon KJ, Harmon KM, Gauger PC, Main RG, Li G. High-throughput whole genome sequencing of porcine reproductive and respiratory syndrome virus from cell culture materials and clinical specimens using next-generation sequencing technology. J Vet Diagn Invest. 2017;29(1):41-50.
47. Langenhorst RJ, Lawson S, Kittawornrat A, Zimmerman JJ, Sun Z, Li Y, Christopher-Hennings J, Nelson EA, Fang Y. Development of a fluorescent microsphere immunoassay for detection of antibodies against porcine reproductive and respiratory syndrome virus using oral fluid samples as an alternative to serum-based assays. Clin Vaccine Immunol. 2012;19(2):180-189.
48. Rotolo ML, Giménez-Lirola L, Ji J, Magtoto R, Henao-Diaz YA, Wang C, Baum DH, Harmon KM, Main RG, Zimmerman JJ. Detection of porcine reproductive and respiratory syndrome virus (PRRSV)-specific IgM-IgA in oral fluid samples reveals PRRSV infection in the presence of maternal antibody. Vet Microbiol. 2018;214:13-20.
49. Detmer SE, Patnayak DP, Jiang Y, Gramer MR, Goyal SM. Detection of Influenza A virus in porcine oral fluid samples. J Vet Diagn Invest. 2011;23(2):241-247.
50. Decorte I, Steensels M, Lambrecht B, Cay AB, De Regge N. Detection and isolation of swine influenza A virus in spiked oral fluid and samples from individually housed, experimentally infected pigs: potential role of porcine oral fluid in active influenza A virus surveillance in swine. PloS one. 2015;10(10):e0139586. doi:10.1371/journal.pone.0139586.
51. Allerson MW, Davies PR, Gramer MR, Torremorell M. Infection dynamics of pandemic 2009 H1N1 influenza virus in a two-site swine herd. Transbound Emerg Dis. 2014;61(6):490-499.
52. Gerber PF, Dawson L, Strugnell B, Burgess R, Brown H, Opriessnig T. Using oral fluids samples for indirect influenza A virus surveillance in farmed UK pigs. Vet Med Sci. 2016;3(1):3-12.
53. Panyasing Y, Goodell C, Kittawornrat A, Wang C, Levis I, Desfresne L, Rauh R, Gauger PC, Zhang J, Lin X, Azeem S, Ghorbani-Nezami S, Yoon KJ, Zimmerman J. Influenza A virus surveillance based on pre-weaning piglet oral fluid samples. Transbound Emerg Dis. 2016;63(5):e328-e338. doi:10.111/tbed.12307.
54. Romagosa A, Gramer M, Joo HS, Torremorell M. Sensitivity of oral fluids for detecting influenza A virus in populations of vaccinated and non-vaccinated pigs. Influenza Other Respir Viruses. 2012;6(2):110-118.
55. Baron S, Singh I, Chopra A, Coppenhaver D, Pan J. Innate antiviral defenses in body fluids and tissues. Antiviral Res. 2000;48:71-89.
56. Hughes HR, Vincent AL, Brockmeier SL, Gauger PC, Pena L, Santos J, Braucher DR, Perez DR, Loving CL. Oral fluids as a live-animal sample source for evaluating cross-reactivity and cross-protection following intranasal influenza A virus vaccination in pigs. Clin Vaccine Immunol. 2015;22(10):1109-1120.
57. Strutzberg-Minder K, Boehmer J, Fischer S, Homuth M, Gomez-Duran O, Finger G, Genzow M. Monitoring influenza A virus infection in pigs by using a competitive enzyme-linked immunosorbent assay to detect virus antibodies in pen-based oral fluid specimens. J Swine Health Prod. 2015;23(3):126-131.
*58. Bower L, Madson D, Hoang H, Sun D, Giménez-Lirola L, Magstadst D, Arruda P, Wilberts B, Yoon K. Utility of oral fluid sampling and testing for monitoring PEDV in herds. Proc AASV. Dallas, Texas. 2014:61-62.
59. Zhang J. Porcine deltacoronavirus: Overview of infection dynamics, diagnostic methods, prevalence and genetic evolution. Virus Res. 2016;226:71-84.
60. Homwong N, Jarvis MC, Lam HC, Diaz A, Rovira A, Nelson M, Marthaler D. Characterization and evolution of porcine deltacoronavirus in the United States. Prev Vet Med. 2016;123:168-174.
61. Opriessnig T, Patterson AR, Elsener J, Meng XJ, Halbur PG. Influence of maternal antibodies on efficacy of porcine circovirus type 2 (PCV2) vaccination to protect pigs from experimental infection with PCV2. Clin Vaccine Immunol. 2008;15(3):397-401.
62. Cheong Y, Oh C, Lee K, Cho KH. Survey of porcine respiratory disease complex-associated pathogens among commercial pig farms in Korea via oral fluid method. J Vet Sci. 2017;18(3):283-289.
63. Wilberts BL, Arruda PH, Kinyon JM, Frana TS, Wang C, Magstadt DR, Madson DM, Patience JF, Burrough ER. Investigation of the impact of increased dietary insoluble fiber through the feeding of distillers dried grains with solubles (DDGS) on the incidence and severity of Brachyspira-associated colitis in pigs. PloS one. 2014;9(12):e114741. doi:10.1371/journal.pone.0114741.
64. Giménez-Lirola LG, Xiao CT, Zavala M, Halbur PG, Opriessnig T. Improving ante mortem diagnosis of Erysipelothrix rhusiopathiae infection by use of oral fluids for bacterial, nucleic acid, and antibody detection. J Microbiol Methods. 2013;92(2):113-121.
*65. US Department of Agriculture. Part IV. Changes in the U.S. Pork Industry 1990-2000. In: National Animal Health Monitoring System Swine 2000. Fort Collins, Colorado: Center for Epidemiology and Animal Health, Animal and Plant Health Inspection Service, US Dept of Agriculture; 2005. Publication #N428.0405.
*66. Desrosiers R. Emerging diseases: The past and the future. Proc AASV. Orlando, Florida. 2015:519-537.
67. Schwabe C. The current epidemiological revolution in veterinary medicine. Part I. Prev Vet Med. 1982;1:5-15.
68. Stevenson GW, Hoang H, Schwartz KJ, Burrough ER, Sun D, Madson D, Cooper VL, Pillatzki A, Gauger P, Schmitt BJ, Koster LG, Killian ML, Yoon KJ. Emergence of porcine epidemic diarrhea virus in the United States: clinical signs, lesions, and viral genomic sequences. J Vet Diagn Invest. 2013;25(5):649-654.
69. Wensvoort G, Terpstra C, Pol JMA, ter Laak EA, Bloemraad M, de Kluyver EP, Kragten C, van Buiten L, den Besten A, Wagenaar F, Broekhuijsen JM, Moonen PLJM, Zetstra T, de Boer EA, Tibben HJ, de Jong MF, van’t Veld P, Groenland GJR, van Gennep JA, Voets MTh, Verheijden JHM, Braamskamp J. Mystery swine disease in The Netherlands: the isolation of Lelystad virus. Vet Q. 1991;13:121-130.
70. Chittick WA, Stensland WR, Prickett JR, Strait EL, Harmon K, Yoon KJ, Wang C, Zimmerman JJ. Comparison of RNA extraction and real-time reverse transcription polymerase chain reaction methods for the detection of porcine reproductive and respiratory syndrome virus in porcine oral fluid specimens. J Vet Diagn Invest. 2011;23(2):248-253.
71. Goodell CK, Zhang J, Strait E, Harmon K, Patnayak D, Otterson T, Culhane M, Christopher-Hennings J, Clement T, Leslie-Steen P, Hesse R, Anderson J, Skarbek K, Vincent A, Kitikoon P, Swenson S, Jenkins-Moore M, McGill J, Rauh R, Nelson W, O’Connell C, Shah R, Wang C, Main R, Zimmerman J. Ring test evaluation of the detection of influenza A virus in swine oral fluids by real-time reverse-transcription polymerase chain reaction and virus isolation. Can J Vet Res. 2016;80(1):12-20.
* Non-refereed references.