| Original research | Peer reviewed |
Cite as: Bhattarai S, Framstad T, Nielsen JP. Stillbirths in relation to sow hematological parameters at farrowing: A cohort study. J Swine Health Prod. 2018;26(4):215-222. https://doi.org/10.54846/jshap/1052
Also available as a PDF.
SummaryObjective: To determine associations between stillbirths and sow hematological parameters at farrowing. Materials and methods: A total of 160 sows from a high-performing Danish farrow-to-finish herd were chosen for the study. Standard hematological parameters were measured in sows within nine days before farrowing. At farrowing, dead piglets were collected and stillborns were identified using a lung floatation technique. The number of live-born piglets and parity of the sow was recorded after termination of farrowing. A generalized linear model was fitted to analyze the associations between each hematological parameter and the probability of stillbirth. Results: The mean (standard deviation) sow hemoglobin concentration before farrowing was 108.5 (8.6) g/L. In total, 29 sows (18.1%) were anemic ie, hemoglobin concentration below 100 g/L. The mean number of total born and stillborn piglets per litter was 16.3 (4.1) and 1.2 (2.2), respectively. The average parity of sows was 2.8 (1.8). Piglet stillbirth was associated with several hematological parameters of the sow, namely hemoglobin concentration, mean cell hemoglobin concentration, mean corpuscular hemoglobin, red blood cell distribution width, hemoglobin distribution width, platelet distribution width, number of reticulocytes, reticulocyte hemoglobin content, and reticulocyte cellular volume. Parity of the sow and total number of piglets born per litter were also associated with stillbirths. Implications: The probability of piglet stillbirth in this study is affected by several hematological parameters of the sow. There is also an association between probability of stillbirth and parity of the sow. | ResumenObjetivo: Determinar la asociación entre los nacidos muertos y los parámetros hematológicos de la hembra durante el parto. Materiales y métodos: Para el estudio, se eligieron un total de 160 hembras de un hato de alto desempeño de parto a finalización Danés. Se midieron los parámetros hematológicos estándar en hembras nueve días antes del parto. En el parto, se recolectaron los lechones muertos, y se identificaron los fetos muertos utilizando una técnica de flotación de pulmón. Se registró el número de lechones nacidos vivos y la paridad de la hembra después de terminar el parto. Se ajustó un modelo linear generalizado para analizar la relación entre cada parámetro hematológico y la probabilidad de muerte fetal. Resultados: La concentración media (desviación estándar) de hemoglobina de la hembra antes del parto fue de 108.5 (8.6) g/L. En total, 29 hembras (18.1%) estuvieron anémicas ie, concentración de hemoglobina por debajo de 100 g/L. El número medio del total de lechones nacidos y muertos por camada fue de 16.3 (4.1) y 1.2 (2.2), respectivamente. La paridad promedio de hembras fue de 2.8 (1.8). Los fetos muertos se relacionaron con varios parámetros hematológicos de la hembra, específicamente la concentración de hemoglobina, concentración media de hemoglobina celular, hemoglobina corpuscular media, amplitud de la distribución de glóbulos rojos, amplitud de la distribución de hemoglobina, amplitud de la distribución de plaquetas, número de reticulocitos, contenido de hemoglobina del reticulocito, y volumen celular del reticulocito. También se asociaron la paridad de la hembra y el número total de lechones nacidos por camada con los nacidos muertos. Implicaciones: La probabilidad de muerte fetal del lechón en este estudio esta afectada por varios parámetros hematológicos de la hembra. También hay una relación entre la probabilidad de muerte fetal y la paridad de la hembra. | ResuméObjectif: Déterminer les associations entre les mortinatalités et les paramètres hématologiques à la mise-bas. Matériels et méthodes: Un total de 160 truies provenant d’un troupeau danois haute performance de type naisseur-finisseur a été choisi pour la présente étude. Les paramètres hématologiques standards ont été mesurés chez des truies dans un délai de neuf jours avant la mise-bas. À la mise-bas, les porcelets morts ont été ramassés et les mort-nés ont été identifiés à l’aide d’une technique de flottaison des poumons. Le nombre de porcelets nés vivants et la parité des truies ont été notés à la fin de la mise-bas. Un modèle linéaire généralisé a été ajusté pour analyser les associations entre chaque paramètre hématologique et la probabilité de porcelets mort-nés. Résultats: La moyenne (écart-type) de la concentration en hémoglobine chez les truies avant la mise-bas était de 108,5 (8,6) g/L. Au total, 29 truies (18,1%) étaient anémiques ie, une concentration en hémoglobine inférieure à 100 g/L. Le nombre moyen de porcelets totaux nés et de porcelets mort-nés par portée était de 16,3 (4,1) et 1,2 (2,2), respectivement. La parité moyenne des truies était de 2,8 (1,8). La présence de porcelets mort-nés était associée avec de nombreux paramètres hématologiques de la truie, nommément la concentration en hémoglobine, la concentration moyenne d’hémoglobine cellulaire, la moyenne d’hémoglobine corpusculaire, l’étendue de la distribution des globules rouges, de l’hémoglobine, et des plaquettes, le nombre de réticulocytes, le contenu en hémoglobine des réticulocytes, et le volume cellulaire des réticulocytes. La parité des truies et le nombre total de porcelets nés par portée ont également été associés avec les mort-nés. Implications: La probabilité de porcelets mort-nés dans la présente étude est affectée par plusieurs paramètres hématologiques de la truie. Il y a également une association entre la probabilité de mortinatalités et la parité de la truie. |
Keywords: swine, hemoglobin, stillbirth
Search the AASV web site
for pages with similar keywords.
Received: June 27, 2017
Accepted: February 5, 2018
In Denmark, stillbirth losses average 1.7 piglets per litter,1 which is a serious economic and welfare issue in pig production. This problem has been increasing worldwide with the selection of sows for greater litter sizes.2-4 Increased litter size results in decreased piglet birth weight and increased within-litter variability, which consequently may result in stillborn piglets.5 Since 2004, Denmark’s breeding strategy has been selection for piglets alive at day five instead of selection for large litter sizes. However, the number of stillborn piglets per litter has stayed constant since 2012.
Interventions to reduce the occurrence of stillbirth are very challenging in herds where stillbirths are not related to obvious infections or management factors. It has been suggested that pathogenic agents contribute to only 30% of stillbirths.6 Several sow and piglet characteristics have been identified as potential risk factors for stillbirths. These risk factors include increased litter size, increased parity of the sow, prolonged duration of parturition, premature rupturing of the umbilical cord, birth in the last third of the birth order, and a sow hemoglobin concentration (Hb) of less than 90 g/L.7-10 Stillbirths due to iron deficiency have been reported in older studies,7,10-12 but the results are inconsistent or not representative of modern pig production.
Although sows get iron from the feed, the oral uptake is not always consistent and adequate.13 Parenteral iron supplementation during pregnancy is uncommon. It has been shown that 75%14,15 of stillborn piglets die during delivery and have lower Hb values than live-born piglets.10,11,16 Furthermore, we have previously shown that Hb in the sow is associated to Hb in the piglets.17 Studies of pregnant women have shown that anemia is associated with fetal mortality, spontaneous abortions, premature births, low birth weight, and immunosuppression.18-24 It can be hypothesized that similar reproductive effects may be observed in sows. It is possible that anemia in sows may decrease the oxygen supply, decrease efficiency of uterine contractions, and cause hypoxia in piglets during parturition, thus increasing the number of stillborns. In this context, the main objective of our study was to investigate the associations between hematological parameters of the sow at farrowing and the probability of stillbirths in offspring. The secondary objectives were to determine the prevalence of anemia in sows and the effect of parity on hematological parameters.
Materials and methods
This was a cohort study using a Danish sow herd. It was carried out between July and October 2013. The study was conducted in accordance with the guidelines of the Danish Ministry of Justice with respect to animal experimentation and care of animals under study. Blood withdrawal was carried out by a skilled person with consideration to the welfare of the pigs.
Herd and sow selection
A high performing Danish farrow-to-finish sow herd was chosen for the study. The herd was selected for convenience and consisted of 1700 sows with 75 farrowings per week. The herd average for number of live-born piglets was 15.3 with 1.1 stillborn piglets per litter. A convenience sample of 160 sows from three consecutive farrowing batches were studied at the time of farrowing. In all selected sows, farrowings were induced with prostaglandin by the herd veterinarian. Farrowing induction was a routine procedure in the herd.
Hematology
Ten milliliters of blood were collected from the jugular vein of sows into EDTA tubes within nine days before farrowing and standard hematological measures were performed. The measured parameters were Hb, erythrocyte count, white blood cell count (both peroxidase method and basophil method), neutrophils (absolute count and percentage), lymphocytes (absolute count and percentage), monocytes (absolute count and percentage), eosinophils (absolute count and percentage), basophils (absolute count and percentage), platelets, mean platelet volume, platelet distribution width (PDW), red blood cell distribution width (RDW), hemoglobin distribution width (HDW), hematocrit, mean cell volume (MCV), mean corpuscular hemoglobin (MCH) and mean cell hemoglobin concentration (MCHC). Reticulocyte indices were also measured which included reticulocyte count (absolute and relative), reticulocyte hemoglobin content (Chr), mean reticulocyte corpuscular hemoglobin concentration, reticulocyte cellular volume (MCVr), reticulocyte red cell distribution width, and reticulocyte hemoglobin distribution width. Hemoglobin values received from the laboratory were multiplied by 16.11 to convert from mmol/L to g/L.25 All laboratory analyses were done using the Advia 2120i Hematology System (Siemens Healthcare Diagnostics Inc, Tarrytown, New York) at the Veterinary Diagnostic Laboratory, Institute for Clinical Veterinary Medicine, University of Copenhagen. All methods were carried out following standard protocols of the manufacturer.
Recording stillborn pigs
Dead piglets collected during and immediately after farrowing were necropsied to determine whether they were stillborn. All fully developed piglets with uninflated lungs were considered stillborn whereas those with floating lungs were considered born alive. A piece of lung was removed using scissors and immersed in a cup of water. When the piece sank in the water, the piglet was categorized as a true stillborn assuming the piglet did not breathe. The number of live-born piglets and parity of sow was recorded after termination of farrowing. The total number of piglets born was calculated as the sum of stillborn and live-born piglets.
Statistical analysis
Data analysis was performed using SAS 9.4 (SAS Institute Inc, Cary, North Carolina). The sows were divided into two categories, anemic (Hb < 100 g/L) and non-anemic (Hb ≥ 100 g/L).26 Additionally, anemia was categorized morphologically into three categories: microcytic (MCV ≤ 63 fL), normocytic (MCV > 63 fL ≤ 75 fL) and macrocytic (MCV > 75 fL). It was further categorized as normochromic (MCHC ≥ 18.62 mmol/L) and hypochromic (MCHC < 18.62 mmol/L). These morphological cut off values were chosen based on normal values for sows two weeks or less before parturition.27 Similarly, three parity ranks were defined: parity rank 1 included first parity sows, parity rank 2 included sows between parities 2 and 4, and parity rank 3 included sows in parities higher than 4.
The difference in hematology between the parity categories was assessed by ANOVA using a general linear model (PROC GLM procedure) in case assumptions for the parametric test were met. Pairwise comparisons across parities were made using Least Square Means with Tukey-Kramer adjustment. Whenever assumptions of parametric test were not met, a Kruskal-Wallis test was used and in case of significance, pairwise comparisons were made using the Dwass-Steel-Critchlow-Fligner method.
A similar method was used to detect differences in the total number of piglets born and stillborn piglets between those categories, as the assumptions for the parametric test were not met.
To study associations between sow hematology and stillbirths, the probability of piglet stillbirth was modelled as the outcome variable. The explanatory variables of primary interest were the measured hematological parameters, which were tested separately. Other explanatory variables in each of the analyses were parity rank of the sow, total number of piglets born, and their interaction. A generalized linear model was fitted to analyze the associations between each measured hematological parameter and the probability of stillbirth. This was done with separate models for each hematological parameter using the PROC LOGISTIC procedure. The variables were removed from the model using backward elimination. Model fit was assessed using Deviance and Pearson Goodness-of-Fit statistics. Predicted probabilities of stillbirths were calculated for each level of Hb based on the final model. Statistical significance was set to P < .05.
Results
Altogether, 160 sows were included in the study. The average parity of the sows was 2.8 (± 1.8) with average total born of 16.3 (± 4.1), and stillborns of 1.2 (± 2.2). In total, 2610 piglets were born, of which 195 were stillborn (7.5%). Seventy-seven sows (48.1%) had no stillborn piglets, 41 sows (25.6%) had 1 stillborn piglet, and the remaining 42 sows (26.2%) had more than one stillborn piglet. Table 1 presents the descriptive summary of the study herd with respect to total number of piglets born, stillborn piglets, and mean Hb of sows within each parity distribution.
Table 1: Descriptive farrowing data and sow hemoglobin by parity
| Sow parity | Sows, n (%) | Hb, mean (SD), g/L | Total-Born Piglets, mean (SD) | Stillborn Piglets, mean (SD) |
|---|---|---|---|---|
| 1 | 41 (25.6) | 113.0 (7.0) | 13.9 (3.4) | 1.4 (2.9) |
| 2 | 45 (28.1) | 107.1 (8.8) | 16.2 (3.3) | 1.2 (2.6) |
| 3 | 29 (18.1) | 106.6 (8.0) | 17.7 (3.2) | 0.7 (1.1) |
| 4 | 19 (11.9) | 109.2 (8.0) | 17.9 (5.0) | 1.2 (1.2) |
| 5 | 7 (4.4) | 104.5 (4.9) | 18.0 (4.3) | 1.8 (1.6) |
| 6 | 11 (6.9) | 109.4 (12.7) | 18.5 (3.8) | 1.7 (1.6) |
| 7 | 5 (3.1) | 100.9 (5.2) | 16.2 (5.2) | 1.2 (1.6) |
| 8 | 1 (0.6) | 109.5 | 6.0 | 0.0 |
| 9 | 2 (1.3) | 100.7 (10.2) | 20.0 (1.4) | 0.5 (0.7) |
| Herd total | 160 | 108.6 (8.6) | 16.3 (4.1) | 1.2 (2.2) |
Hb = hemoglobin; SD = standard deviation.
Prevalence of anemia in sows
Altogether, 29 sows (18.1%) were anemic with Hb values below 100 g/L. On average, these sows had 1.7 (± 2.6) stillborn piglets compared to 1.1 (± 2.1) stillborn piglets from non-anemic sows, which had Hb values equal to or greater than 100 g/L. Morphological characterization of anemia revealed that 39 sows had microcytic blood cells, whereas 121 sows had normocytic blood cells. Similarly, 32 sows had hypochromic blood cells, whereas 128 had normochromic blood cells. Only nine sows had both microcytic and hypochromic blood cells. Other sow hematological values are presented in Table 2.
Table 2: Mean (SD) sow hematological values for different parity ranks at farrowing
| Hematological parameters | Unit | Parity rank* | P | Herd average | ||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | ||||
| RBC | × 1012 cells/L | 5.75 (0.39)a | 5.38 (0.47)b | 5.00 (0.45)c | < .001 | 5.41 (0.51) |
| Hct | L/L | 0.36 (0.02)a | 0.35 (0.02)b | 0.34 (0.02)b | < .001 | 0.35 (0.02) |
| Hb | g/L | 113.00 (6.95)a | 107.38 (8.34)b | 105.76 (9.63)b | < .001 | 108.56 (8.61) |
| MCV | fL | 63.98 (2.47)b | 65.10 (2.79)b | 68.63 (2.57)a | < .001 | 65.39 (3.06) |
| MCHC | mmol/L | 19.08 (0.77) | 19.07 (0.58) | 19.15 (0.46) | .83 | 19.08 (0.61) |
| MCH | fmol | 1.22 (0.06)b | 1.24 (0.06)b | 1.31 (0.05)a | < .001 | 1.24 (0.07) |
| HDW | mmol/L | 1.18 (0.09) | 1.18 (0.17) | 1.15 (0.13) | .36 | 1.18 (0.15) |
| Platelets | × 109 cells/L | 160.29 (55.23) | 152.80 (64.29) | 155.53 (55.72) | .80 | 155.16 (60.47) |
| MPV | fL | 9.84 (1.85) | 9.85 (1.86) | 9.63 (1.75) | .88 | 9.81 (1.83) |
| PDW | % | 59.77 (13.42)a | 57.13 (12.23)ab | 50.51 (6.48)b | .02 | 56.73 (12.14) |
| WBC | × 109 cells/L | 15.77 (3.10)a | 12.95 (3.04)b | 11.18 (2.10)c | < .001 | 13.38 (3.29) |
| RDW | % | 16.70 (0.99)a | 16.34 (1.43)a | 15.53 (1.33)b | < .001 | 16.30 (1.36) |
| Mono, count | × 109 cells/L | 0.80 (0.22)a | 0.56 (0.15)b | 0.47 (0.12)c | < .001 | 0.61 (0.20) |
| Lymp, count | × 109 cells/L | 6.54 (1.23)a | 4.54 (1.31)bc | 4.23 (0.79)c | < .001 | 5.00 (1.52) |
| Neut, count | × 109 cells/L | 7.30 (3.02) | 6.88 (3.04) | 5.73 (2.07) | .06 | 6.80 (2.93) |
| Eos, count | × 109 cells/L | 0.92 (0.39)a | 0.78 (0.42)ab | 0.60 (0.25)b | .002 | 0.79 (0.40) |
| Baso, count | × 109 cells/L | 0.08 (0.06)a | 0.05 (0.01)b | 0.03 (0.01)c | < .001 | 0.05 (0.03) |
| Mono, diff | % | 5.18 (1.39)a | 4.46 (1.10)b | 4.27 (1.06)b | .001 | 4.62 (1.22) |
| Lymp, diff | % | 42.50 (8.84)a | 36.39 (11.75)b | 38.73 (8.90)ab | < .001 | 38.34 (10.90) |
| Neut, diff | % | 45.13 (10.21)a | 51.71 (12.49)b | 50.10 (9.99)ab | < .001 | 49.76 (11.83) |
| Eos, diff | % | 5.92 (2.49) | 6.11 (3.03) | 5.63 (2.65) | .69 | 5.98 (2.83) |
| Baso, diff | % | 0.53 (0.33)a | 0.39 (0.14)b | 0.33 (0.10)b | < .001 | 0.41 (0.21) |
| Retic, count | × 109 cells/L | 87.06 (28.34)a | 75.26 (35.59)bc | 62.48 (28.48)c | < .001 | 76.21 (33.53) |
| Retic relative count | % | 1.52 (0.54) | 1.42 (0.80) | 1.27 (0.66) | .09 | 1.42 (0.72) |
| MCVr | fL | 84.20 (3.51)b | 85.25 (3.84)b | 87.66 (2.94)a | < .001 | 85.37 (3.77) |
| CHCMr | mmol/L | 16.18 (0.41) | 16.24 (0.47) | 16.38 (0.40) | .20 | 16.25 (0.45) |
| Chr | fmol | 1.35 (0.06)b | 1.37 (0.06)b | 1.42 (0.05)a | < .001 | 1.37 (0.06) |
| RDWr | % | 15.24 (1.13)a | 15.27 (1.67)a | 14.64 (2.39)b | .01 | 15.16 (1.70) |
| HDWr | mmol/L | 1.55 (0.13) | 1.61 (0.21) | 1.65 (0.30) | .30 | 1.60 (0.21) |
* Parity rank1 included first parity sows, parity rank 2 included sows between parities 2 and 4, and parity rank 3 included sows in parities higher than 4.
abc Means within a row with different superscripts are significantly different (P < .05; ANOVA in case assumptions of parametric test were met, Kruskal-Wallis test in case assumptions of parametric test were not met).
SD = standard deviation; RBC = red blood cell count; Hct = hematocrit; Hb = hemoglobin; MCV = mean corpuscular volume; MCHC = mean cell hemoglobin concentration; MCH = mean corpuscular hemoglobin; HDW = hemoglobin distribution width; MPV = mean platelet volume; PDW = platelet distribution width; WBC = white blood cell count; RDW = red blood cell distribution width; Mono = monocytes; Lymp = lymphocytes; Neut = neutrophils; Eos = eosinophils; Baso = basophils; diff = differential; Rectic = reticulocyte; MCVr = reticulocyte cellular volume; CHCMr = mean reticulocyte corpuscular hemoglobin concentration; Chr = reticulocyte hemoglobin content; RDWr = reticulocyte distribution width; HDWr = reticulocyte hemoglobin distribution width.
Differences across parities
There were 41 parity rank 1 sows, 93 sows in parity rank 2, and 26 sows in parity rank 3. A significant difference in Hb levels among the three parity ranks was found (P < .001). Parity rank 1 sows had significantly higher Hb (113.0 ± 6.9 g/L) compared to parity rank 2 (107.4 ± 8.3 g/L) and parity rank 3 (105.8 ± 9.6 g/L) sows (P = .001 in both cases). There was no difference in Hb values between parity rank 2 and parity rank 3 sows (P = .65). The differences in other hematological parameters across parity ranks are presented in Table 2. The total number of piglets born was different among the three parity ranks (P < .001). Parity rank 1 sows had significantly fewer total born piglets (13.9 ± 3.4) compared to parity rank 2 (17.0 ± 3.7) and parity rank 3 (17.6 ± 4.6) sows (P < .001 and P = .0025, respectively). No difference was found in the total number of piglets born between parity rank 2 and parity rank 3 sows (P = .92). Similarly, there was no difference in the number of stillborn piglets among the parity ranks (P = .14).
Stillbirths in relation to sow hematological parameters
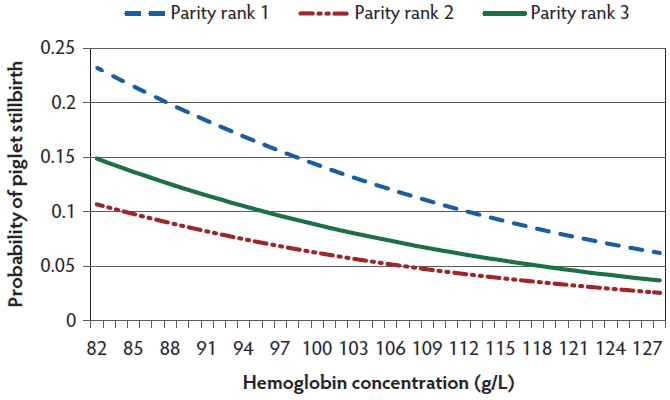
The results from the final generalized linear model measuring associations between hematology parameters and probability of stillbirth are shown in Table 3. Piglet stillbirths were associated with several hematological parameters, namely Hb (Figure 1), MCH, MCHC, RDW, HDW, PDW, the number of reticulocytes, Chr, and MCVr. The probability of stillbirth in relation to these hematological parameters was dependent on parity of the sow and total number of piglets born per litter. No interaction was found between parity of the sow and total number of piglets born per litter in any of the analysis.
Table 3: Effect of sow hematology at farrowing on the probability of stillborn piglets per litter
| Hematological parameters | Probability estimate | Standard error | P* |
|---|---|---|---|
| Hemoglobin (g/L) | -0.0330 | 0.0096 | < .001 |
| Intercept | 0.0829 | 1.1498 | .943 |
| Parity rank 1†| 0.5487 | 0.1363 | < .001 |
| Parity rank 2†| -0.3780 | 0.1025 | < .001 |
| Total born | 0.05487 | 0.1363 | .012 |
| MCH (fmol) | -4.1942 | 1.1743 | < .001 |
| Intercept | 1.4101 | 1.4651 | .336 |
| Parity rank 1 | 0.2988 | 0.1355 | .027 |
| Parity rank 2 | -0.4481 | 0.1069 | < .001 |
| Total born | 0.0822 | 0.0244 | < .001 |
| MCHC (mmol/L) | -0.6375 | 0.1313 | < .001 |
| Intercept | 8.1094 | 2.4371 | < .001 |
| Parity rank 1 | 0.4604 | 0.1334 | < .001 |
| Parity rank 2 | -0.4074 | 0.1037 | <.001 |
| Total born | 0.0921 | 0.0249 | < .001 |
| RDW (%) | -0.2193 | 0.0648 | < .001 |
| Intercept | 0.0229 | 1.1480 | .984 |
| Parity rank 1 | 0.5292 | 0.1351 | < .001 |
| Parity rank 2 | -0.3208 | 0.1023 | .002 |
| Total born | 0.0615 | 0.0231 | .008 |
| HDW (mmol/L) | -2.0607 | 0.6569 | .002 |
| Intercept | -1.2259 | 0.8637 | .156 |
| Parity rank 1 | 0.4655 | 0.1320 | < .001 |
| Parity rank 2 | -0.3507 | 0.1022 | < .001 |
| Total born | 0.0691 | 0.0233 | .003 |
| PDW (%) | -0.0166 | 0.00714 | .012 |
| Intercept | -2.6484 | 0.5857 | < .001 |
| Parity rank 1 | 0.4674 | 0.1313 | < .001 |
| Parity rank 2 | -0.3226 | 0.1022 | .002 |
| Total born | 0.0651 | 0.0234 | .005 |
| Reticulocytes (× 109 cells/L) | -0.00540 | 0.00272 | .047 |
| Intercept | -3.2448 | 0.4536 | < .001 |
| Parity rank 1 | 0.4889 | 0.1347 | < .001 |
| Parity rank 2 | -0.3561 | 0.1023 | < .001 |
| Total born | 0.0701 | 0.0232 | .003 |
| Chr (fmol) | -3.8509 | 1.2227 | .002 |
| Intercept | 1.4260 | 1.6451 | .386 |
| Parity rank 1 | 0.3322 | 0.1329 | .012 |
| Parity rank 2 | -0.4156 | 0.1051 | < .001 |
| Total born | 0.0880 | 0.0247 | < .001 |
| MCVr (fL) | -0.0499 | 0.0215 | .020 |
| Intercept | 0.5436 | 1.8341 | .767 |
| Parity rank 1 | 0.3540 | 0.1324 | .008 |
| Parity rank 2 | -0.3812 | 0.1035 | < .001 |
| Total born | 0.0751 | 0.0236 | .001 |
* Statistical analysis was done using a generalized linear model. The probability of piglet stillbirth was modeled as the outcome variable with sow hematological parameters, sow parity rank, total number of piglets born, and their interaction as explanatory variables. Parity rank 3 is the reference group in each of the analysis.
† Parity rank1 included first parity sows, parity rank 2 included sows between parities 2 and 4, and parity rank 3 included sows in parities higher than 4.
MCH = mean corpuscular hemoglobin; MCHC = mean cell hemoglobin concentration; RDW = red blood cell distribution width; HDW = hemoglobin distribution width; PDW = platelet distribution width: Chr = reticulocyte hemoglobin content; MCVr = reticulocyte mean corpuscular volume.
Figure 1: Probability of stillbirths in relation to sow hemoglobin concentration at farrowing. Parity rank1 included first parity sows, parity rank 2 included sows between parities 2 and 4, and parity rank 3 included sows in parities higher than 4. Probability was estimated with 16 total born piglets using the final generalized linear model (P < .001).
Discussion
The herd selected for this study had good health status and high productivity with 15.3 live-born and 1.1 stillborn piglets per litter. In this study, stillborn piglets were observed in 83 (51.9%) litters and the stillborn percentage was relatively low (7.4%) compared to the average in Denmark1 (9.6%) which may be related to good farrowing surveillance and use of prostaglandin for farrowing induction. This is in agreement with other studies that have shown reduced stillborn piglets per litter in attended farrowings compared to non-attended farrowings.28,29 Similarly, induced farrowings result in a decreased number of stillbirths compared to non-induced farrowings.30 Furthermore, the stillborn piglets reported in this study are true stillborn piglets identified by lung floatation technique, whereas the national figures are based on numbers reported by workers at the farm using visual judgement. The stillbirth rate was similar or higher than reported in earlier international literature which lies between 5.6 to 7.5%.31,32 In these studies, a smaller litter size was observed, 12.2 and 13.5, compared to 16.3 total born piglets in the present study.31,33 Nevertheless, good farrowing surveillance and use of prostaglandin in our study may have influenced the effect of sow hematology on the stillbirth rate. Furthermore, different sow and piglet factors reported to be associated with piglet stillbirth,33 such as farrowing duration, sow body condition, and piglet birth order, were not included in this study.
The mean sow Hb values from this study were below the normal reference interval (110 to 145 g/L) for sows two weeks or less before parturition.27 However, Hb reference ranges vary greatly between breeds, age, season, physiological status, sample size, other management factors, and the laboratory measurement techniques. The Hb values in the study sows decreased after first parity, which is in agreement with other studies.32
This study indicates that stillbirths are negatively associated to Hb and other hematological values related to physiological performance of the sow at farrowing. The association between stillbirths and hematological values in the sow may be related to oxygen supply during farrowing or related to the nutritional iron deficiency in the sow. High hematological values of the sow may also reflect the efficiency of uterine contractions and the vigor of the litter at the onset of parturition. This might have a positive effect in reducing the number of stillborn piglets.
Both the indices of mature erythrocytes (Hb, MCH, MCHC, RDW, HDW) and indices of immature erythrocytes (reticulocytes, Chr, MCVr) showed an association with stillbirths. Indices of immature erythrocytes (eg, reticulocytes) show more recent bone marrow activity because of their short life span as compared to the indices of mature erythrocytes.34,35 Therefore, the stillbirths associated with immature erythrocyte indices may be related to sow physiological characteristics during or shortly before farrowing, although blood samples were taken in this study within nine days before farrowing. However, changes in mature erythrocyte indices associated with stillbirths are also related to hematological changes long before farrowing. The change in mature erythrocyte indices could also be related to piglet development in the uterus before parturition. Further investigations are required to study this effect.
Considerably increased RDW, HDW, PDW, and reticulocytes in the sow can reflect iron deficiency and therefore, the probability of stillbirths would be expected to increase. However, this was not seen in our study because all these parameters showed a negative association with the proportion of stillbirths. The role of hematological parameters other than Hb in stillbirths has not been studied before and the exact role is therefore unknown.
In a Canadian study, an association between the probability of stillbirth and reduced Hb in piglets was found, but no association was observed between stillbirth and sow Hb in the final statistical model.11 It has been reported that stillborn piglets have lower Hb values than live-born piglets.10,16 We have previously shown that Hb values in newborn piglets are related to Hb values in the sow.17 Therefore it seems that Hb levels of both the sow and piglets are important factors related to stillbirth.
Some sows in this herd had microcytic or hypochromic blood cells, though the number of sows that had both microcytic and hypochromic blood cells was very few. Microcytic-hypochromic anemia is one of the striking features of iron deficiency. Nevertheless, iron deficiency is the main cause of microcytic anemia in which the red blood cells appear smaller. Lead poisoning and vitamin B6 (pyridoxine) deficiency also cause microcytic anemia but these conditions are not reported in sows under commercial conditions.
This study shows an association between probability of stillborn piglets and parity of the sow. Stillbirth probability in parity rank 1 and 3 sows was higher compared to parity rank 2 sows. This result is consistent with the findings of Leenhouwers et al15 who observed a greater number of stillbirths per litter in first parity sows than in second parity sows. The number of stillbirths then increased between the second and fifth parity. Canario et al36 also found a greater probability of stillbirths in first parity sows compared to second parity sows. A larger number of stillbirths in first parity sows could be related to too narrow a birth canal or a small uterus.15,36,37 The stillbirths in higher parity sows could be related to poor muscle tone, increased farrowing duration, and pathological changes in the reproductive tract.38
The probability of stillbirth was dependent on the total number of piglets born. This is in agreement with previous studies which report higher stillbirths with increased litter size.2,3 Selection for increased litter size may result in decreased piglet birth weight and increased within-litter variability, which consequently results in more stillborn piglets.5 Studies have also shown that increased litter size results in longer farrowing duration increasing the risk of piglet hypoxia due to detachment of the placenta or rupture of the umbilical cord.11,33,36,39
It has been estimated that of all stillborn piglets, most of them die during farrowing and only a few of them die either shortly before or immediately after farrowing. Such differentiation of stillborn piglets was not made in the current study. The role of hematological parameters in the farrowing process is obscure. A possible explanation for the association between Hb and other hematological parameters and stillbirths could be decreased oxygen supply in the piglets due to low iron status in the sow. This suggests the possibility of decreasing the number of stillborn piglets by improving the sow hematological status. The main limitation of this study is that only one sow herd was investigated, therefore future studies on additional herds are warranted. Furthermore, studies are needed to investigate whether sow Hb values can be increased, which could serve as a herd intervention to reduce the number of stillborn piglets.
Implications
- In this study, the probability of piglet stillbirth is affected by several hematological parameters of the sow.
- Piglet stillbirths may be reduced by modifying hematological levels of the sow.
- Further studies are needed to investigate whether sow Hb can be increased (eg, iron supplementation) to have better oxygen carrying capacity.
Acknowledgements
The authors wish to thank Pharmacosmos A/S for funding the blood testing and transport during the study. Anna Kathrine Jensen is acknowledged for assistance in the herd.
Conflict of interest
Jens Peter Nielsen has consulted for Pharmacosmos A/S which financed the laboratory testing of samples in this study.
Disclaimer
Scientific manuscripts published in the Journal of Swine Health and Production are peer reviewed. However, information on medications, feed, and management techniques may be specific to the research or commercial situation presented in the manuscript. It is the responsibility of the reader to use information responsibly and in accordance with the rules and regulations governing research or the practice of veterinary medicine in their country or region.
References
*1. Jessen O. National average productivity in pig production in 2015 (Landsgennemsnit for productivitet i svineproduktionen 2015). http://svineproduktion.dk/Publikationer/Kilder/Notater/2016/1611.aspx. Published June 2016. Accessed April 6, 2017.
2. Rosendo A, Druet T, Gogué J, Canario L, Bidanel J. Correlated responses for litter traits to six generations of selection for ovulation rate or prenatal survival in French Large White pigs. J Anim Sci. 2007;85(7):1615-1624.
3. Hanenberg E, Knol E, Merks J. Estimates of genetic parameters for reproduction traits at different parities in Dutch Landrace pigs. Livest Prod Sci. 2001;69(2):179-186.
*4. PigCHAMP. 2016 Benchmarking Summaries. http://www.pigchamp.com/benchmarking/benchmarking-summaries. Published 2017. Accessed January 11, 2018.
5. Wolf J, Žáková E, Groeneveld E. Within-litter variation of birth weight in hyperprolific Czech Large White sows and its relation to litter size traits, stillborn piglets and losses until weaning. Livest Sci. 2008;115(2-3):195-205.
6. Vanroose G, de Kruif A, Van Soom A. Embryonic mortality and embryo–pathogen interactions. Anim Reprod Sci. 2000;60-61:131-143.
7. Moore R, Redmond H, Livingston C Jr. Iron deficiency anemia as a cause of stillbirths in swine. J Am Vet Med Assoc. 1965;147(7):746-748.
*8. Randall G. Studying stillbirths. Pig Farming. 1972;20(suppl):53-55.
9. Bille N, Nielsen N, Larsen J, Svendsen J. Preweaning mortality in pigs. II. The perinatal period. Nord Vet Med. 1974;26:294-313.
10. Rootwelt V, Reksen O, Farstad W, Framstad T. Associations between intrapartum death and piglet, placental, and umbilical characteristics. J Anim Sci. 2012;90(12):4289-4296.
11. Zaleski HM, Hacker RR. Variables related to the progress of parturition and probability of stillbirth in swine. Can Vet J. 1993;34(2):109-113.
12. Archibald R, Hancock EE. Iron deficiency – stillbirth of swine. Can J Comp Med. 1939; 3(5):134.
13. Ullrey D, Miller E, West D, Schmidt D, Seerley R, Hoefer J, Luecke R. Oral and parenteral administration of iron in the prevention and treatment of baby pig anemia. J Anim Sci. 1959;18(1):256-263.
14. Glastonbury J. Preweaning mortality in the pig. Aust Vet J. 1977;53(7):310-314.
15. Leenhouwers JI, van der Lende T, Knol EF. Analysis of stillbirth in different lines of pig. Livest Prod Sci. 1999;57(3):243-253.
16. Svetina A, Vrabac L, Belić M, Turk R. Relation between erythrocyte parameters and stillbirth in piglets. Veterinarski arhiv. 2006;76(4):297-303.
*17. Jensen AK, Pedersen KS, Nielsen JP. Association between blood haemoglobin concentration in sows and neonatal piglets. In: Proceedings of the 5th European Symposium of Porcine Health Management; May 22-24 2013; Edinburgh, United Kingdom.
18. Tomashek KM, Ananth CV, Cogswell ME. Risk of stillbirth in relation to maternal haemoglobin concentration during pregnancy. Matern Child Nutr. 2006;2(1):19-28.
19. Zhou LM, Yang WW, Hua JZ, Deng CQ, Tao X, Stoltzfus RJ. Relation of hemoglobin measured at different times in pregnancy to preterm birth and low birth weight in Shanghai, China. Am J Epidemiol. 1998;148(10):998-1006.
20. Ren A, Wang J, Ye R, Li S, Liu J, Li Z. Low first-trimester hemoglobin and low birth weight, preterm birth and small for gestational age newborns. Int J Gynaecol Obstet. 2007;98(2):124-128.
21. Scanlon KS, Yip R, Schieve LA, Cogswell ME. High and low hemoglobin levels during pregnancy: differential risks for preterm birth and small for gestational age. Obstet Gynecol. 2000;96(5, Part 1):741-748.
22. Kandoi A, Bhatia B, Pandey L, Pandey S, Sen P, Satya K. Cellular immunity status in anaemia in pregnancy. Indian J Med Res. 1991;94:11-15.
23. Dallman PR. Iron deficiency and the immune response. Am J Clin Nutr. 1987;46(2):329-334.
24. Prema K, Ramalakshmi B, Madhavapeddi R, Babu S. Immune status of anaemic pregnant women. Br J Obstet Gynaecol. 1982;89(3):222-225.
25. Bhattarai S, Nielsen JP. Early indicators of iron deficiency in large piglets at weaning. J Swine Health Prod. 2015;23(1):10-17.
26. Egeli AK, Framstad T, Grønningen D.The effect of peroral administration of amino acid-chelated iron to pregnant sows in preventing sow and piglet anaemia. Acta Vet Scand. 1998;39(1):77-87.
27. Thorn CE. Hematology of the pig. In: Weiss DJ, Wardrop KJ, eds. Schalm’s Veterinary Hematology. Ames, Iowa: Wiley-Blackwell; 2010:848.
28. Holyoake PK, Dial GD, Trigg T, King VL. Reducing pig mortality through supervision during the perinatal period. J Anim Sci. 1995;73(12):3543-3551.
29. White K, Anderson D, Bate LA. Increasing piglet survival through an improved farrowing management protocol. Can J Anim Sci. 1996;76(4):491-495.
30. Cerne F, Jöchle W. Clinical evaluations of a new prostaglandin analog in pigs: 1. Control of parturition and of the MMA-syndrome. Theriogenology. 1981;16(4):459-467.
31. Vanderhaeghe C, Dewulf J, De Vliegher S, Papadopoulos G, de Kruif A, Maes D. Longitudinal field study to assess sow level risk factors associated with stillborn piglets. Anim Reprod Sci. 2010;120(1):78-83.
32. Normand V, Perrin H, Auvigne V, Robert N, Laval A. Anaemia in the sow: a cohort study to assess factors with an impact on haemoglobin concentration, and the influence of haemoglobin concentration on the reproductive performance. Vet Rec. 2012;171:350.
33. Borges VF, Bernardi ML, Bortolozzo FP, Wentz I. Risk factors for stillbirth and foetal mummification in four Brazilian swine herds. Prev Vet Med. 2005;70(3):165-176.
34. Macdougall IC, Cavill I, Hulme B, Bain B, McGregor E, McKay P, Sanders E, Coles GA, Williams JD. Detection of functional iron deficiency during erythropoietin treatment: a new approach. BMJ. 1992;304(6821):225-226.
35. Mast AE, Blinder MA, Dietzen DJ. Reticulocyte hemoglobin content. Am J Hematol. 2008;83(4):307-310.
36. Canario L, Cantoni E, Le Bihan E, Caritez JC, Billon Y, Bidanel JP, Foulley JL. Between-breed variability of stillbirth and its relationship with sow and piglet characteristics. J Anim Sci. 2006;84(12):3185-3196.
37. Le Cozler Y, Guyomarc’h C, Pichodo X, Quinio P-Y, Pellois H. Factors associated with stillborn and mummified piglets in high-prolific sows. Anim Res. 2002;51:261-268.
38. English P, Morrison V. Causes and prevention of piglet mortality. Pig News Inf. 1984;5(4):369-376.
39. Lucia Jr T, Corrêa MN, Deschamps JC, Bianchi I, Donin MA, Machado AC, Meincke W, Matheus JE. Risk factors for stillbirths in two swine farms in the south of Brazil. Prev Vet Med. 2002;53(4):285-292.
* Non-refereed references.