| Original research | Peer reviewed |
Cite as: Craig JR, Collins CL, Athorn RZ, et al. Investigating the reproductive performance of gilt progeny entering the breeding herd. J Swine Health Prod. 2017;25(5):230–237.. https://doi.org/10.54846/jshap/1001
Also available as a PDF.
SummaryObjective: To quantify the performance of gilt progeny in the F1 breeding herd at a large swine farm in New South Wales, Australia (Rivalea Australia Pty Ltd). Materials and methods: Performance data on all gilts selected for entrance to the commercial breeding herd from January 2014 until December 2015 were included in this study. Comparisons were made between gilt and sow progeny in terms of the proportion of animals to reach first breeding, performance to parity 4, and longevity to parity 3. Results: Gilt progeny were lighter than sow progeny at each live weight measurement (P < .001), and had a higher P2 backfat level at selection than sow progeny (P = .02) at the same live weight. Gilt progeny selected into the breeding herd reached first breeding before 220 days of age less often than sow progeny (P < .001) and were 1 day older at first breeding (P = .003). Sow progeny had a lower farrowing rate from this breeding (P < .001). After the first breeding, there were few differences in performance indices between groups for the first four parities. There were no statistically significant differences between the groups in terms of longevity indices. Implications: Fewer gilt progeny may be selected to enter the breeding herd; however, after farrowing their first litter, selected gilt progeny perform just as well as sow progeny. While it is recommended to continue to include gilt progeny in the replacement-gilt selection process, further research in this field is recommended. | ResumenObjetivo: Cuantificar el desempeño de la descendencia de primerizas en el hato de cría F1 en una granja porcina grande en Nuevo Sur Gales, Australia (Rivalea Australia Pty Ltd). Materiales y métodos: En este estudio, se incluyó la información del desempeño de todas las primerizas seleccionadas para entrar al hato de cría comercial entre enero del 2014 hasta diciembre del 2015. Se comparó, entre las descendencias de primerizas y hembras destetadas, la proporción de animales que llegaron a primera inseminación, desempeño hasta la paridad 4, y longevidad hasta la paridad 3. Resultados: La descendencia de las primerizas fue más ligera que la descendencia de las hembras destetadas en cada medición de peso vivo (P < .001), y a la selección, tuvieron más grasa dorsal al nivel P2 (P = .02) al mismo peso vivo. La descendencia de las primerizas seleccionadas para entrar al hato de cría, llegaron a su primera inseminación, antes de los 220 días de edad, con menos frecuencia que la descendencia de las hembras destetadas (P < .001) y también tenían 1 día más de edad (P = .003). La descendencia de las hembras destetadas mostró una porcentaje de fertilidad más bajo en esta inseminación (P < .001). Después de la primera inseminación, se encontraron pocas diferencias en los índices de desempeño entre los grupos en las primeras cuatro paridades. No hubo diferencias estadísticamente significativas entre los grupos en términos de índices de longevidad. Implicaciones: Se puede seleccionar menos progenie de primerizas para ser introducidas al hato; sin embargo, después de la primera camada, la progenie de las primerizas seleccionadas tiene el mismo comportamiento que las hijas de hembras. Aunque se recomienda continuar incluyendo la progenie de primerizas dentro del proceso de reemplazo de primerizas, se recomienda más investigación en este campo. | ResuméObjectif: Quantifier les performances de la progéniture de cochettes dans le troupeau reproducteur F1 d’une grosse ferme porcine dans la région de New South Wales, Australie (Rivalea Australia Pty Ltd). Matériels et méthodes: Les données de performance de toutes les cochettes sélectionnées pour être introduite dans le troupeau reproducteur commercial entre janvier 2014 et décembre 2015 ont été incluses dans cette étude. Des comparaisons ont été faites entre la progéniture des cochettes et de truies en termes de proportion d’animaux atteignant le premier accouplement, de performances jusqu’à la parité 4, et la longévité jusqu’à parité 3. Résultats: La progéniture des cochettes étaient moins lourdes que celle des truies à chaque point de mesure du poids (P < 0,001) et avait une épaisseur de gras dorsal P2 plus grande au moment de la sélection que la progéniture des truies (P = 0,02) au même poids vif. La progéniture des cochettes sélectionnée pour introduction dans le troupeau reproducteur a été accouplée avant l’âge de 220 jours moins souvent que la progéniture des truies (P < 0,001) et était 1 jour plus âgé au premier accouplement (P = 0,003). La progéniture des truies avait un taux inférieur de mise-bas relié à cet accouplement (P < 0,001). Après le premier accouplement, il y avait peu de différences dans les indices de performance entre les groupes pour les quatre premières parités. Il n’y avait aucune différence statistiquement significative entre les groupes en termes d’indices de longévité. Implications: Un nombre moindre de la progéniture des cochettes pourrait être sélectionné pour introduction dans le troupeau reproducteur; toutefois, suite à la mise-bas de leur première portée, la progéniture sélectionnée des cochettes performe aussi bien que la progéniture des truies. Bien qu’il soit recommandé de continuer à introduire la progéniture des cochettes dans le processus de sélection de remplacement des cochettes, des études supplémentaires dans ce domaine sont recommandées. |
Keywords: swine, gilt progeny, selection, breeding, reproductive performance
Search the AASV web site
for pages with similar keywords.
Received: September 7, 2016
Accepted: March, 7, 2017
Gilts represent a significant proportion of the Australian breeding herd, with recent sow turnover rates in Australia reported at 56.1%, and with 22.7% of sows bred being primiparous.1 First litter progeny born to these sows (“gilt progeny”) are eligible for selection as replacement gilts themselves in nucleus and F1 breeding herds.
Gilt progeny, however, are generally born2,3 and weaned4,5 lighter than progeny born to multiparous sows, are lighter at the conclusion of the finishing stage,6 and exhibit higher rates of disease and mortality in the early stages of development before and immediately after weaning.3,7 Differences in growth performance may be a consequence of breeding gilts at a young age, when they are still partitioning energy into their own growth rather than the growth of their fetuses,8 and when uterine capacity may be limiting.9,10 Higher morbidity and mortality rates in gilt progeny may be caused by differences in colostrum intake, quality, and absorption, as colostrum from gilts may be lower in yield11 and may contain lower concentrations of immunoglobulins (Ig)12-14 and growth factors15,16 than colostrum from sows of higher parities. These characteristics may have negative implications for the selection of gilt progeny as replacements in the breeding herd and their reproductive performance and overall longevity.
Gilt progeny are more likely to be selected into nucleus herds that utilise estimated breeding values (EBVs) in their selection process as a result of increased genetic turnover. In F1 multiplier herds, which may not have EBVs calculated, having lighter body weights at selection as a result of slower growth rates early in life may cause a greater proportion of gilt progeny to fail to be selected for breeding. Little is known about the effect of dam parity on reproductive performance of the resulting progeny; however, there is evidence to suggest that being born to a gilt can result in lower re-breeding rates and prolonged wean-to-estrus intervals (WEIs).17 Additionally, females that are compromised in terms of birth weight,18 colostrum intake and immune status,19,20 and growth rate and live weight around the time of selection and first breeding,21-23 have been shown to exhibit a poorer reproductive capacity.
Research in this field is warranted to give an understanding of the effects of selecting gilt progeny as breeding females in order to determine whether it is economically viable to involve these smaller, slower growing progeny in the selection process. If these progeny are compromised in terms of reproductive capacity and longevity in the breeding herd due to the shortcomings mentioned, producers could make decisions about their selection processes to improve herd efficiency. The purpose of this study was to benchmark the reproductive performance of F1 gilts born to primiparous sows (gilt progeny) compared to that of gilts born to multiparous sows (sow progeny) and investigate their reproductive outcomes in the breeding herd. It was hypothesized that gilt progeny would take longer, or indeed fail, to reach first breeding more often, and would have higher rates of gestation failure, lower litter sizes at birth and weaning, longer WEIs, and poorer overall reproductive longevity.
Materials and methods
Animals
This experiment involved collection of retrospective production data records under commercial field conditions. In this case, animals were not manipulated beyond what would be required for diagnostic purposes and were adequately housed and humanely cared for according to the Model Code of Practice for the Welfare of Animals: Pigs (Australia).
Retrospective production records for a total of 18,136 gilts (Primegro; bred on farm) selected to enter the multiplier (F1) breeding herd at Rivalea Australia’s site in Corowa, New South Wales, from 1 January 2014 to 31 December 2015, were included in this study. This included 3164 gilt progeny (parity 1) and 14,972 sow progeny (parities 2 to 9; average 3.6). Records analyzed prior to selection were therefore included only for gilts that were selected to the breeding herd, as including data from animals not selected, but eligible for selection, was beyond the scope of this study.
Within this multiplier herd, gilts were selected on-site at approximately 23 to 24 weeks of age. Selection criteria included live weight (gilts had to be heavier than 70 kg at selection to be used for breeding); body, vulva, and udder conformation; teat number; and absence of physical defects such as hernias or lameness. Selection was carried out each week by a small group of trained staff, with personnel rotated each day. These selection criteria were different from those used for the nucleus herd, which included calculation of EBVs on the basis of reproductive and growth performance of relatives, live weight and backfat at selection, and numerous other records.
These animals were managed under commercial conditions at Rivalea Australia’s Corowa site. The site consisted of five farms, all of which housed gestating sows in group pens throughout gestation in various group sizes depending on farm (space allowance approximately 2 m2 per sow). Once selected, gilts were kept for approximately 5 weeks at the parent farm, after which they were transported to the breeding barn of one of the five individual farms for boar exposure and estrus detection from this period onwards (approximately 28 to 29 weeks of age, depending on farm). Gilts were then brought to the designated breeding area at least once daily and exposed to a number of “teaser” boars to stimulate puberty. Gilts were bred by artificial insemination at <>the second observed estrus; however, they might also have been bred at first or third (or later) estrus depending on the farm, time of year, and management recommendation indicated by the approximate weight at each observed estrus (measured by the Allometric Growth Tape for Gilts; Swine Reproduction and Development Program (SRDP), University of Alberta, Edmonton, Canada). The growth tape approximated the live weight of the animal at estrus according to the circumference of the girth at the level of the shoulder with recommendation of either breeding or measuring again at the next observed estrus (101 to 135 kg), breeding at the observed estrus (136 to 150 kg), or not breeding (< 100 kg or > 150 kg) on the basis of this approximation.
Gilts were given ad libitum access to a number of commercial weaner and grower diets from weaning until selection, and a specific gilt developer diet from selection until first breeding. In gestation, gilts and sows were fed approximately 2.3 to 2.5 kg per day of a commercial gestation diet up until farrowing. Access to feed was ad libitum during lactation, except in the first 4 days after farrowing where they were fed on a step-up program.
Data collection
Data was extracted from Rivalea Australia’s record-keeping program (PigFM). All records for all females selected during the experimental period were used in the analysis. This meant that females were at different stages of their reproductive life cycle at the end of the recording period; however, this was accounted for in the statistical analysis. Records analyzed prior to selection included birth litter size (BLS; n = 18,136), birth weight (BWT, kg; n = 12,815), 21-day weight (21WT, kg; n = 9263), teat number at birth (Teat#; n = 14,156), post-weaning weight (approximately 2 weeks post weaning; PWWT, kg; n = 3224), selection weight (at approximately 23 to 24 weeks of age; SelWT, kg; n = 13,201), and selection backfat (P2, mm; n = 3929). Live weights at 21 days of age and PWWT of a subset of these gilts were obtained from an ongoing subsequent project (R. Z. Athorn, K. L. Bunter, J. R. Craig; unpublished data, 2017).
Gilts were categorized into quartile groups according to their birth and selection weights, with the groups being light (< 1.39 kg at birth and < 95 kg at selection), medium (1.39 to 1.59 kg; 95 to 102 kg), heavy (1.60 to 1.83 kg; 103 to 110 kg), and extra heavy (> 1.83 kg; > 110 kg).
Records analyzed after selection included age at first observed estrus (not recorded for every gilt; AgeE1; days; n = 2640), age at first breeding (whether successful or not; AgeB1; days; n = 14,077), days between first observed estrus and first breeding (B1-E1; days; n = 2390), approximate weight at first breeding (measured using the growth tape, SRDP; B1WT; kg; n = 10,448), and days between selection and first breeding (B1-Sel; days; n = 14,077). Age at breeding (Age; days), gestation length (GL; days), number born alive (BA), number of stillbirths (SB), number of mummified fetuses (Mumm), total born (TB), lactation length (LL), number of pigs weaned (#W), and subsequent WEI were recorded at each parity achieved in the recording period, regardless of the number of the breeding at which this parity was achieved. Records analyzed for lifetime performance within the recording period included traits relating to sow medications, such as total number of medication events (Med#; n = 18,136) and age first medicated (AgeMed; days; n = 2338). Average WEI (AveWEI; days; n = 8266), total breedings (TotB; n = 14,077), total litters produced (TotL; n = 14,077), and total number of reproductive failures (returns, abortions, negative tests, etc; #RF; n = 14,077) were also analyzed, along with age (AgeRem; days) and parity (ParRem) at death or removal from the herd (n = 3332).
Statistical analysis
Data were analyzed using SPSS software (IBM SPSS; Version 21.0). Continuous variables (eg, first breeding age, number weaned) were analyzed using the MIXED procedure, with dam treatment (gilt progeny versus sow progeny) as a fixed factor, and other blocking and (or) nuisance factors and covariates included in the final model as appropriate. Outliers (> 1.5 times the interquartile range from the mean) or obvious data input errors were excluded from the analysis. Nuisance factors and covariates found to have significant effects on some of the traits measured included birth month (BMth), birth litter size (BLS), age (Age), and weight (WT) of the animal at measurement, farrowing barn (Barn[Farm]), breeding month, total breedings (TotB), and age at the end of the experimental period (Ageatend), and these were included in the analysis as appropriate. There was no effect of farm on any trait measured, and this was therefore omitted from the overall model.
Five binomial traits were set up to evaluate first breeding achievement and (or) success and longevity to parity 3, based on appropriate ages at which to reach these milestones referenced in the literature,24,25 and calculated from gilts that reached these milestones during the experimental period: bred prior to 220 days of age (first bred at or before 220 days of age; females at least 220 days of age by the end of the experimental period), bred prior to 270 days of age (first bred at or before 270 days of age, of females at least 270 days of age by the end of the experimental period), removed before first breeding (removed from herd before being bred at least once, of females that were not bred at or before 270 days of age), reached parity 3 (farrowed a third litter at or before 700 days of age, of females at least 700 days of age by the end of the experimental period), and removed before parity 3 (removed prior to farrowing a third litter, of females that had not farrowed a third litter at or before 700 days of age).
A limit was set on the age of the sows at the end of the experimental period to include only sows that had reached the age at which they would have the opportunity to achieve these milestones. The success of the first breeding was analyzed on the subset of sows that had achieved a first breeding, regardless of the age at which this was reached. For the females removed prior to first breeding or parity 3 within the appropriate age ranges, removals were grouped as reproductive, health, structural, or other reasons, and analyzed as binomial traits.
An additional binomial trait (Medicated) was set up to assess the frequency of sows medicated at least once before reaching parity 3, and this was based on the subset of sows that had successfully reached parity 3 within the experimental period. Medications recorded after sows had reached parity 3 were not included in this analysis. Binomial variables and ratios of birth and selection weight categories were analyzed using chi square (χ2). Values of P < .05 were considered significant and values of P < .10 were considered trends.
Results
Live weight
Sow progeny were heavier (P < .001) than gilt progeny at all periods where a live weight was obtained (Table 1). Birth weight of gilt progeny was even lighter when correcting for the smaller litter size (total born) of their birth litter (12.39 ± 0.07 pigs for gilt litters versus 13.71 ± 0.05 for sow litters). Gilt progeny had a higher (P < .001) number of animals in the light birth-weight group than sow progeny (39.2% and 23.0%, respectively), and this was also the case at selection (32.0% and 25.8%, respectively). Sow progeny grew faster (P < .001) than gilt progeny from birth until selection (601 ± 6 g per day versus 581 ± 6 g per day, respectively). Age at selection (AgeSel) tended to be higher (P = .06) for gilt progeny, and therefore models for selection parameters were adjusted accordingly, where the effect of AgeSel was significant (Table 1). At selection, there was no difference in backfat between groups (gilt versus sow progeny, 14.9 ± 0.4 mm versus 15.0 ± 0.4 mm, respectively; P = .66). However, when corrected for their lighter body weight at this time, gilt progeny (15.5 ± 0.3 mm) had greater backfat (P = .02) than sow progeny (15.2 ± 0.2 mm).
Table 1: Estimated marginal means and statistical models used for the mixed models analysis of growth traits up until selection and reproductive traits from selection to first breeding for gilt progeny (GP) and sow progeny (SP) selected to enter the Rivalea (Australia) F1 breeding herd between 1 January 2014 and 31 December 2015
| Trait | Model | GP | SP | P |
|---|---|---|---|---|
| Live weight | ||||
| BWT (kg) | y = Tmt + BMth | 1.44 ± 0.01 | 1.64 ± 0.01 | < .001 |
| 21WT (kg) | y = Tmt + BMth + BLS + Age21WT | 5.47 ± 0.08 | 6.58 ± 0.08 | < .001 |
| PWWT (kg) | y = Tmt + BMth + BLS + AgePW | 11.0 ± 0.3 | 12.7 ± 0.3 | < .001 |
| SelWT (kg) | y = Tmt + BMth + BLS + AgeSel | 99.1 ± 0.9 | 102.7 ± 0.9 | < .001 |
| B1WT (kg)* | y = Tmt + BMth + BLS + AgeB1 | 141.0 ± 0.5 | 142.7 ± 0.4 | < .001 |
| First breeding | ||||
| AgeSel (days) | y = Tmt + BMth | 169.3 ± 0.6 | 169.2 ± 0.6 | .06 |
| AgeE1 (days) | y = Tmt + BMth + AgeSel | 200.0 ± 0.7 | 199.9 ± 0.6 | .79 |
| AgeB1 (days) | y = Tmt + BMth + BLS + AgeSel | 223.6 ± 1.2 | 222.4 ± 1.1 | .003 |
| Sel-B1 (days) | y = Tmt + BMth + BLS + AgeSel | 54.5 ± 1.2 | 53.2 ± 1.1 | .003 |
* Measured using the Allometric Growth Tape for Gilts (Swine Reproduction and Development Program, University of Alberta, Edmonton Canada). Data are expressed as mean ± standard error and P < .05 was considered significant (chi-square analysis).
BWT = birth weight; 21WT = 21-day weight; PWWT = post-weaning weight; SelWT = weight at selection (approximately 23-24 weeks of age); B1WT = weight at first breeding; AgeSel = age at selection; AgeE1 = age at first estrus; AgeB1 = age at first breeding; Sel-B1 = days from selection to breeding; Tmt = dam treatment (gilt versus sow); BMth = birth month; BLS = birth litter size; Age21WT = age at 21-day weight; AgePW = age at post-weaning weight.
First breeding
There was no difference (P = .79) between gilt progeny and sow progeny in terms of age at which first estrus was observed. However, age at first breeding was higher in gilt progeny (P = .003; Table 1) and gilt progeny had a greater (P = .01) number of days between detection of first estrus and first breeding in the gilts that had their first estrus recorded. From selection, gilt progeny took approximately 1 more day (P = .003) to reach first breeding than sow progeny.
Fewer (P < .001) selected gilt progeny were bred by 220 days and 270 days of age than selected sow progeny (Table 2). As a proportion of gilts not bred prior to 270 days of age, more (P = .04) gilt progeny were removed from the herd than sow progeny, while more sow progeny remained active in the herd (Active in herd; Table 2). Of the females removed from the herd before first breeding, more (P < .001) gilt progeny were removed for reproductive reasons (ie, anestrus) than sow progeny, whereas more (P = .01) sow progeny were removed for health reasons (eg, sudden death, ill thrift), and tended to be removed more often (P = .09) for structural reasons (eg, lame, prolapse, udder defects; Figure 1).
Table 2: Results (means) from the chi-square (χ2) analysis of binomial traits from first breeding until parity 3 compared between gilt progeny (GP) and sow progeny (SP)*
| Trait | GP (%) | SP (%) | χ2 | P |
|---|---|---|---|---|
| Selection to first breeding | ||||
| Bred prior to 220 days of age† | 40.5 | 44.4 | 14.61 | < .001 |
| Bred prior to 270 days of age‡ | 80.7 | 84.4 | 21.10 | < .001 |
| Not bred prior to 270 days of age‡ | 19.3 | 15.6 | 21.10 | < .001 |
| Removed | 88.4 | 84.7 | 4.29 | .04 |
| Active in herd§ | 11.6 | 15.3 | 4.29 | .04 |
| First breeding FR | 86.4 | 82.6 | 15.74 | < .001 |
| Longevity to P3¶ | ||||
| Reached P3¶ | 47.5 | 49.7 | 0.89 | .35 |
| Did not reach P3¶ | 52.5 | 50.3 | 0.89 | .35 |
| Removed | 93.9 | 94.2 | 0.03 | .86 |
| Active in herd** | 6.1 | 5.8 | 0.03 | .86 |
| Medicated | 26.3 | 27.9 | 0.38 | .54 |
* Chi-square (χ2) test analysis for binomial traits, described in Table 1; P < .05 was considered significant.
† Of females ≥ 220 days of age at the end of the experimental period.
‡ Of females ≥ 270 days of age at the end of the experimental period.
§ Gilts not bred most likely due to failing to reach puberty or management decisions (eg, not at optimal breeding weight), but remain in the herd and are eligible to be bred (have not died or been removed, such that Removed + Active in herd = 100%).
¶ Of females ≥ 700 days of age at the end of the experimental period.
** Sows that have not farrowed their third litter most likely due to prolonged non-productive days, but remain in the herd and are eligible to reach parity 3 (have not died or been removed, such that Removed + Active in herd = 100%).
FR = farrowing rate; P3 = parity 3.
Figure 1: Removal reasons (A) prior to first breeding (females ≥ 270 days of age by the end of the experimental period) and (B) prior to parity 3 (females ≥ 700 days of age by the end of the experimental period) for gilt progeny (GP) and sow progeny (SP; Table 1) analysed using chi-square (χ2). No symbol indicates no significant difference between GP and SP (P ≥ .10); * P < .10 indicates a trend; † indicates a significant difference at P < .05; ‡ indicates a significant difference at P < .001.
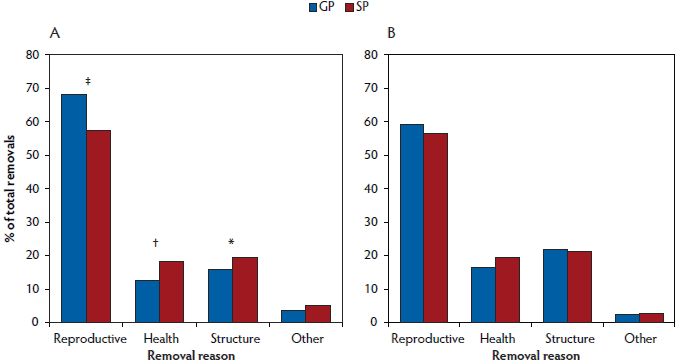
Of the gilts that had been first bred in the experimental period, more (P < .001) sow progeny were bred unsuccessfully than gilt progeny, resulting in a lower farrowing rate (Table 2), with more pregnancies failing due to reproductive reasons (Figure 1) as signified by return to estrus, negative pregnancy test, abortion, etc.
Lifetime reproductive performance
There was no significant difference in total born between the two groups at parity 1 (P = .51; data not shown). Gilt progeny tended to have fewer (P = .09) born alive at their first parity than sow progeny when adjusted for total born (10.78 ± 0.02 versus 10.83 ± 0.03 piglets, respectively), and fewer (P = .02) piglets weaned than sow progeny (9.21 ± 0.07 versus 9.34 ± 0.08 piglets, respectively). There were no differences (P ≥ .05) between the groups in terms of number of stillbirths or number of mummified fetuses (data not shown). There were few differences between the treatment groups for any trait analyzed in the subsequent parities (2 to 4; data not shown). Between weaning the second litter and the subsequent breeding, gilt progeny tended (P = .05) to have a longer WEI than sow progeny (5.91 ± 0.21 versus 5.48 ± 0.08 days, respectively). At parity 3, gilt progeny tended (P = .09) to have a lower total born (TB) than sow progeny (13.25 ± 0.16 versus 13.53 ± 0.08 piglets, respectively); however, this difference was not reflected at other parities. There were no differences (P = .54) between numbers of females medicated in either progeny group (Table 2). Sow progeny were medicated more often (P = .02) in their reproductive lifetime than gilt progeny (0.28 ± 0.01 versus 0.24 ± 0.02 medication events per sow, respectively).
Longevity
There were no differences (P ≥ .10) between gilt and sow progeny in terms of longevity in the herd to parity 3 (Table 2). There was no difference (P ≥ .10) between groups in terms of average WEI, total breedings, litters and reproductive failures, and age and parity at removal (data not shown). Reasons for removals prior to parity 3 did not differ between gilt and sow progeny (Figure 1).
Discussion
The overall objective of this study was to evaluate, in a retrospective manner, the reproductive performance and longevity in the breeding herd of progeny born to primiparous sows (“gilt progeny”) selected as replacement females. It was found that, in accordance with previous studies,5,6,26 (selected) gilt progeny were born lighter, grew more slowly, and were therefore lighter at later ages, such as at 21 days of age, 2 weeks after weaning, at selection, and at first breeding. As this study included only gilts selected to stay in the breeding herd, these figures may be even more disparate if the data for females that were not selected or eligible for selection due to lighter body weights, morbidity, or mortality were able to be included in the analysis.
Gilt progeny had more backfat than sow progeny at selection after adjusting for their lower body weight. This may be due to differences in birth weight, as some studies27-29 report that low birth weight piglets (LBW; < 1.2 kg) have a higher fat-to-lean ratio at slaughter (or in this case, at selection). This may be due to increased adipocyte numbers in the carcass as the result of heightened activity of fatty acid synthase and malic enzyme in backfat tissue.27 Low birth weight pigs also have fewer secondary muscle fibers at birth, which may translate into less lean muscle at older ages.30
Collectively, these results suggest that any differences in growth over the lifetime of a selected gilt born to a gilt are direct results of being born and weaned lighter than sow progeny. Strategies to increase birth weights and (or) growth rates in the pre-weaning period may improve the reproductive performance of these gilts. However, improving birth weights of gilt progeny may be difficult, as pressure to breed gilts earlier in life24 means their parity 1 dams are still partitioning energy into their own growth and energy metabolism,8,31-33 and may not have the uterine and (or) mammary capacity to support such large litters. Therefore, improving growth during the pre-weaning period using techniques such as cross-fostering34,35 and feeding supplemental milk,26 may be an opportunity to improve the subsequent growth of gilt progeny to improve their chances of being selected for the breeding herd and of being more reproductively successful.
The results of this study suggest that gilt progeny have higher rates of anestrus and take approximately a day longer to reach first breeding than their sow progeny counterparts. This is in accordance with other studies that found that low birth weight,19,36 restricted access to colostrum,20,37 and low growth rates22,38 in gilts can result in prolonged days from entry to puberty and first breeding and (or) slower rates of sexual maturation. Lighter gilts at selection have been shown to have lower levels of estradiol, IGF-I, medium to heavy follicles, and lighter reproductive tracts than heavier gilts,39 which may suggest that lighter gilt progeny may be less sexually developed than sow progeny at selection. However, age at first observed estrus in the two progeny groups in the present study did not differ significantly, which may suggest that age at first breeding was prolonged in gilt progeny due to these gilts not being at a desired weight (as estimated by allometric growth tape) by their first estrus rather than as a result of being more immature reproductively. However, it is important to note that in this commercial system, age at first observed estrus is not always recorded, which may be a confounding influence. The result that gilt and sow progeny reached first estrus at the same age should therefore be interpreted with some caution. With this in mind, the finding in the present study that sow progeny had a lower farrowing rate at first breeding than gilt progeny was unexpected. One study17 found that younger gilts at first breeding were more likely to have been bred more than once before farrowing, which is consistent with the current results, as sow progeny were approximately 1 day younger at first breeding. It may be possible that gilt progeny that are underdeveloped reproductively are removed during the selection processes, as they are below the weight threshold at that period. Larger sow progeny may be selected into the breeding herd, but underlying reproductive issues may not be identified until the time of first breeding, where these higher rates of reproductive loss occur. The higher proportion of gilt progeny under this weight threshold would experience increased selection pressure, which may result in the better breeding females reaching the first breeding and therefore increasing farrowing rate in these animals.
The higher number of sow progeny being removed before their first breeding for structural reasons may be due to their higher growth rates, as heavier, faster growing gilts tend to have an increased incidence of lameness as the weight load on the hooves and legs increases.40,41 The fact that more sow progeny were removed for health reasons and had more medications per sow than gilt progeny is surprising, as other authors have found that gilt progeny have higher morbidity and mortality rates than sow progeny.5,7,26 However, much of this prior research focuses on disease rates earlier in life, and little evidence is available for differences in morbidity and mortality of gilt progeny compared to sow progeny in later life. This again may reflect smaller, unthrifty gilt progeny not being selected for breeding in this particular herd.
Contrary to the current hypothesis, after gilt progeny were bred at least once, they were generally equivalent to sow progeny in terms of reproductive performance and longevity characteristics. Gilt progeny tended to farrow fewer live piglets at their first parity than sow progeny, which is in agreement with Vallet et al,19 who found that females born lighter had a shorter uterine length at puberty, which may represent lighter-born gilt progeny. However this difference was not seen at later parities, which may indicate that these females caught up in terms of reproductive capacity by these later ages. Unfortunately, observed estrus was not always recorded in this production system, and this may have a confounding influence on factors such as farrowing rate and litter size if, for example, more gilt progeny than sow progeny were bred on the second estrus.
Progeny born to gilts39 and low-growth-rate gilts17 have been known to have longer WEIs than their heavier or faster growing counterparts. The WEI after parity 1 did not differ between gilt and sow progeny in the current study. This is in contrast to Tummaruk et al,17 who found that gilt progeny had a significantly longer WEI after parity 1 than progeny born to parity 4 and 5 sows. There were a few differences between the groups in terms of performance indicators at later parities (ie, WEI after parity 2); however, in the current study, these were not replicated at other parities and therefore seem to be anomalies. It would be interesting to see if these results could be replicated in other herds, as there are no apparent reasons for these seemingly random differences to occur.
It was further hypothesized that gilt progeny would not persist in the herd to the same degree as sow progeny, as low birth weight,18 slower growth rates,17,23 and higher age at first breeding24,42 have all been associated with impaired sow longevity. However this was not the case in this dataset, with both groups exhibiting the same percentage of sows reaching parity 3. Future studies should focus on investigating the longevity of both gilt and sow progeny beyond parity 3, to explore whether these differences become more apparent later in life.
It is possible that due to lower growth rates in gilt progeny, these females are under the weight limit at selection and are therefore culled before entry into the breeding herd. This would result in better quality gilt progeny being selected for the breeding herd, which may be a reason for the lack of differences in reproductive performance and longevity between gilt and sow progeny. Unfortunately, investigating the proportion of gilt progeny selected from the gilt pool available for selection was beyond the scope of this study, as records were not kept for gilts culled at selection. Further research into this area is recommended to confirm these assumptions that gilt progeny are selected less frequently due to weight restrictions, among other restrictions at selection.
As gilts born to primiparous sows are the result of increased genetic turnover, these progeny often have higher EBVs and may be selected preferentially into nucleus herds as a result (J. Harper, Rivalea Australia Pty Ltd, oral communication, 2017). Gilt progeny selected into nucleus herds may therefore have more reproductive problems than sow progeny, which should be a target of research in the future. Longevity per se is not the priority in these herds, as sows are culled or moved out of the nucleus earlier in their reproductive lifetime for genetic turnover gains. It would be of interest, however, to quantify the effects of dam parity on effectiveness of their progeny as breeding sires to further evaluate the usefulness of gilt progeny as breeding animals, with one study suggesting that the amount of colostrum and milk consumed during the pre-weaning period can affect the reproductive performance of boars.43
In conclusion, gilt progeny are more likely than sow progeny to exhibit anestrus before optimal time for first breeding, and are hence more likely to be culled from the breeding herd in that period. However, once bred, gilt progeny in this study performed just as well in the breeding herd as sow progeny. To the best of these authors’ knowledge, this is the first study to quantify the differences between gilt progeny and sow progeny selected for breeding in a commercial herd in Australia. As this is a new area of research, further investigation of the impact of gilt progeny in the breeding herd is warranted. It is recommended that further research should focus on improving growth and health of gilt progeny, especially in the vital pre-weaning period. Selection practices may need to be reviewed in light of this new information, and future research should focus on suggesting selection benchmarks and improving management practices for gilt progeny in the breeding herd to improve their lifetime productivity.
Implications
- Under the conditions of this study, gilt progeny are born lighter and grow more slowly than sow progeny throughout their lifetime in the growing herd.
- Under the conditions of this study, while gilt progeny selected into the breeding herd are less likely to reach first breeding than sow progeny due to anestrus, gilt progeny have a higher farrowing rate at first breeding, which may be a result of increased selection pressure.
- After being bred for the first time, gilt progeny perform just as well reproductively as their sow progeny counterparts (born alive, number weaned, etc, at least up until parity 4), and their longevity in the herd does not differ (at least up until parity 3) under the conditions of the current study.
- Further research is warranted to determine what proportion of gilt progeny eligible for selection is selected to enter the breeding herd, in order to make decisions on the necessity and (or) appropriate timing for selection of these females.
Acknowledgements
The authors would like to acknowledge Australian Pork Limited (APL) for their generous financial support towards this research project.
Jessica Craig’s PhD candidature is supported by an Australian Postgraduate Award from Murdoch University.
Conflict of interest
Jessica Craig, Dr Cherie Collins, and Dr Rebecca Athorn are employed by Rivalea (Australia) Pty Ltd in the Research and Innovation Department.
Disclaimer
Scientific manuscripts published in the Journal of Swine Health and Production are peer reviewed. However, information on medications, feed, and management techniques may be specific to the research or commercial situation presented in the manuscript. It is the responsibility of the reader to use information responsibly and in accordance with the rules and regulations governing research or the practice of veterinary medicine in their country or region.
References
*1. Australian Pork Limited. Australian Pig Annual 2012-2013. Deakin West, ACT, Australia, June 2013:13.
2. Hendrix WF, Kelley KW, Gaskins CT, Hinrichs DJ. Porcine neonatal survival and serum gamma globulins. J Anim Sci. 1978;47:1281–1286.
3. Miller YJ, Collins AM, Emery D, Begg DJ, Smits RJ, Holyoake PK. Piglet performance and immunity is determined by the parity of both the birth dam and the rearing dam. Anim Prod Sci. 2012;53:46–51.
4. Wilson ER, Johnson RK. Adjustment of 21-day litter weight for number of pigs nursed for purebred and crossbred dams. J Anim Sci. 1980;51:37–42.
5. Carney-Hinkle EE, Tran H, Bundy JW, Moreno R, Miller PS, Burkey TE. Effect of dam parity on litter performance, transfer of passive immunity, and progeny microbial ecology. J Anim Sci. 2013;91:2885–2893.
6. Gatford KL, Smits RJ, Collins CL, Argente C, De Blasio MJ, Roberts CT, Nottle MB, Kind KL, van Wettere WHEJ, Owens JA. Progeny outcomes following maternal treatment with porcine somatotropin during pregnancy. Manipulating Pig Production XII, Werribee, Australia; 2009;103.
*7. Holyoake PK. Dam parity affects the performance of nursery pigs. 19th IPVS Cong. Copenhagen, Denmark; 2006:149.
8. Sinclair AG, Edwards SA, Hoste S, McCartney A, Fowler VR. Partitioning of dietary protein during lactation in the Meishan synthetic and European White breeds of pig. Anim Sci. 1996;62:355–362.
9. Gluckman PD, Hanson MA. Maternal constraint of fetal growth and its consequences. Seminars in Fetal and Neonatal Medicine. 2004;9:419–425.
10. Redmer DA, Wallace JM, Reynolds LP. Effect of nutrient intake during pregnancy on fetal and placental growth and vascular development. Domest Anim Endocrinol. 2004;27:199–217.
11. Devillers N, Farmer C, Le Dividich J, Prunier A. Variability of colostrum yield and colostrum intake in pigs. Animal. 2007;1:1033–1041.
12. Klobasa F, Butler JE, Werhahn E, Habe F. Maternal-neonatal immunoregulation in swine. II. Influence of multiparity on de novo immunoglobulin synthesis by piglets. Vet Immunol Immunopathol. 1986;11:149–159.
13. Inoue T. Possible factors influencing immunoglobulin A concentration in swine colostrum. Am J Vet Res. 1981;42:533–536.
14. Inoue T, Kitano K, Inoue K. Possible factors influencing the immunoglobulin G concentration in swine colostrum. Am J Vet Res. 1980;41:1134–1136.
15. Averette LA, Odle J, Monaco MH, Donovan SM. Dietary fat during pregnancy and lactation increases milk fat and insulin-like growth factor I concentrations and improves neonatal growth rates in swine. J Nutr. 1999;129:2123–2129.
16. Monaco MH, Gronlund DE, Bleck GT, Hurley WL, Wheeler MB, Donovan SM. Mammary specific transgenic over-expression of insulin-like growth factor-I (IGF-I) increases pig milk IGF-I and IGF binding proteins, with no effect on milk composition or yield. Transgenic Res. 2005;14:761–773.
17. Tummaruk P, Lundeheim N, Einarsson S, Dalin A. Effect of birth litter size, birth parity number, growth rate, backfat thickness and age at first mating of gilts on their reproductive performance as sows. Anim Reprod Sci. 2001;66:225–237.
18. Magnabosco D, Bernardi ML, Wentz I, Cunha ECP, Bortolozzo FP. Low birth weight affects lifetime productive performance and longevity of female swine. Livest Sci. 2016;184:119–125.
19. Vallet JL, Calderón-Díaz JA, Stalder KJ, Phillips C, Cushman RA, Miles JR, Rempel LA, Rohrer GA, Lents CA, Freking BA, Nonneman DJ. Litter-of-origin trait effects on gilt development. J Anim Sci. 2016;94:96–105.
20. Vallet JL, Miles JR, Rempel LA, Nonneman DJ, Lents CA. Relationships between day one piglet serum immunoglobulin immunocrit and subsequent growth, puberty attainment, litter size, and lactation performance. J Anim Sci. 2015;93:2722–2729.
21. Kummer R, Bernardi ML, Wentz I, Bortolozzo FP. Reproductive performance of high growth rate gilts inseminated at an early age. Anim Reprod Sci. 2006;96:47–53.
22. Amaral Filha WS, Bernardi ML, Wentz I, Bortolozzo FP. Growth rate and age at boar exposure as factors influencing gilt puberty. Livest Sci. 2009;120:51–57.
23. Roongsitthichai A, Cheuchuchart P, Chatwijitkul S, Chantarothai O, Tummaruk P. Influence of age at first estrus, body weight, and average daily gain of replacement gilts on their subsequent reproductive performance as sows. Livest Sci. 2013;151:238–245.
24. Schukken YH, Buurman J, Huirne RB, Willemse AH, Vernooy JC, van den Broek J, Verheijden JH. Evaluation of optimal age at first conception in gilts from data collected in commercial swine herds. J Anim Sci. 1994;72:1387–1392.
25. Serenius T, Stalder KJ. Length of productive life of crossbred sows is affected by farm management, leg conformation, sow’s own prolificacy, sow’s origin parity and genetics. Animal. 2007;1:745–750.
26. Miller YJ, Collins AM, Smits RJ, Thomson PC, Holyoake PK. Providing supplemental milk to piglets preweaning improves the growth but not survival of gilt progeny compared with sow progeny. J Anim Sci. 2012;90:5078–5085.
27. Gondret F, Lefaucheur L, Juin H, Louveau I, Lebret B. Low birth weight is associated with enlarged muscle fiber area and impaired meat tenderness of the longissimus muscle in pigs. J Anim Sci. 2006;84:93–103.
28. Rehfeldt C, Kuhn G. Consequences of birth weight for postnatal growth performance and carcass quality in pigs as related to myogenesis. J Anim Sci. 2006;84:E113–E123.
29. Collins CL, Leury BJ, Tatham BG, Henman DJ, Dunshea FR. Pigs born light increase adipose tissue deposition following a restricted protein intake during early growth. Manipulating Pig Production XI. Brisbane, Australia; 2007:185.
30. Aberle ED. Myofiber differentiation in skeletal muscles of newborn runt and normal weight pigs. J Anim Sci. 1984;59:1651–1656.
31. Clowes EJ, Williams IH, Baracos VE, Pluske JR, Cegielski AC, Zak LJ, Aherne FX. Feeding lactating primiparous sows to establish three divergent metabolic states: II. Effect on nitrogen partitioning and skeletal muscle composition. J Anim Sci. 1998;76:1154–1164.
32. Pluske JR, Williams IH, Zak LJ, Clowes EJ, Cegielski AC, Aherne FX. Feeding lactating primiparous sows to establish three divergent metabolic states: III. Milk production and pig growth. J Anim Sci. 1998;76:1165–1171.
33. Zak LJ, Williams IH, Foxcroft GR, Pluske JR, Cegielski AC, Clowes EJ, Aherne FX. Feeding lactating primiparous sows to establish three divergent metabolic states: I. Associated endocrine changes and postweaning reproductive performance. J Anim Sci. 1998;76:1145–1153.
*34. Flowers WL. New ideas about gilt development and management. Proc 2005 Manitoba Swine Seminar. Winnipeg, Manitoba, Canada; 2005:11.
35. Smits RJ, Collins CL. Progeny reared by their birth dam do not outperform progeny crossfostered to a similar parity dam. Manipulating Pig Production XII. Werribee, Australia; 2009:143.
36. Magnabosco D, Cunha ECP, Bernardi ML, Wentz I, Bortolozzo FP. Impact of the birth weight of Landrace × Large White dam line gilts on mortality, culling and growth performance until selection for breeding herd. Acta Sci Vet. 2015;43:1274.
37. Chen JC, Frankshun A, Wiley AA, Miller DJ, Welch KA, Ho T, Bartol FF, Bagnell CA. Milk-borne lactocrine-acting factors affect gene expression patterns in the developing neonatal porcine uterus. Reproduction. 2011;141:675–683.
38. Tummaruk P, Tantasuparuk W, Techakumphu M, Kunavongkrit A. The association between growth rate, body weight, backfat thickness and age at first observed oestrus in crossbred Landrace × Yorkshire gilts. Anim Reprod Sci. 2009;110(1–2):108–122.
39. van Wettere WHEJ. Management and Nutrition of the Replacement Gilt [PhD thesis]. The University of Adelaide, Australia; 2008.
40. Jørgensen B, Sørensen MT. Different rearing intensities of gilts: II. Effects on subsequent leg weakness and longevity. Livest Prod Sci. 1998;54:167–171.
41. Prunier A, Heinonen M, Quesnel H. High physiological demands in intensively raised pigs: impact on health and welfare. Animal. 2010;4:886–898.
42. Koketsu Y, Takahashi H, Akachi K. Longevity, lifetime pig production and productivity, and age at first conception in a cohort of gilts observed over six years on commercial farms. J Vet Med Sci. 1999;61:1001–1005.
43. Rahman KM, Lovich JE, Lam C, Camp ME, Wiley AA, Bartol FF, Bagnell CA. Nursing supports neonatal porcine testicular development. Domest Anim Endocrinol. 2014;48:84–92.
* Non-refereed references.