| Original research | Peer reviewed |
Cite as: Pierdon MK, Martell AL, Parsons TD. Use of ropes to collect oral fluids from gestating sows housed in dynamic groups and fed via electronic sow feeder. J Swine Health Prod. 2016;24(5):246–252. https://doi.org/10.54846/jshap/942
Also available as a PDF.
SummaryObjectives: The primary objective of this study was to understand how group-housed sows interact with ropes as a tool for collecting oral fluids. The secondary objective was to provide evidence that oral fluids collected from gestating sows housed in pre-implantation dynamic groups can be a useful sample for porcine reproductive and respiratory syndrome (PRRS) surveillance. Materials and methods: Oral-fluid samples were collected 1 day per week for 3 weeks at a 750-sow PRRS-negative facility with two pens housing pre-implantation dynamic groups for gestating sows fed via an electronic sow feeder (ESF) system. Ropes were placed and activity filmed with handheld cameras. Videos were analyzed for number of sows to chew, time to first chew (TFC), and number of aggressive events. Serum samples were collected from a subset of sows that had contributed oral fluids on this farm, as well as from sows on a second similar farm that was PRRS-positive. Results: The average number of sows contacting a rope during sampling was 19.9 ± 1.2 (n = 13 videos). Repeated sampling significantly influenced TFC (Kruskal-Wallis; P < .05). Oral-fluid PRRS enzyme-linked immunosorbent assay sample-to-positive (S:P) ratios for individual ropes correlated with the mean serum S:P ratio of a subset of 10 sows that contacted the rope. Implication: Rope sampling will likely provide a method for readily collecting oral-fluid samples from sows housed in dynamic groups and fed with an ESF. | ResumenObjetivos: El objetivo principal de este estudio fue entender como interactúan las hembras alojadas en grupos con cuerdas como material para recolectar fluidos orales. El objetivo secundario fue proveer evidencia de que los fluidos orales colectados de hembras gestantes alojadas en grupos dinámicos pre implantación puede ser una muestra útil para el monitoreo del síndrome respiratorio y reproductivo porcino (PRRS por sus siglas en inglés). Materiales y métodos: Se colectaron muestras de fluidos orales 1 día a la semana por 3 semanas en un edificio de 750 hembras negativo al PRRS con dos corrales alojando grupos dinámicos pre implantación para hembras gestantes alimentadas vía un sistema alimentador de hembras electrónico (ESF por sus siglas en inglés). Se colocaron cuerdas y se filmó la actividad con cámaras manuales. Se analizaron los videos para ver cuántas hembras masticaron, tiempo para la primera masticación (TFC por sus siglas en inglés), y número de eventos agresivos. Se colectaron muestras de suero de un subconjunto de hembras que habían contribuido fluidos orales en esta granja, así como de hembras en una segunda granja similar que resultó positiva al PRRS. Resultados: El número promedio de hembras que hicieron contacto una cuerda durante el muestreo fue 19.9 ± 1.2 (n = 13 videos). El muestreo repetido influenció significativamente el TFC (Kruskal-Wallis; P < .05). Los índices muestra a positivo (S:P por sus siglas en inglés) del ensayo por inmunoabsorción ligado a enzimas del PRRS del fluido oral para las cuerdas individuales se correlacionaron con el ratio S:P del suero promedio de un subconjunto de 10 hembras que hicieron contacto con la cuerda. Implicación: El muestreo de cuerda probablemente proveerá un método para colectar fácilmente muestras de fluido oral de hembras alojadas en grupos dinámicos y alimentados con un ESF. | ResuméObjectifs: L’objectif primaire de la présente étude était de comprendre comment les truies logées en groupe interagissent avec des cordes comme outil de prélèvement de fluide oral. Le second objectif était de fournir des évidences que les fluides oraux prélevés chez des truies gestantes logées dans des groupes dynamiques pré-implantation peuvent être des échantillons utiles pour la surveillance du syndrome reproducteur et respiratoire porcin (SRRP). Matériels et méthodes: Des échantillons de fluides oraux ont été prélevés 1 jour par semaine pendant 3 semaines, sur un site hébergeant 750 truies négatives pour le SRRP, dans deux parcs logeant des groupes dynamiques pré-implantation de truies gestantes nourries via un distributeur électronique d’aliment pour truie (DEAT). Des cordes ont été placées et les activités filmées avec des caméras tenues à la main. Les vidéos ont été analysées pour déterminer le nombre de truies qui ont mâché, le délai avant la première mâchée (DPM), et le nombre d’évènements agressifs. Des échantillons de sérum ont été prélevés sur cette ferme d’un sous-groupe de truies qui avaient contribué des fluides oraux, de même que de truies sur une autre ferme similaire dont les animaux étaient positifs pour SRRP. Résultats: Le nombre moyen de truies en contact avec une corde durant l’échantillonnage était 19,9 ± 1,2 (n = 13 vidéos). Des échantillonnages répétés ont influencé de manière significative le DPM (Kruskal-Wallis; P < .05). Les rapports échantillon-résultats positifs (E:P) pour l’épreuve immuno-enzymatique de détection du SRRP à partir des fluides oraux pour des cordes individuelles étaient corrélés avec le rapport sérique moyen E:P d’un sous-groupe de 10 truies qui ont été en contact avec la corde. Implication: L’échantillonnage au moyen d’une corde sera fort probablement une méthode pour prélever facilement des échantillons de fluides oraux à partir de truies logées en groupes dynamiques et nourris avec un DEAT.
|
Keywords: swine, group housing, oral fluids, pen gestation, porcine reproductive and respiratory syndrome virus testing, PRRS, PRRSV
Search the AASV web site
for pages with similar keywords.
Received: August 28, 2015
Accepted: March 22, 2016
Oral fluids as a diagnostic sample to detect pathogens in swine was first described in the 1970s.1 The use of rope to obtain samples of oral fluids from growing swine is a more recent advance.2.3 This approach has been applied as a diagnostic tool for successful detection of pathogens in pigs at many additional stages of production: suckling piglets,4 replacement gilts,5 and individually housed boars6 and sows.7 Collection of oral fluids from sows in gestation stalls with individual ropes may not be practical when seeking a minimum sample size of 30 or more. However, group-housed sows do not face this same constraint, as multiple animals can access a single rope, and thus rope sampling promises to be much more efficient in pens of sows. To our knowledge, no research has been published on the use of ropes for collection of oral fluids for disease monitoring in group-housed sows.
Environmental enrichment studies have shown that sows housed in groups chew on cotton ropes.8 However, several factors need to be addressed when examining how to optimize the use of ropes for collection of oral fluids from gestating sows. First, while over 80% of the growing pigs in a pen interact with the rope in 60 minutes,9 similar information is not available for sows. Thus, it is important to understand how many sows in a given pen chew on the rope. Second, unlike growing pigs, sows are maintained in a herd for years instead of months and could be sampled repeatedly during their lives. The number of individually housed boars and sows that can be successfully sampled increases with repeated exposure to a rope.6,7 Thus, it is also critical to understand if repeated sampling impacts the number of animals interacting with the rope in group-housed sows. Third, it is also important to determine if oral fluids from the same or a different population of animals is captured when ropes are repeatedly introduced to the same group of animals. And finally, the specific animal interacting with the rope is likely also important. Social hierarchy develops when gestating sows are housed in groups, impacting aggression10 and the order in which they eat,11,12 and may impact their interaction with novel objects such as ropes. Furthermore, the social rank of individuals within the group has been shown to influence the animals’ immune stimulation and subsequently may influence disease status.13 Several different types of housing systems are employed for gestating sows that impact the number of animals in a pen, the size of the pen, the shape of the pen, and likely the way sows interact with the ropes hung in the pen. This study explored the applicability of oral-fluid testing in group-housed sows (> 100 sows per pen) with sows mixed 1 to 3 days after the last insemination and prior to implantation of the embryos (pre-implantation groups). These groups were also dynamic, since sows were removed to go to farrowing every other week, and sows were added to the pen every other week.14,15 This study was designed to examine how many animals the rope sample represents, how experience impacts the time it takes sows to interact with the rope, and how social status affects oral-fluid sampling in terms of the animals that interact with the rope, in a single type of group sow housing.
Materials and methods
Each farm had current Pork Quality Assurance certification, which provides guidelines that directed animal care.
Study overview
The primary objective of this study was to understand how group-housed sows interact with ropes as a tool for collecting oral fluids. The aim was to quantify the number of sows that interacted with ropes during a short sampling period (approximately 60 minutes) and to explore a limited number of factors that, on the basis of our experience in pen gestation, had the potential to impact sow-rope interactions. For a variety of logistical and biosecurity reasons, this part of the study was carried out on a farm negative for porcine reproductive and respiratory syndrome (PRRS). This farm is referred to as Study Farm 1.
A secondary objective of this study was to provide some evidence that oral fluids collected from gestating sows housed in pre-implantation dynamic groups can be a useful sample for PRRS surveillance. A second farm, which was PRRS-positive, was recruited to participate in the study, and this farm is referred to as Study Farm 2. The second farm was chosen on the basis of its similarities to the initial study farm. The details of the two study farms are described subsequently. Data was collected in August of 2013 on Study Farm 1 and September of 2013 on Study Farm 2.
Description of study farms
Study Farm 1. The main part of the study, conducted on Study Farm 1, was used for the collection of all behavioral data presented. The farm was an owner-operated, 700-sow, farrow-to-wean, PRRS-negative facility that had managed gestating sows housed in pre-implantation dynamic groups and fed with electronic sow feeding since 2007. Sows (PIC 1050; PIC, Hendersonville, Tennessee) were housed in two pens, and gilts were housed in a separate pen. Our study was conducted only in pens containing sows.
Study Farm 2. This farm was recruited to supplement findings on the utility of oral-fluid samples from group-housed sows for PRRS surveillance. The farm was an owner-operated, 1400-sow, farrow-to-wean, PRRS-positive facility. At the time of the study, the facility was weaning PRRS-positive pigs, determined by polymerase chain reaction testing, and was vaccinating quarterly with a modified-live PRRS vaccine (Ingelvac PRRS MLV; Boehringer Ingelheim Vetmedica Inc, St Joseph, Missouri). This facility had managed gestating sows, housed in dynamic pre-implantation groups and fed with electronic sow feeding, since 2008. Choice Genetics CG32 sows (Choice Genetics, West Des Moines, Iowa) were housed in four pens, with gilts housed in a separate pen. The study was conducted only in pens containing sows.
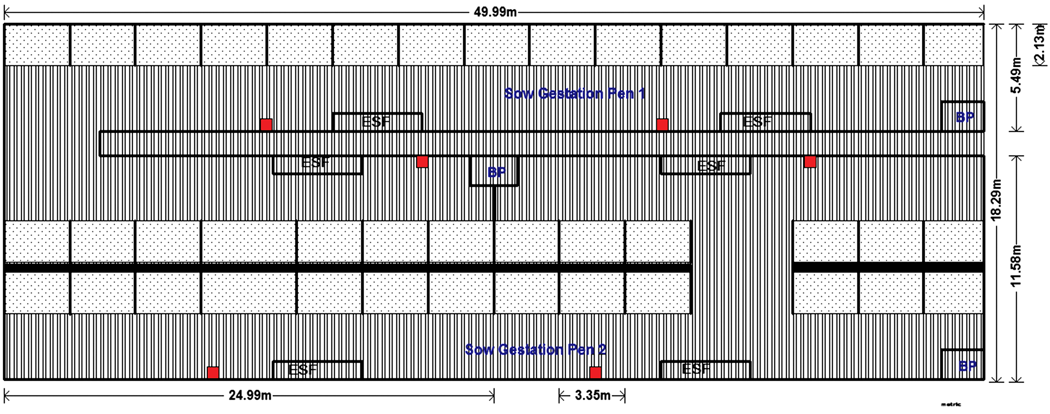
Common practices and designs of the two study farms. Each sow pen housed approximately175 animals and was equipped with three electronic sow-feeding stations (Compident VI; Schauer Agrotronics, Prambachkirchen, Austria). The feed system turned on daily at midnight and closed when all animals had eaten, which typically was between 2 and 4 pm. Sows were fed a standard corn-soy diet according to their body condition. About 30 newly bred sows, or approximately 17% of the pen inventory, were introduced to a pen every 14 days, after the movement of a corresponding number of late-term sows to farrowing. Pens were designed to house sows with a space allocation of 1.9 to 2.1 m2 per sow. Flooring was slatted, with the exception of solid areas provided for lying and sleeping (Figure 1).
Figure 1: Schematic of sow gestation area on study farm and placement of ropes for collection of oral fluids. Study Farm 1 was a farrow-to-wean sow farm where gestating sows and gilts were housed in pre-implantation, dynamic groups. Animals were fed via electronic sow feeding stations (ESFs). Gilts were housed separately from older parity sows. Each sow gestated in one of two pens that each contained three ESF stations. The flooring was totally slatted except for the 2.10 × 3.35-meter sleeping areas in each pen that had raised, solid concrete bases (stippled areas). The gestation area included three 1.8 × 2.1-meter boar pens (BP) that could be used for automated heat detection. Behavioral observations were carried out in both sow pens. Ropes were placed approximately 3 meters along the fence line from the feeder entrance (red squares). Sow interactions with the ropes were recorded by an individual with a handheld video camera outside the pen near each rope.
Behavioral observations
Behavioral data was collected only on Farm 1.
Sow-rope interactions
Data collection. Sow interactions with both the rope and her cohort at the rope site were video recorded via handheld cameras (Handycam; Sony, New York, New York). The observers holding the cameras also called out the sows’ ear tag numbers as the sows contacted the rope to individually identify sows. This information was recorded on the audio track of the video recording and was available for subsequent analysis. Video recording started immediately after placing the ropes in the pen at approximately 8 am. Videos were of varying lengths due to the challenges associated with collecting behavioral data in an on-farm setting, but data analysis was capped at the first 55 minutes of each video to standardize the length.
Data analysis. Videos were analyzed off-line using the Noldus Observer XT V. 10.5 software (Noldus Information Technology Inc, Leesburg, Virginia) to identify sow-rope and sow-sow interactions. A chew event was defined as the rope being in the mouth of a sow. An aggressive event was defined as a sow biting or head butting another sow at the rope. The following metrics were tallied for each video from the individual sow events: number of sows to chew on a rope (NSC), time to first chew on a rope (TFC), and number of aggressive events at the rope (NAE). The cameras recorded at 60 frames per second and thus provide an effective temporal resolution in our measurements of one data point every 16.7 milliseconds (ms). The software is configured to report the temporal resolution of data collection as 0.02 seconds after converting 16.7 ms to seconds and then rounding to two significant figures. A replicate (n) was defined as the observations from a site where ropes were hung that were video-taped with a single camera. Rope-hanging sites were randomly assigned, using a random number generator, to have either one or two ropes.
Feed rank. Feed order was saved daily by the ESF computer and listed times and amounts of feed eaten by individual sows at an ESF station. Most sows ate their daily allotment in a single trip to the feeder. However, occasionally a sow consumed her feed over multiple visits. The time of record in these rare cases was the feeding at which she consumed the largest portion of her daily ration. A feed rank (FR) was determined for each sow over a week period by taking the average daily feed order over the 7-day period preceding a Wednesday behavioral observation day. It was possible for sows to have fewer than seven observations for feed order over a 7-day period, as some animals may have missed an occasional meal or because of movement of animals in and out of the pen. In approximately 99% of sows in each pen, five observations were used for the weekly feed-rank calculations. It was not possible to retroactively capture the feed-order data prior to the first day of data collection, and thus feed rank was calculated only for the second and third days of data collection. Accordingly, feed-rank correlations were limited to the final 2 days of behavioral observations.
Oral-fluid collection
Farm 1. Oral-fluid samples were collected for behavioral observations starting at approximately 8:00 am and continuing for at least 55 minutes once a week on Wednesdays for 3 consecutive weeks. Cotton ropes, 1.6 cm in diameter (Troyer’s Rope Co, Conneautville, Pennsylvania), were tied to the fence-line of the pen, single or paired, 3 meters from the feeder entrance (Figure 1), at a height of 1 meter from the floor, leaving approximately 0.5 meter of rope exposed to the sows in the pen.16 Single ropes were hung alone, whereas paired ropes were hung 0.75 meter apart in the same location. Oral fluids collected on Day 1 were submitted for PRRS antibody testing. Oral fluids were harvested by gathering the rope in a plastic bag, grasping the rope, and pulling it from the bag.
Farm 2. Oral fluids were also collected on Farm 2, which had historically tested PRRS-positive. A single 1.6-cm diameter cotton rope was placed approximately 3 meters from the entrance of a feeder in each of three different pens at approximately 8:00 am and collected 1 hour later. Individual sows contacting the rope during this hour were identified for subsequent serological testing. Oral fluids were harvested as described for Farm 1.
Serological data collection
Farm 1. For comparison with oral-fluid samples, blood was collected on Day 2 from 17 sows that were verified by video to have chewed on the rope from which oral fluids had been collected on Day 1. Blood samples were collected from restrained sows via venipuncture of the anterior vena cava.
Farm 2. Sows that chewed on the ropes were marked by an observer. Ten of the marked sows from each rope were then restrained and blood samples were collected as described for Study Farm 1.
Laboratory testing
All blood samples were tested with the Idexx PRRS enzyme-linked immunosorbent assay (ELISA) X3 Ab test (Idexx Laboratories, Inc, Westbrook, Maine), and oral-fluid samples were tested by Idexx PRRS Oral Fluids Ab test (Idexx Laboratories, Inc) at the Iowa State University Veterinary Diagnostic Laboratory. All oral-fluid and serum samples were maintained on ice from collection until receipt at the laboratory. Samples were tested individually and the resulting sample-to-positive (S:P) ratios scored as positive or negative, with an S:P ratio of ≥ 0.4 considered positive.
Statistical evaluation
Data analysis was performed using STATA version 13.1 (StataCorp LP, College Station, Texas). According to the Shapiro Wilk test, NSC and NAE were normally distributed and thus these data were analyzed using a two-way analysis of variance (ANOVA) with sampling day (DAY) and number of ropes (ROPES) as main effects. A Kruskal-Wallis test was used to test similar relationships for TFC, which was not normally distributed. Spearman’s correlation test was used to examine correlations between continuous variables (oral-fluids ELISA and serological ELISA), and a point biserial correlation test was employed for correlations involving binary data (initiate aggression, “yes” or “no;” chew, “yes” or “no”). Normally distributed behavioral data are presented either as a daily mean, which represents the mean value of all replicates on that day, or as an overall mean with the standard error of the mean (SEM), which is the mean value for all replicates in the study. For the variables that were not normally distributed, behavioral data are presented as the median value for each day or as an overall median with the interquartile range (IQR), which represents the median value across all replicates in the study. Only significant interactions are reported. As location was not varied as part of the study design, the location of the ropes was not analyzed.
Results
Number of sows to chew
The overall average NSC at a rope site was 19.9 ± 1.2 (Table 1). Number of sows to chew increased numerically from a mean of 15.7 to 21.7 over the 3 days of collection, but DAY did not significantly influence NSC (P > .05) (Table 1). ROPES also did not influence NSC (P > .05).
Table 1: Behavioral observations of sows interacting with ropes used for oral-fluid collection (Farm 1)*
| Day | n | Mean no. of sows to chew | Median time to first chew (seconds) | Mean no. of aggressive events |
|---|---|---|---|---|
| 1 | 3 | 15.7 | 558.0a | 14.3c |
| 2 | 4 | 20.5 | 174.4 | 28.0 |
| 3 | 6 | 21.7 | 24.7b | 38.5d |
| All | 13 | 19.9 | 234.1 | 29.7 |
* Study described in Figure 1. Mean number of sows to chew, median time to first chew, and mean number of aggressive events are summarized for the 55 minutes of video data on different experimental days; n is the number of experimental replications on each day.
a,b Values with different superscripts within a column are significantly different within the main effect (P < .01; two-way ANOVA with DAY and ROPES as main effects).
c,d Values with different superscripts within a column are significantly different within the main effect (P < .05; two-way ANOVA with DAY and ROPES as main effects).
DAY = sampling day; ROPES = number of ropes (one or two)
Time to first chew
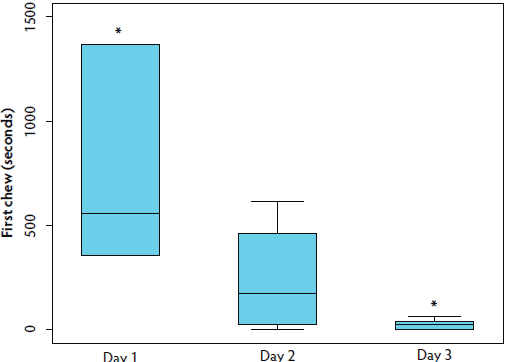
The range of time to first chew (TFC) across all replicates was 0.01 to 1367.04 seconds, with a median of 43.7 ± 345.7 seconds. ROPES did not influence TFC (P > .05), but there was a significant effect of DAY on TFC (Table 1; Kruskal-Wallis; P < .05). Sows initially approached the rope more than 20 times faster on day 3 than on day 1 (P < .01), as both median time to first chew and the IQR decreased with repeated rope sampling in the pen (Figure 2).
Figure 2: Study described in Figure 1. The duration of time required for the initial animal to chew on a rope (TFC) decreased with repeated sampling of group-housed gestating sows. Boxplot demonstrates that both the median TFC, as well as the interquartile range, decreased with repeated sampling. Time points marked by an asterisk differ significantly (Kruskal-Wallis; P < .01).
Number of aggressive events
The overall average NAE in the 55 minutes analyzed was 29.7 ± 4.5 (Table 1). The two-way ANOVA showed there was a significant effect of DAY on NAE (P < .05), as they doubled between day 1 and day 3. ROPES did not influence NAE (P > .05).
Feed rank
On sampling day 2, sows that ate later in the day (lower FR) were more likely to chew on a rope (correlation [r] = 0.15; P < .01), and of the sows that chewed on a rope, those with a higher FR were more likely to initiate aggression at the rope (r = -0.34; P < .05). The same results were repeated on sampling day 3, where sows with a lower FR were more likely to chew on a rope (r = 0.16; P < .01), but of the sows chewing on a rope, the ones with higher FR were more likely to initiate aggression (r = -0.43; P < .01).
Serology
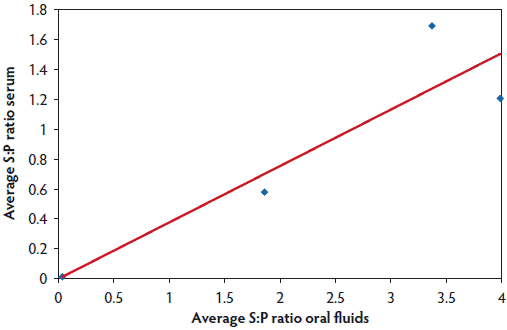
The pen-level oral-fluid ELISA result was indicative of the individual sow serum ELISA findings. The pen-based oral-fluid samples were PPRS-positive when there were serologically PRRS-positive sows in the pen that had sampled the rope (Table 2). Furthermore, the magnitude of the oral-fluid ELISA S:P ratio was positively correlated with the serum ELISA S:P ratio of sows that had chewed on the rope and were sampled for serological testing (r = 0.79; P < .001). Finally, the S:P ratios for oral-fluid ELISAs on samples collected from individual ropes increased numerically as the average serum S:P ratios of a subset of the sows that sampled the rope increased (Figure 3).
Table 2: Proportion of sows seropositive for PRRS and comparison of S:P ratios for serum and oral-fluid samples*
| Farm | No. of serum samples | No. (proportion) of positive serum samples | Mean serum S:P ratio (range) | Mean oral-fluid S:P ratio |
|---|---|---|---|---|
| 1 | 17 | 0 (0) | 0.013 (0.0 – 0.051) | 0.038 |
| 2 | 10 | 8 (0.8) | 1.206 (0.086 – 3.064) | 3.985 |
| 2 | 10 | 3 (0.3) | 0.580 (0.093 – 2.039) | 1.859 |
| 2 | 10 | 10 (0.1) | 1.693 (0.53 – 2.874) | 3.370 |
* Study described in Figure 1. Blood samples were collected from sows that chewed on the ropes. Serum samples were tested with the PRRS X3 Ab ELISA (Idexx Laboratories, Inc, Westbrook, Maine) and oral-fluid samples were tested by PRRS Oral Fluids Ab ELISA (Idexx Laboratories, Inc). An S:P ratio ≥ 0.4 was considered positive.
PRRS = porcine reproductive and respiratory syndrome; S:P = sample-to-positive ratio; ELISA = enzyme-linked immunosorbent assay.
Figure 3: Study described in Figure 1. Blood samples and oral fluids were collected from a subset of 10 sows for testing using an enzyme-linked immunosorbent assay (ELISA): the PRRS X3 Ab ELISA for serum (Idexx Laboratories, Inc, Westbrook, Maine), and the PRRS X3 Oral Fluids ELISA for oral-fluid samples (Idexx Laboratories, Inc). Oral-fluid average sample-to-positive (S:P) ratio increased as the average S:P ratio of serum from sows that interacted with the rope increased. The average S:P ratio of the subset of sows that were marked as chewing on the rope is plotted against the average oral-fluid ELISA S:P ratio for pairs of ropes hung in four different pens. The line highlights the relationship between individual serum ELISA values and the ELISA values for the collective oral-fluid samples obtained from these same group-housed sows (linear fit described by y = 3.78 × – 0.0015, R² = 0.83).
Discussion
The work described here provides the first evidence to support the feasibility of oral-fluid collection for disease surveillance in group-housed gestating sows. On average, approximately 20 sows contacted a rope placed near the entrance of an ESF station. While the time for the first animal in the pen to chew on the rope decreased and the number of aggressive events at the rope increased following weekly sample collection, the total number of sows contributing to an oral-fluid sample did not change, given repeated exposure to the rope. Interestingly, the number of aggressive events at the rope correlated with feed rank, a proxy for social heirarchy.11,12 Dominant animals were more likely to be involved in fights at the rope, but, perhaps counter intuitively, animals with a lower social status were more likely to sample the rope. Finally, the mean serum S:P ratios correlated with the S:P ratios of the pen-level oral-fluid samples.
Our findings on the number of animals to sample the rope support this technique as a possible sampling protocol for dynamic pre-implantation groups like those in the farms studied here. The placement of ropes approximately 3 meters from the entrances of two to four different feeders is predicted to generate samples that would contain oral fluids from 30 or more different sows in the barn. There are, however, many different options available for group housing gestating sows, and further work will be needed to understand how generally applicable these findings are to other types of gestational group housing.
The time of day that the ropes are placed in the pen is likely to impact the outcome of sampling, given that the activity level of sows in an ESF pen is not constant across the day. From the time the feeding system turns on, activity increases over an 8- to 12-hour period and then starts to decrease as the majority of the animals are fed and the stations close for the day.17,18 In this study, the optimal time of day to sample was not investigated specifically, but sampling time was chosen on the basis of our previous research and clinical experience with group-housed sows being fed by ESF. Our goal was to place the ropes more or less halfway through their daily feeding cycle, while the feeders were still open and the sows were still eating. The start of the feeding cycle varies from farm to farm, and accordingly, the absolute time for sampling may be farm-specific. However, we suggest that determining the sampling time relative to the start and finish times of the feeding cycle is an important consideration, especially when sampling in dynamic pre-implantation groups fed by ESF, to ensure that sows are still active and feeding when investigators are attempting to sample.
Our studies revealed that the TFC in a pen decreased with subsequent rope sampling in the pen; however, the overall number of sows to chew was not affected by sampling history. A similar effect on experience was reported for rope testing of individually housed sows. Pepin et al7 report an increase in the number of sows successfully sampled with repeated exposure to rope testing, but did not study whether the latency of animals that chewed on a rope depended on experience. The practical implication for the TFC on repeated sampling is that when sampling for the first time in a pen, oral-fluid collection may take longer (median difference in TFC of approximately 9 minutes). The range of time it took for sows to approach the rope is important as well, because producers and veterinarians should not be discouraged if it takes over 20 minutes for the sows to approach the rope the first time sampling is attempted.
Sows that had a lower feed rank (ie, sows that ate later in the day relative to other sows) chewed on a rope more often. One interpretation of these findings is that frequency of rope chewing is inversely correlated with social status. However, alternatively, we would suggest that these observations are more likely explained by the time of sampling coordinating with the time of feeding of these sows. Thus, the timing of rope placement likely will impact the part of the social hierarchy that is captured by the oral-fluid sampling, and placing the ropes at a point earlier in the feeding cycle may sample sows with a higher feed rank and associated higher social status.11,12
Aggressive events at the rope exhibited a positive correlation with repeated oral-fluid collection in the pen, as well as social hierarchy. However, neither impacted the total number of sows to interact with the rope. It is interesting that, despite this increase in aggressive events at the rope, these aggressive events do not limit the number of sows sampling the rope, ie, dominant sows are not successful in guarding the ropes.
We observed a correlation between the oral-fluid PRRS ELISA S:P ratios and the serum S:P ratios of a subset of sows that chewed on the rope. Even when only 30% of the blood-tested sows contributing to the rope sample were seropositive on PRRS ELISA, the oral-fluid sample was positive on PRRS ELISA. The exact contribution that a given sow makes to the oral-fluid sample will depend on her serum S:P ratio and the amount of saliva she contributes to the sample. The results show that the magnitude of the oral-fluid S:P ratio was better correlated with the maximum value of an individual sow’s S:P ratio than with the number of seropositive sows or the mean S:P ratio of the blood-sampled sows at the rope. These findings highlight that the oral-fluid sample S:P ratios are useful for PRRS surveillance at the herd level, but their interpretation is likely more complicated than the simple arithmetic mean of individual serum S:P ratios.
This study documents that it is possible to collect oral fluids from group-housed sows, as reported for individually housed boars6 or sows.7 Our findings on sows housed in pre-implantation dynamic groups suggests that collecting and testing oral-fluid samples could be an effective and sensitive method for exposure screening for pathogens with validated oral-fluid diagnostic tests. It also highlights how more work is needed to understand the limitations of this approach in herds with low prevalence of seropositive animals or in other types of group-housing systems. Further work is also needed to investigate how disease presence may alter both sow behavior and social structure, as well as potentially influence the specific animals that interact with the rope.
Implications
• Rope sampling will likely provide a method for readily collecting oral-fluid samples from sows housed in dynamic groups and fed with an electronic sow feeder.
• The subset of sows sampled from the pen will most likely depend on the point during the course of the daily feeding cycle when ropes are hung, as sows interacting with the rope likely correspond to those currently gathering to enter the ESF station to feed.
• The results of this study suggest that, under similar conditions, hanging two to four ropes per pen for approximately 1 hour, with each rope placed about 3 meters from the entrance of an ESF station, should capture an oral-fluid sample that represents 30 or more sows when at least two pens are sampled.
Acknowledgements
This work was supported by Pennsylvania Pork Producers Council, Pennsylvania Soybean Growers Board, and USDA PRRS CAPII.
Conflict of interest
None reported.
Disclaimer
Scientific manuscripts published in the Journal of Swine Health and Production are peer reviewed. However, information on medications, feed, and management techniques may be specific to the research or commercial situation presented in the manuscript. It is the responsibility of the reader to use information responsibly and in accordance with the rules and regulations governing research or the practice of veterinary medicine in their country or region.
References
1. Corthier G. Swine Fever: influence of passive immunity on pig immune response following vaccination with a live virus vaccine (Thiverval strain). Annales de Recherches Vétérinaires [Annals of Veterinary Research]. 1976;7:361–372.
2. Prickett J, Zimmerman J. The development of oral fluid based diagnostics and applications in veterinary medicine. Anim Health Res Rev. 2010;11:207–216.
3. Prickett JR, Kim W, Simer R, Yoon K, Zimmerman J. Oral-fluid samples for surveillance of commercial growing pigs for porcine reproductive and respiratory syndrome virus and porcine circovirus type 2 infections. J Swine Health Prod. 2008;16:86–91.
*4. Spronk G, Nerem J, Hanson D, Wayne S, Dee S. Oral fluids as an alternative technology in practice. Proc Iowa Dis Conf. 2011;127–131.
*5. Day D, Baysinger A, Hartsook G. PRRS Diagnostics: correlation of serum, tonsil scrapings, and oral fluids in gilts with natural exposure. Proc AASV. Phoenix, Arizona. 2011:81–82.
6. Kittawornrat A, Prickett J, Chittick W, Wanga C, Engle M, Johnson J, Patnayak D, Schwartz T, Whitney D, Olsena C, Schwartz K, Zimmerman J. Porcine reproductive and respiratory syndrome virus (PRRSV) in serum and oral fluid samples from individual boars: Will oral fluid replace serum for PRRSV surveillance? Virus Res. 2010;154:170–176.
7. Pepin B, Fanfang L, Main R, Ramirez A, Zimmerman J. Collection of oral fluids from individually housed sows. J Swine Health Prod. 2015;23:35–37.
8. Elmore M, Garner J, Johnson A, Kirkden R, Richert B, Pajor E. Getting around social status: Motivation and enrichment use of dominant and subordinate sows in a group setting. Appl Anim Behav Sci. 2011;133:154–163.
9. Seddon Y, Guy J, Edwards SA. Optimising oral fluid collection from groups of pigs: Effect of housing system and provision of ropes. Vet J. 2012;193:180–184.
10. Jensen P. An analysis of agonistic interaction patterns in group-housed dry sows: Aggression regulation through an “avoidance order.” App Anim Ethol. 1982;9:47–61.
11. Chapinal N, Ruiz-de-la-Torre J, Cerisuelo A, Baucells M, Gasa J, Manteca X. Feeder use patterns in group-housed pregnant sows fed with an unprotected electronic sow feeder (Fitmix). J Appl Anim Welf Sci. 2008;11:319–336.
12. Hunter E, Broom D, Edwards S, Sibly S. Social hierarchy and feeder access in a group of 20 sows using a computer-controlled feeder. Anim Prod. 1988;47:139–148.
13. Tuchscherer M, Puppe B, Tuchscherer A, Kanitz E. Effects of social status after mixing on immune, metabolic, and endocrine responses in pigs. Phys Behav. 1998;64:353–360.
14. Bench CJ, Rioja-Lang FC, Hayne SM, Gon-you HW. Group gestation housing with individual feeding – I: How feeding regime, resource allocation, and genetic factors affect sow welfare. Livest Sci. 2013;1522: 208–217.
*15. Parsons T. Making electronic sow feeding work in the United States: Static versus dynamic animal flows. Proc Leman Conf. 2011:203–205.
16. Prickett J, Johnson J. Prognostic profiling of swine populations using oral fluid samples. Veterinary Diagnostic Laboratory, Iowa State University, Ames, Iowa. Available at https://vetmed.iastate.edu/vdpam/research/disease-topics/swine/oral-fluids. Accessed 26 May 2016.
*17. Pavlovic L, Dunipace S, Horback T, Parsons T. Behavioral assessment of gestating sows via ear-mounted accelerometers. Proc AASV. Dallas, Texas. 2014:353.
*18. Sullivan K, Parsons T. Evaluation of sleep-wake cycle and behavioral indicators of REM in gestating sows. Proc AASV. Orlando, Florida. 2013:285.
*Non-refereed references.