| Production Tool | Peer reviewed |
Cite as: Peters BM, Krantz SA, Holtkamp DJ, et al. Reference values for immunocrit ratios to assess maternal antibody uptake in 1-day-old piglets. J Swine Health Prod. 2016;24(1):36–41.
Also available as a PDF.
SummaryColostrum intake is an essential component for piglet survival. Assessing colostrum intake, and consequently transfer of immunoglobulins (Igs), has been difficult to quantitate in swine. In this study, the authors first sought to subjectively determine the least stressful method to collect necessary sample quantities for Ig quantitation. The immunocrit ratio (IR) method was used to quantify a benchmark Ig level for a commercial production system. Lastly, the authors sought to identify associations between IR and production parameters. The cephalic vein provided consistent sample volumes and caused minimal animal distress. Additionally, a small volume of serum (30 μL) can be used for IR testing. An IR benchmark was determined to be 0.098 for this production system. For this study, no significant associations were found between pre-weaning mortality or average daily weight gain and IR. Birth weight and parity had significant effects (P < .05) on IR, with parity 1 litters having lower IR than higher parity litters. Using the IR technique to identify IR benchmarks for piglets will help producers improve colostrum intake opportunities in piglets with suboptimal Ig levels. The IR method ascertains whether piglets are receiving adequate maternal antibodies until their own immune systems are developed. | ResumenEl consumo de calostro es un componente esencial para la supervivencia del lechón. La evaluación del consumo de calostro, y por ende la transferencia de inmunoglobulinas (Igs por sus siglas en inglés), ha sido difícil de cuantificar en cerdos. En este estudio, primero, los autores investigaron como determinar subjetivamente el método menos estresante para recolectar la cantidad de muestra necesaria para la cuantificación de la Ig. Se utilizó el método de índice de inmunocrito (IR por sus siglas en inglés) para cuantificar un nivel comparativo para la Ig para un sistema de producción comercial. Segundo, los autores buscaron identificar asociaciones entre el IR y los parámetros de producción. La vena cefálica proporcionó volúmenes de muestra consistentes y causó mínima molestia al animal. Además, un pequeño volumen de suero (30 µL) puede utilizarse para pruebas de IR. Se determinó un punto de referencia de IR de 0.098 para este sistema de producción. En este estudio, no se encontraron asociaciones significativas entre la mortalidad predestete o la ganancia media de peso diaria y el IR. El peso al nacimiento y la paridad tuvieron efectos significativos (P < .05) en el IR, las camadas de paridad 1 tuvieron un IR más bajo que las camadas de paridad más alta. La utilización de la técnica IR para identificar los puntos de referencia IR para lechones ayudará a los productores a mejorar las oportunidades de consumo de calostro en lechones con niveles de Ig subóptimas. El método IR comprueba si los lechones están recibiendo anticuerpos maternos adecuados hasta que su propio sistema inmunológico se desarrolle. | ResuméLa prise de colostrum est un élément essentiel pour la survie des porcelets. L’évaluation de la prise de colostrum, et par conséquent le transfert d’immunoglobulines (Igs), a été difficile à quantifier chez le porc. Dans la présente étude, les auteurs ont premièrement visé à déterminer subjectivement la méthode la moins stressante pour prélever les quantités nécessaires d’échantillon pour la quantification d’Ig. La méthode du ratio d’immunocrite (IR) a été utilisée afin de quantifier un niveau étalon d’Ig pour le système commercial de production. Finalement, les auteurs ont voulu identifier les associations entre IR et les paramètres de production. L’utilisation de la veine céphalique a permis d’obtenir des volumes constants d’échantillons et causait un minimum de détresse chez les animaux. De plus, un faible volume de sérum (30 µL) peut être utilisé pour l’épreuve d’IR. Une valeur étalon d’IR de 0,098 fut déterminée pour ce système de production. Pour la présente étude, aucune association significative ne fut établie entre la mortalité pré-sevrage ou le gain de poids journalier moyen et l’IR. Le poids à la naissance et la parité avaient des effets significatifs (P < 0,05) sur l’IR, les portées de parité 1 ayant un IR plus faible que les portées de parités plus élevés. En utilisant la technique d’IR pour identifier les balises d’IR pour les porcelets aidera les producteurs à améliorer les opportunités de prises de colostrum chez les porcelets avec des niveaux sub-optimaux d’Ig. La méthode d’IR détermine si les porcelets reçoivent des quantités adéquates d’anticorps maternels jusqu’à ce que leur propre système immunitaire soit développé. |
Keywords: swine, colostrum, on-farm, immunocrit, immunity
Search the AASV web site
for pages with similar keywords.
Received: November 12, 2014
Accepted: August 10, 2015
Passive immunoglobulin (Ig) uptake via colostrum ingestion is of paramount importance for development of a newborn piglet’s immune system. Colostrum contains antibodies and cells, such as macrophages and B and T lymphocytes, involved in both innate and humoral immunity.1-3 Compared to older animals, a newborn piglet’s immune system has a limited capacity to synthesize Igs for the first 3 to 6 weeks of life.4 A piglet’s ability to uptake these constituents influences its survival during the first several weeks of life, as these components are the newborn piglet’s only source of protection against pathogens.5,6 In studies evaluating the effect of time on the Ig content of sow colostrum, the highest concentration of IgG in colostrum is seen at the start of parturition. After 24 hours, the level decreases to basal levels for sow milk.7 While the industry does not currently have a means of inducing continuously high globulin concentrations in sow milk, it is possible to implement colostrum management practices in order to promote optimal nursing during the time when piglet enterocytes allow peak globulin absorption. However, to date, it has been difficult to routinely quantify Igs to assess the need for colostrum management. Even though cattle and swine share the same mechanism of maternal immunity, until 1980, there was limited research regarding the quantification of swine Ig transfer.8,9 To amend this oversight, Yaguchi et al8 used technologies such as refractometry, electrophoresis, and spectrophotometry. In an attempt to make Ig quantitation more accessible to producers, a sandwich enzyme-linked immunosorbent assay (ELISA) was developed in 1985.10 Unfortunately, this ELISA still requires 3 hours of incubation time post sample collection. Recently, Vallet et al11 developed a simple, rapid, and inexpensive method to measure passive transfer of Igs from dam to piglet. Vallet et al11 tested serum samples from 1-day-old piglets via the immunocrit method to generate an immunocrit ratio (IR). In the immunocrit procedure, aliquots of serum and 40% ammonium sulfate solution are mixed to precipitate the Ig proteins present. The length of precipitate formed in the hematocrit tube is then compared to the residual serum solution in the tube to calculate the IR as a decimal. Using this method, a research swine herd IR benchmark was established in early 2013.11 However, benchmark immunocrit values and the ability to assess colostrum management with IR in commercial situations have not been validated. Therefore, the three objectives of this study were to evaluate alternative sampling techniques in neonatal piglets for the IR method, generate a benchmark for a desirable IR value in commercial populations, and determine whether an association exists between IR and production parameters such as wean weight and pre-weaning mortality.
Materials and methods
All animals in this study were raised and handled on commercial farms that are Pork Quality Assurance Plus certified and adhere to standards set forth by the National Pork Board. Due to the minimally invasive procedures utilized, an Institutional Animal Care and Use Committee protocol was not required.
The sows in the nine farms in this study were either C29 or 1070 PIC females (PIC, Hendersonville, Tennessee). These sows were housed in farrowing stalls, shared a common feed source, and were naive to porcine reproductive and respiratory syndrome virus. Pre-weaning mortality on these farms for the previous 6-month period ranged from 8% to 12%.
Sample collection
Blood samples for this study were obtained from piglets in all nine sow units within the same commercial production system. To first determine the most effective sampling method, a sample size of seventeen 24-hour- old piglets (± 12 hours) were selected from various litters within the same farrowing room. Selection of piglets was based on the ease of access to handlers. These piglets were assigned to various blood collection techniques via the cephalic vein or the medial or lateral saphenous veins. Blood collection via tail docking was attempted on several piglets from each of these groups. Blood collection at the time of tail docking would have provided the most practical collection method. Ideally, blood that pooled at the site of tail docking would have been suctioned into a 1-mL syringe. Unfortunately, an insufficient amount of sample (blood) pooled at the docking site. Using a 1-mL syringe and a 22-gauge × ¾-inch needle, 1 mL of blood was collected from each piglet to ensure an adequate volume (30 μL) of serum for testing. Piglet sample group size (n = 17) was determined by convenience while factoring in venous access and practicality for producers (no power studies were performed to determine this sample size). In order to not disrupt the lactation cycle of the litter, no more than two piglets were selected from the same sow. The degree of stress experienced by the animal was subjectively evaluated on the basis of the authors’ observations of subject vocalization and attempts to avoid restraint. A technique was considered successful if 1 mL of blood was collected from at least three piglets from the sample group of 17.
Once the successful sampling technique was found (via cephalic vein), 1 mL of blood from a new selection of 17 piglets was collected to determine serum volume necessary (30 or 50 μL) to evaluate IR results. Requiring less sample volume would prove beneficial in cases where a veterinarian wished to evaluate colostrum intake in a particularly dehydrated or unthrifty piglet.
On the basis of these results, blood collection via the cephalic vein was chosen for the larger study in which 779 piglets were sampled.
For the larger study, 30 litters were sampled from each of the nine sow farms. A light (< 1.25 kg), medium (1.25 to 1.75 kg), and heavy (> 1.75 kg) piglet was chosen, on the basis of birth weight, from each trial sow per farm. Sex, birth weight, sow parity, birth dam’s ID, total born alive in the litter in which the piglet was born, time of birth (am or pm), farrowing date, wean weight, and litter mortality were recorded for each piglet. Because previous research has stressed the importance of timing and piglet age with regard to passive Ig transfer, piglet birth time was approximated.7 The birth-time designation was determined as follows: any piglet born between 7 am and 2:59 pm was designated an am piglet. This designation signified that caretakers were present to observe parturition and assist with drying and nursing if deemed necessary. A pm birth was assigned to any piglet born between 3 pm and the following morning at 7 am when workers returned. Adjusted wean weights were calculated by multiplying average daily gain (kg) by 21 days. Blood (1 mL) was collected via the cephalic vein of piglets at approximately 24 hours of age by using a 22-gauge × ¾-inch needle and a 1-mL syringe (Figure 1). Samples were processed as previously described by Vallet et al.11 Briefly, blood was allowed to clot overnight. Serum was separated and centrifuged at 1350g for 10 minutes. Next, 50-μL serum samples were mixed with 50 μL of 40% ammonium sulfate ([NH4]2SO4) in dolphin-nosed tubes. Using hematocrit microcapillary tubes, the mixture was centrifuged at 14,800g with the following adjustment in procedure: serum centrifugation time was increased from 5 to 10 minutes, as recommended to improve serum separation).12 All blood samples were processed within 24 hours of collection.
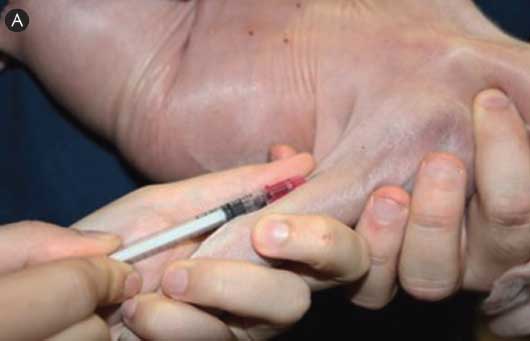
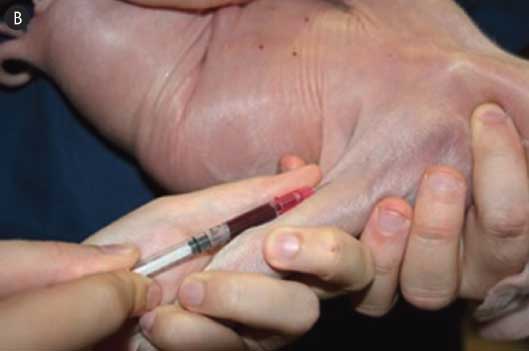
Figure 1: Illustration of a technique to draw blood from the cephalic vein using a 22-gauge, 3/4-inch needle to minimize stress to 1-day-old pigs. One mL of blood was more than sufficient to obtain a final volume of 50 μL serum, which can be used for the immunocrit ratio test to monitor colostrum intake.
Statistical analysis
A paired t test was performed on the IRs of 17 piglets to first determine the serum sample size (30 or 50 μL) needed to provide comparable results to the volume (50 μL) previously validated by Vallet et al.11 For this study, the piglet was the experimental unit. Descriptive statistics, including mean, standard deviation (SD), and maximum and minimum values, were calculated for IR by farm, and scatter plots of IR were generated. Mean IRs were calculated for each farm by parity (P1, P2, P3, P4+), birth weight (< 1.25 kg, 1.25 to 1.75 kg, and > 1.75 kg), and adjusted wean weight (< 5 kg, 5 to 7 kg, and > 7 kg). For each farm, the average IR and SD were calculated.
The effect of IR on average daily gain (ADG) was analyzed using a mixed linear regression model with IR, birth weight, am or pm birth, sex, parity, and total born alive as fixed effects and farm and sow as random effects. Birth weight was modeled as a continuous variable. The effects of IR, am or pm birth, sex, parity, and total born alive on pre-weaning mortality were analyzed using a logistic regression model with the GLIMMIX procedure (SAS 9.2; SAS Institute, Cary, North Carolina). Values of P < .05 were considered significant. To determine a practical IR sample size, the SD for this production system (0.026) was used in addition to estimating calculated precision (distance from the mean to limit), based on a 95% confidence limit.
Results
Sampling technique
The jugular and vena cava veins were excluded as potential blood collection sites due to the difficulty in consistent accurate venipuncture of these vessels in neonatal pigs. Tail docking, a normal production procedure commonly performed at 5 days of age, likewise proved to be an insufficient means of collecting a significant volume of serum to analyze. Sample collection from the saphenous veins yielded the desired quantity of blood (1 mL), but results were not easily replicated while attempting to humanely restrain the animal. The cephalic vein provided consistent sample volumes and required minimal restraint and distress to the animal.
There was no significant difference in resulting IR values (mean ± SD; n = 17) when comparing 30-µL (0.113 ± 0.018) and 50-μL (0.114 ± 0.018) samples (P = .37). While a volume of 0.5 mL blood per piglet was determined to provide a sufficient amount of serum for the immunocrit test, a 1-mL sample was consistently collected for each piglet in this trial in order to account for potential retesting or extreme results.
Commercial production system benchmark
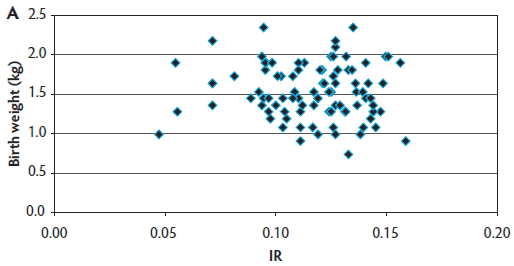
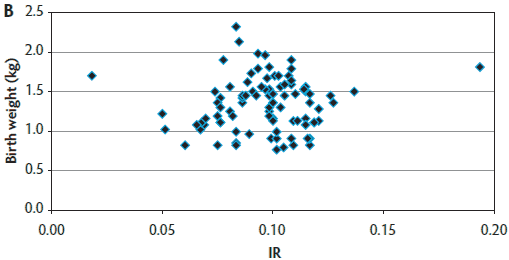
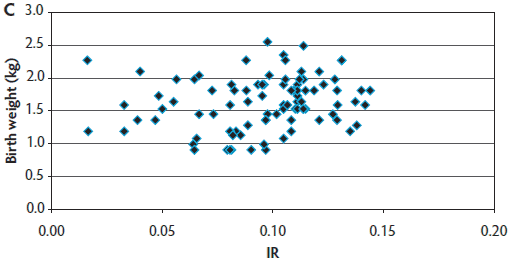
The target number of 90 blood samples collected per farm was reached for most sow farms (farms 1, 2, 3, 5, 6, and 9). However, in farms 4, 7, and 8, that target sample size was not achieved (actual sample size was 89, 69, and 81, respectively) because those sow farms had smaller sow-herd inventories and fewer farrowings occurring in a given week. Therefore, a total of 779 blood samples were obtained for immunocrit ratio measurements in this production system. A mean IR of 0.098 ± 0.026 was found for the entire operation (Table 1). Figure 2 provides an example of the uniformity a producer should strive to create within a herd. Farm 1 had the highest mean IR values and lowest variability (Figure 2A). Farm 9 consistently had the lowest IR values (Figure 2B). Farm 7 had mid-range IR values and the highest variability (Figure 2C). Across these three farms (1, 7, and 9) and the other farms (data not shown for farms 2 through 6 and Farm 8), it was demonstrated that small piglets can achieve a high IR and that heavier birth weight piglets may have lower IRs. In five of nine sow farms (56%), P1 litters had the lowest IRs when compared with older parity litters, reinforcing the importance of managing parity distribution in the herd. In six of nine farms (66%), the highest IR values were found in piglets with the heaviest birth weight and with heavier weaned weights. On the basis of the calculated precision, a sample size of 30 piglets (distance from the mean of 0.009) was found to be adequate to evaluate colostrum management strategies on farms, because 30 piglets generated the shortest distance from the mean to limit, compared with other sample sizes tested.
Table 1: Average immunocrit ratios (IR) in 779 blood samples from 24-hour-old piglets in nine commercial sow farms belonging to one swine production system*
| Sow farm | Average IR (± SD) | Minimum | Maximum |
|---|---|---|---|
| 1 | 0.116 (± 0.023) | 0.047 | 0.158 |
| 2 | 0.101 (± 0.028) | 0.016 | 0.148 |
| 3 | 0.099 (± 0.022) | 0.046 | 0.172 |
| 4 | 0.098 (± 0.028) | 0.015 | 0.156 |
| 5 | 0.096 (± 0.022) | 0.051 | 0.193 |
| 6 | 0.095 (± 0.029) | 0.056 | 0.129 |
| 7 | 0.095 (± 0.029) | 0.015 | 0.167 |
| 8 | 0.093 (± 0.026) | 0.015 | 0.138 |
| 9 | 0.090 (± 0.025) | 0.008 | 0.163 |
| Production system | 0.098 (± 0.026) | 0.008 | 0.163 |
* Farms with IR averages lower than that of the whole production system (bold) were targeted for intervention to improve colostrum management. Immunocrit ratio measurement: 50 μL of serum was mixed with 50 μL of ammonium sulfate solution in a dolphin-nosed tube, then centrifuged in a hematocrit microcapillary tube for 10 minutes. The IR was calculated by dividing the length of the precipitate in the tube (mm) by the length of the entire sample (mm).
SD = standard deviation.
Figure 2: Examples of immunocrit ratios (IR) in relation to piglet birth weight. Panel A: Farm 1 had consistently high IR values (most IR values are clumped together); Panel B: Farm 9 had consistently low IR values; and Panel C: Farm 7 had mid-range IR values and high variability.
IR and production parameters
No significant associations were found between pre-weaning mortality or ADG and IR. Birth weight was the only significant independent variable for ADG (P < .05). Parity had a significant effect on IR (P < .05), with P1 litters having lower IR than higher parity litters.
Discussion
The first goal of this study was to determine a blood collection method that was less stressful to the piglet than methods used previously in research settings.8, 11 The cephalic vein was easily accessed and repeatedly provided adequate sample volumes, and this method was minimally stressful to the animal. Consistent blood sample collection was expedited by adequate lighting, choosing well-hydrated piglets, and using experienced handlers. Hydration status was subjectively determined by assessing piglet body condition score and general attitude. If the needle slipped from the vein mid-draw, another method was used to collect the blood that pooled at the puncture site. Any contaminants, such as piglet moisture-absorbent powder, easily separated out upon centrifugation inside the hematocrit tubes.
For the immunocrit test, the paired t test results indicated that if a sample yielded less serum than anticipated, a smaller serum sample could be used. This validation will prove useful in situations when it is difficult to collect a sample from an animal, particularly in cases where piglets are severely dehydrated or in piglets with relatively low birth weights (< 0.68 kg).13 Modification of the collection method and the volume of blood necessary increases handler awareness of piglet welfare.
Vallet et al11 reported that an immunocrit ratio of 0.125 coincided with high piglet survivability in a research setting. This value was used as a desirable benchmark reference for the current study. Our data suggests that IR averages are lower in commercial settings. An explanation for this difference might be that the study of Vallet et al11 had a larger sample size (number of animals) within a single farm. In the present study, there was a small sample size per farm. The average IR of 0.098 (± 0.026) for the entire production system was used as a benchmark in the current study. Colostrum management could be improved within this production system by focusing efforts on the four farms (farms 6, 7, 8, and 9) with the lowest IRs. The highest-ranking IR value (0.116 ± 0.023) was identified as an achievable benchmark for commercial operations.
Low birth weights did not appear to condemn pigs to poor colostrum intake (as evaluated by indirect measurement of immunoglobulins using IR), and improving and monitoring colostrum intake among all pigs, regardless of birth weight, may be beneficial. One possible method to provide equal suckling opportunity for pigs within a litter is split suckling. Split suckling is a technique used to minimize competition amongst littermates by allowing half of the litter to nurse colostrum for 1 to 2 hours and then allowing the second half of the litter to nurse. Additionally, caretakers should assess the likelihood of colostrum ingestion on abdomen fullness rather than relying solely on piglet birth weight. These findings suggest that the producer’s goal, in addition to producing top-quality animals, should be to achieve consistent Ig intake, with fewer than 15% of pigs below an IR of 0.116.
It was interesting to observe that all piglets born in the medium-range weight group (1.25 to 1.75 kg) had the lowest IR. This result could be due to the attention caregivers gave to the lighter and heavier piglets during split suckling and suggests that care also needs to be given to the medium-sized piglet.
Contrary to reports by Vallet et al,11 a statistically significant association was not found between IR and pre-weaning mortality or ADG in this study of commercial animals. Despite this difference in findings, it is still a logical goal to strive to create a herd that is immunologically as uniform as possible. The antibodies that are garnered from colostrum ingestion still represent the piglet’s sole source of defense against pathogens during the first 4 to 6 weeks of life. Ensuring adequate colostrum intake during the first 24 hours of life acts as a preventive measure against future infections and resulting production losses.
Parity was the only variable that impacted IR. Producers with high replacement rates might benefit from the knowledge that offspring of P1 sows appear to be at an immunological disadvantage. For producers with high replacement rates and concurrent average IRs below commercial values, P1 sow offspring should be the caretakers’ primary focus in moving forward with colostrum- management improvement strategies.
The supply cost for performing an IR test is approximately $1.22 (US$) per piglet (2013 estimate). This accounts for the 1-mL syringe, a 22-gauge × ¾-inch needle, hematocrit tube, and blood tube needed per sample. Additional variable costs depend on the veterinarian’s access to and quantity purchased of the following: a micropipette, micropipette tips, ammonium sulfate, a standard centrifuge, and a hematocrit centrifuge. Because a sample size of 30 piglets was adequate to evaluate colostrum management strategies on farms, a recommendation is to choose 1-day-old piglets from 10 different litters of mixed sow parities and select one light, one medium, and one heavy piglet per litter as a representative sample of the population. The immunocrit values in the commercial farms reported here can be used as benchmarks to monitor colostrum management practices.
Implications
• The cephalic vein is a reasonable site for collecting blood samples from neonatal pigs.
• A serum volume of 30 µL may be used for the described immunocrit method.
• A sample size of 30 piglets per farm, including one light, one medium, and one heavy piglet from 10 litters of different parities, is a good target size to survey colostrum management on a sow farm.
• Sampling piglets once or twice per year and comparing their immunocrit ratios to the herd average will help to assess on-farm colostrum management. This system has the potential to attain an IR of 0.116. Individual production systems should ideally perform the immunocrit procedure in order to establish their system’s goal IR.
• Under the conditions of this study, pigs from P1 litters have significantly lower IRs, which may place them at a greater immunological disadvantage.
Acknowledgements
The authors would like to thank Katelyn Watt-Barker for aiding in sample collection, Deb Amodie and Dr Robyn Fleck for their support with the descriptive statistics and sample size calculations, and the University of Tennessee at Martin for the use of laboratory equipment.
Conflict of interest
None reported.
Disclaimer
Scientific manuscripts published in the Journal of Swine Health and Production are peer reviewed. However, information on medications, feed, and management techniques may be specific to the research or commercial situation presented in the manuscript. It is the responsibility of the reader to use information responsibly and in accordance with the rules and regulations governing research or the practice of veterinary medicine in their country or region.
References
1. Le Jan C. A study by flow cytometry of lymphocytes in sow colostrum. Res Vet Sci. 1994;57:300–304.
2. Bandrick M, Ariza-Nieto C, Baidoo S, Molitor T. Colostral antibody-mediated and cell-mediated immunity contributes to innate and antigen-specific immunity in piglets. Dev Comp Immunol. 2014:43;114–120.
3. Wagstrom E, Yoon K, Zimmerman J. Immune components in porcine mammary secretions. Viral Immunol. 2000;13:383–397.
4. McGlone J, Pond W. Applied anatomy and physiology related to blood sampling, hematology, and immunology. In: McGlone J, Pond W. Piglet Production: Biological Principles and Applications. Clifton Park, New York: Delmar Learning; 2003:34–50.
5. Quesnel H, Farmer C, Devillers N. Colostrum intake: influence on piglet performance and factors of variation. Livest Sci. 2012;146:105–114.
6. Rooke J, Bland I. The acquisition of passive immunity in the new-born piglet. Livest Prod Sci. 2002;78:13–23.
7. Frenyo V, Pethes G, Antal T, Szabo I. Changes in colostral and serum IgG content in swine in relation to time. Vet Res Commun.1980/1981;4:275–282.
8. Yaguchi H, Murata H, Kagota K, Namioka S. Studies on the relationship between the serum gamma globulin levels of neonatal piglets and their mortality during the first two months of life: an evaluation for the ammonium sulphate reaction. Brit Vet J. 1980;136:63–70.
9. Butler J, Lager K, Splichal I, Francis D, Kacskovics I, Sinkora M, Wertz N, Sun J, Zhao Y, Brown W, DeWald R, Dierks S, Muyldermans S, Lunney J, McCray P, Rogers C, Welsh M, Navarro P, Klobasa F, Habe F, Ramsoondar J. The piglet as a model for B cell and immune system development. Vet Immunol Immunopathol. 2009;128:147–170.
10. Varley M, Rucklidge G, Wilkinson R, Maitland A. Enzyme-linked immunosorbent assay for the measurement of immunoglobulin G concentrations in porcine plasma and colostrum. Res Vet Sci.1985; 38:279–281.
11. Vallet JL, Miles JR, Rempel LA. Simple novel measure of passive transfer of maternal immunoglobulin is predictive of preweaning mortality in piglets. Vet J. 2013;195:91–97.
12. Vallet JL, Miles JR, Rempel LA, Nonneman DJ, Lents CA. Relationships between day one piglet serum immunoglobulin immunocrit and subsequent growth, puberty attainment, litter size, and lactation performance. J Anim Sci. 2015;93:2722-2729.
13. Cabrera RA, Lin X, Campbell JM, Moeser AJ, Odle J. Influence of birth order, birth weight, colostrum and serum immunoglobulin G on neonatal piglet survival. J Anim Sci Biotechnol. 2012;3:1–10.