| Case Report | Peer reviewed |
Reporte de un caso que describe el curso clínico de la diarrea epidémica porcina en un centro de sementales comercial y el regreso del centro a servicio después de la inoculación del hato completo con el virus de la diarrea epidémica porcina
Cite as: McCarty M, Petznick T, Kuster C, et al. Case report describing the clinical course of porcine epidemic diarrhea in a commercial boar stud and return of the stud to service after whole-herd inoculation with porcine epidemic diarrhea virus. J Swine Health Prod. 2015;23(5):264–271.
Also available as a PDF.
SummaryIn January 2014, an outbreak of porcine epidemic diarrhea (PED) occurred in a PED-naive commercial boar stud. This report documents the outbreak following whole-herd PED virus (PEDV) inoculation with fecal material, cleaning procedures, sentinel exposure, and semen supply to naive sow farms. Boar saliva samples were diagnostically comparable to rectal swabs and collection dummy Swiffer (Proctor and Gamble, Cincinnati, Ohio) samples for PEDV RNA detection. Viral RNA was not detected in semen samples collected during the outbreak, yet reproductive organs from sacrificed boars 5 days after exposure were positive by polymerase chain reaction. Placed sentinel and replacement animals in the stud remained clinically negative following cleaning procedures, and semen shipments started 13 weeks post inoculation (WPI) to one PED-naive sow farm, with six other naive sow farms resuming shipments after 17 WPI. All sow farms remained naive 10 months later. This report demonstrates that it is possible for a commercial boar stud to experience a PED outbreak without infecting naive sow farms at the onset, retain valuable genetics, and resume semen delivery to PED-naive sow farms after cleaning, disinfection, and testing, without infecting sows upon re-opening. | ResumenEn enero 2014, ocurrió un brote de diarrea epidémica porcina (PED por sus siglas en inglés) en una centro de sementales comercial libre al PED. Este reporte documenta el brote seguido de la inoculación del centro completo con el virus del PED (PEDV por sus siglas en inglés) con materia fecal, procedimientos de limpieza, exposición a centinelas, y abastecimiento de semen a granjas de hembras libres de la enfermedad. Las muestras de saliva de los machos fueron comparables diagnósticamente con muestras rectales y los Swiffer (Proctor and Gamble, Cincinnati, Ohio) del banco de recolección en busca de la detección del PEDV RNA. No se detectó RNA viral en muestras de semen recolectadas durante el brote, sin embargo los órganos reproductivos de machos sacrificados 5 días después de la exposición resultaron positivos a la reacción en cadena de polimerasa. Los centinelas colocados y los animales de remplazo en la granja permanecieron negativos después de los procedimientos de limpieza, y el envío de semen inició 13 semanas después de la inoculación (WPI por sus siglas en inglés) a una granja de hembras libre de PED, otras seis granjas de hembras libres reanudaron la recepción después de 17 WPI. Todas las granjas de hembras permanecieron libres10 meses después. Este reporte demuestra que es posible que una centro de machos comercial experimente un brote de PED sin infectar granjas de hembras libres al inicio del brote, retenga genética de valor, y reanude la entrega de semen a granjas de hembras libres a la PED después de limpieza, desinfección, y pruebas, sin infectar hembras al reabrir. | ResuméEn janvier 2014, une éclosion de diarrhée épidémique porcine (DEP) est survenue dans une verraterie commerciale naive pour la DEP. Le présent rapport documente l’éclosion survenue suivant l’inoculation du troupeau au complet avec le virus de la DEP (VDEP) en utilisant du matériel fécal, les procédures de nettoyage, l’exposition d’animaux sentinelles, et la fourniture de semence à des fermes de truies naives. D’un point de vue détection de l’ARN du VDEP, les échantillons de salive de verrat étaient comparables à des écouvillons rectaux et des prélèvements effectués sur les mannequins de collecte à l’aide de Swiffer (Proctor and Gamble, Cincinnati, Ohio). L’ARN viral ne fut pas détecté dans les échantillons de semence prélevés durant l’éclosion malgré que des échantillons provenant des organes reproducteurs de verrats sacrifiés 5 jours suivant l’exposition étaient positifs par réaction d’amplification par la polymérase. Les sentinelles et les animaux de remplacement dans la verraterie sont demeurés cliniquement négatifs suivant les procédures de nettoyage, et les expéditions de semence commencées 13 semaines post inoculation (SPI) a une ferme de truies naives pour la DEP, et six autres fermes de truies naives commençant à recevoir de la semence 17 SPI. Toutes les fermes de truies sont demeurées naives 10 mois plus tard. Ce rapport démontre qu’il est possible pour une verraterie commerciale de subir une éclosion de DEP sans que des fermes de truies naives ne soient infectées au début de l’éclosion, de conserver la valeur génétique du troupeau, et de recommencer la livraison de semence à des fermes de truies naives pour la DEP après nettoyage, désinfection, et tests de détection, sans infecter des truies suite à la remise en opération. |
Keywords: swine, porcine epidemic diarrhea virus, artificial insemination, boar stud, PEDV
Search the AASV web site
for pages with similar keywords.
Received: December 2, 2014
Accepted: January 20, 2015
Porcine epidemic diarrhea virus (PEDV) was first identified in the United States in late April 2013.1 Since then it has spread rapidly across the country and caused significant production and economic losses, with estimates of 7 to 8 million pigs lost from June 2013 to April 2014.2 While strides have been made in prevention and clinical management in other segments of production, better information is needed to answer questions regarding the course of porcine epidemic diarrhea (PED) in artificial insemination boars, its effects on semen quality and production, and risk to sow farms sourcing from a previously infected stud. Of particular concern is the question of whether PEDV can be shed in semen.
Although confirmation of PEDV infection has been described within numerous sow farms, nursery facilities, and grow-finish barns throughout the United States, infection within a commercial boar stud has not yet been formally documented. Likewise, the veterinary literature lacks reports of mature boar infection. Up to this point, commercial boar studs faced the very real risk of depopulation if infected with PEDV, with the loss of valuable genotypes and inherent slow recovery to previous production levels after restocking with young boars. Given PEDV’s predilection for enterocytes,3 the ability to retain exposed boars, maintain a mature age structure, observe a prudent herd closure time, and re-open without infecting downstream sow farms was theoretically possible, but not yet proven. To the authors’ knowledge, this is the first North American PED case report of its kind, specific to artificial insemination boars, that demonstrates the ability to retain previously infected boars and resume service to naive sow farms without transmitting the virus.
Case history
In January 2014, PEDV entered a boar stud in northeast Nebraska that was negative for porcine reproductive and respiratory syndrome virus. This is a facility under veterinary care and certified by Pork Quality Assurance (PQA; National Pork Board).
Rapid detection, intentional whole-herd exposure, and boar retention provided a unique opportunity to capture much-needed data and set the conditions for this clinical case report. At the time of the outbreak, the boar stud held approximately 200 boars in the main barn, with 30 boars present in a connected on-site isolation barn. The site is fully filtered from October 1 to June 1 each year. During times of filtration, the load-out area has a positive pressure system to prevent back-draft of air. The load-out area is used for removal of dead animals, garbage, or other items exiting the site. No known biosecurity breakdowns occurred at this site. The closest known PEDV-positive farm at the time was approximately 11.2 kilometers away.
On January 23, in the afternoon, after boar collection had been completed for the day and semen had been shipped to naive farms, diarrhea was observed in four boars in the main barn. All farms were contacted to monitor closely for clinical signs, and semen held at the boar stud for post-production analysis was sent to GeneSeek, Inc (Lincoln, Nebraska) for PEDV testing by polymerase chain reaction (PCR). All samples tested negative. The next morning, 15 boars in the main barn had diarrhea, and semen collection was halted for the day. Fecal samples were collected and transported to GeneSeek, Inc, for PEDV PCR testing. Results were received the same afternoon, with all submitted samples positive for PEDV RNA (Figure 1).
Figure 1: Timeline of events for a porcine epidemic diarrhea (PED) outbreak in a commercial boar stud, detailing clinical disease, diagnostic testing, sentinel pig exposure, and resuming of semen delivery to PED-naive sow farms after whole-herd oral inoculation with fecal material. On January 23, 2014, an outbreak of diarrhea was observed in a genetic boar stud housing approximately 200 boars in the main building, and 30 boars in a connected on-site isolation barn. Rectal swabs were collected from a total of 30 conveniently selected cohort boars and tested for porcine epidemic diarrhea virus (PEDV) by PCR. Beginning immediately after the first set of samples were collected (day 0), whole-herd PEDV inoculation was performed by spraying into the mouth of each boar fecal material collected from diarrheic boars. Inoculation of boars that did not show clinical signs was repeated 3 days post inoculation (DPI) using fresh fecal material and refrigerated aliquots of the first inoculum. Fecal and oral swabs were collected from cohort boars to evaluate viral shedding, and environmental samples were tested for PEDV genetic material.
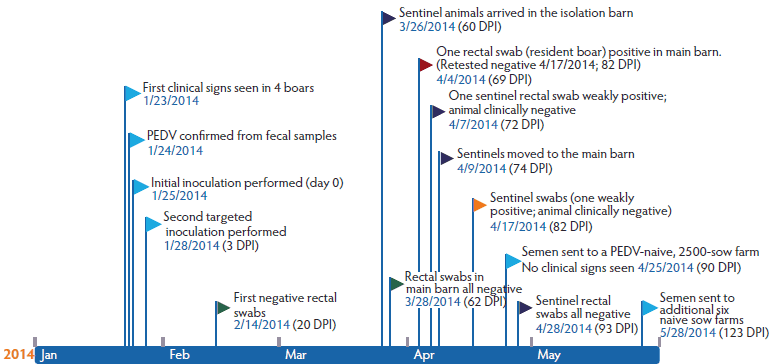
Investigative design, sample collection, and laboratory testing
On the morning of January 25, whole-herd PEDV inoculation was performed using fecal material from diarrheic boars that was sprayed into the mouth of each boar. Oral inoculation of boars that had not previously shown clinical signs was repeated 3 days later using fresh fecal material and refrigerated aliquots of the first inoculum (Figure 1). Immediately prior to inoculation, 30 mature boars in the main stud, not showing clinical signs of PEDV infection and not located directly beside a boar that was showing clinical signs, were conveniently selected for prospective diagnostic monitoring. This was done in an effort to time the initial exposure to PEDV inoculation with feces from boars that were either PCR-positive or showing clinical signs, rather than to prior exposure. Rectal swabs were collected from these 30 boars using individual sterile cotton swabs that were placed in 0.5 mL sterile saline in a 5-mL snap-cap tube (Falcon tube; Corning, New York).
Rectal swab samples were subsequently collected from the 30 cohort boars on days post inoculation (DPI) 1-8, 13, 20, 27, 34, 41, 48, 55, 62, and 69 (Table 1) for testing at Iowa State University Veterinary Diagnostic Laboratory (ISU VDL; Ames, Iowa) using a previously described PEDV N-gene-based real-time reverse transcriptase- (RT-) PCR.4 Because of financial testing constraints, a subset of 10 boars from the original cohort of 30 were conveniently selected for additional collection and testing of semen, serum, and oral-swab samples during the study period (Table 2 and Table 3). Semen was collected utilizing the double gloved-hand method for minimum contamination, with subsamples obtained for further testing in the on-site semen-processing laboratory using aseptic technique.5 Oral swabs were collected using a sterile cotton swab that was inserted between the lip and gum while boars were mounted on the dummy. Samples were then placed in BD Universal Viral Transport System vials (UVT; Franklin Lakes, New Jersey). Collected semen samples were processed at the ISU VDL as previously reported6 and individually assayed for PEDV RNA using a described PCR protocol.4 After semen samples were collected from the boars, unscented, dry Swiffer pads (Proctor and Gamble, Cincinnati, Ohio) were soaked in 10 mL of sterile saline, used to wipe the collection dummies, and then placed in sealed plastic bags. Oral swabs and Swiffer pads were assayed using the same PCR protocol with individual results from 0 to 3 DPI and pooled results thereafter. Serum indirect fluorescent antibody (IFA) testing was performed as previously described.4
Table 1: Summary results for cohort boars tested for porcine epidemic diarrhea virus (PEDV) by polymerase chain reaction (PCR) on rectal swabs*
| Day post inoculation | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 13 | 20 | 27 | 34 | 41 | 48 | 55 | 62 | 69 | |
| n | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 29† | 28† | 27† | 27 | 27 | 27 | 27 | 27 |
| No. positive | 1 | 9 | 25 | 30 | 29 | 30 | 30 | 30 | 30 | 30 | 24 | 22 | 16 | 24 | 4 | 2 | 0 | 1‡ |
| Min Ct | 34.5 | 13.0 | 14.6 | 15.0 | 14.7 | 14.7 | 12.6 | 13.9 | 16.7 | 19.4 | 26.2 | 27.8 | 28.6 | 27.2 | 31.9 | 32.1 | 0.0 | 34.0 |
| Max Ct | 34.5 | 34.7 | 34.9 | 34.0 | 33.0 | 30.0 | 33.0 | 30.3 | 33.1 | 34.3 | 34.4 | 34.7 | 34.8 | 34.5 | 34.6 | 33.3 | 0.0 | 34.0 |
| Mean Ct | 34.5 | 26.3 | 26.6 | 21.9 | 20.1 | 19.9 | 18.7 | 19.9 | 23.1 | 28.9 | 31.1 | 31.7 | 32.7 | 31.9 | 33.5 | 32.7 | 0.0 | 34.0 |
* Study described in Figure 1.
† On each day, one boar was euthanized due to lameness or was unexpectedly found dead.
‡ Retest on this boar the following week was negative.
Ct = cycling threshold; Min = minimum; max = maximum.
Table 2: Results testing for porcine epidemic diarrhea virus (PEDV) by PCR on oral swabs obtained from boars during semen collection*
| DPI | No. of samples† | Pooled | PCR results | |
|---|---|---|---|---|
| Positive | Negative | |||
| 0 | 10 | No | 0 | 10 |
| 6 | 9 | No | 9 | 0 |
| 13 | 9 | Yes | 2 | 0 |
| 20 | 9 | Yes | 0 | 2 |
| 27 | 9 | Yes | 0 | 2 |
* Study described in Figure 1. Of the 30 boars described, 10 were chosen for additional, once-weekly collection of oral fluids by swabbing the mouth while the boar was mounted on a dummy. A sterile cotton swab was inserted between the lip and gum and then was placed in virus transport medium. Transport medium was pooled (two pools; one pool of five and one pool of four samples) for testing at 13, 20, and 27 DPI.
† One boar was removed in the first week because of lameness.
PCR = polymerase chain reaction; DPI = days post inoculation.
Table 3: Results of PCR testing of environmental samples obtained from a collection dummy in a boar stud recently exposed (day 0) to porcine epidemic diarrhea virus (PEDV) by whole-herd oral inoculation*
| DPI | No. of samples | Pooled | Positive | Negative |
|---|---|---|---|---|
| 0 | 10 | No | 3 | 7 |
| 2 | 5 | No | 5 | 0 |
| 3 | 6 | No | 6 | 0 |
| 6 | 6 | Yes | 1 | 0 |
| 20 | 6 | Yes | 1 | 0 |
| 27 | 4 | Yes | 0 | 1 |
| 34 | 6 | Yes | 1 | 0 |
| 48 | 6 | Yes | 0 | 1 |
| 55 | 6 | Yes | 0 | 1 |
* Study described in Figure 1. Unscented, dry Swiffer pads (Proctor and Gamble, Cincinnati, Ohio) soaked in 10 mL of sterile saline were used to wipe down dummies after semen collection. Pads were placed in sealed plastic bags and tested individually (up to 3 DPI) or pooled (one pool of four or six samples) thereafter.
PCR = polymerase chain reaction; DPI = days post inoculation.
Extended semen samples were submitted to Kuster Research and Consulting Inc (Geneseo, Illinois) for semen quality evaluation, including computer-aided sperm analysis motility (Integrated Visual Optical System; Hamilton Thorne Biosciences, Beverly, Massachusetts), full morphology differential (manual; trained technician), and flow cytometry analysis (Guava EasyCyte Plus; Millipore Corp, Hayward, California). A non-infected boar stud that mirrored the infected site in key aspects (negative control site), including geographic proximity (approximately 14.5 kilometers), shared farm personnel prior to the PED break, genotypes, and production protocols, was chosen for comparison of semen quality. Extended semen samples collected from 10 genotype- and age-matched boars at the negative control site were submitted once a week for 8 weeks to provide comparative semen-quality data. Semen-quality parameters were analyzed by repeated measures analysis of variance (ANOVA), with Tukey’s HSD test used to investigate pair-wise comparisons where significant differences (P < .05) were noted (Statistix 10.0; Analytical Software, Tallahassee, Florida).
Three non-cohort boars in the main barn were chosen for necropsy to determine if there was evidence of PEDV infection present in reproductive organs that could lead to direct semen shedding. All three boars had shown clinical signs for 1 to 2 days prior to necropsy at 5 DPI. All reproductive organs were harvested with the intent of preventing contamination from the environment or the intestinal contents, with sections of each of the following collected for histopathology, immunohistochemistry (IHC), and PCR testing: testes, epididymides, bulbourethral gland, seminal vesicles, prostate, and penis. Intestinal samples were also collected.
Cleaning and disinfection procedures
Initial cleaning of the main and isolation barns consisted of removing all visible organic material from equipment and floors with a hot-water power washer (87ºC) using Biosolve detergent (DuPont, Wilmington, Delaware), then sanitizing with Clorox bleach (Oakland, California). Lemon juice was also used in both barns to remove hard water stains and biofilm, with the additional use of Synergize (Reno, Nevada), a quaternary ammonium-glutaraldehyde disinfectant, in isolation only. The cleaning procedure commenced 14 and 20 DPI in the main and isolation barns, respectively. Boars in isolation at the time of the outbreak were moved into the main barn at 20 DPI.
Unscented Swiffer pads were used to sample for PEDV RNA in cleaned and disinfected areas. Samples were collected from all aspects of isolation, including the shower area, medication room, boar stalls, feed boxes, collection area, and miscellaneous equipment. In addition, laboratory and semen pick-up locations, including insulated coolers, semen cups, carts, floor, countertops, computer, microscope, cool room, and anteroom floor were tested using the Swiffer pad protocol as described.
Sentinel animals
After cleaning and disinfection, seven commercial, PEDV-naive sentinel grower pigs of mixed gender, weighing approximately 57 kg, were placed in the empty isolation barn 9 weeks post inoculation (WPI). These sentinels were then moved into the main barn 11 WPI (Figure 1). Rectal swabs were obtained from sentinels 10 days after they were placed in isolation, and 8 and 15 days after they were moved to the main barn. In addition, 40 naive replacement boars were placed in the isolation barn 14 WPI and moved to the main barn, with direct contact with previously infected boars, at 17 WPI.
Results and outcomes
The most intense period of clinical disease after inoculation occurred 4 to 6 DPI, with evidence of watery diarrhea, reduced feed intake, lethargy, and occasional vomiting (3.28%). All but seven boars in the entire stud had recorded clinical signs consistent with PED. The last clinical signs were noted in the main barn on February 7, 2014, at 13 DPI. One boar of the 10 initially designated for prospective diagnostic monitoring was removed in the first week due to lameness that prevented semen collection.
At the initial sampling (day 0 immediately before oral inoculation), rectal swabs from 29 of the 30 cohort boars were negative by PCR, with cycle threshold (Ct) cutoff > 35. In the single positive boar, quantity of virus was low (Ct = 34.5). At 3 DPI, all 30 boars were positive by fecal PCR for PEDV, with Ct values ranging from 15.0 to 34.0 (Table 1). With the exception of one boar at 4 DPI, all others remained PCR-positive from 3 to 13 DPI. Inconsistent fecal PEDV shedding was apparent thereafter in the study population (Table 1). At approximately 9 WPI, all 30 cohort boars tested negative by PCR on rectal swabs. One boar tested PCR-positive at 69 DPI after testing negative on the 3 previous weeks. On retest the following week, the rectal swab from this boar once again tested negative.
All semen samples were negative by PEDV PCR on day 0 immediately before oral inoculation with feedback material. Semen samples from all boars at subsequent collection time points were also negative. Serum samples were negative for PEDV antibody by IFA at 1 DPI, and all were positive at 21 DPI. Oral swabs from all sampled boars were PCR-negative on day 0 immediately before oral inoculation. All oral-fluid swabs were positive at 6 DPI (Ct = 25 to 32) and remained positive through 13 DPI (Ct = 32 to 33). Thereafter, all pools were negative for PEDV genomic material (Table 2).
Small intestinal samples from all three necropsied boars showed histopathological changes consistent with PEDV infection, including villus atrophy with variable enterocyte degeneration or attenuation and mild non-suppurative cellular inflammation within the lamina propria. Viral antigen was also detected by IHC in affected sections. In contrast, reproductive organs of all three boars were unremarkable histologically, and PEDV antigen was not detected in testes, epididymis, seminal vesicle, bulbourethral gland, prostate, or penile tissue. However, testicular tissue from two of the boars and penile tissue from the third were PCR-positive (Ct = 29.6 to 34.3).
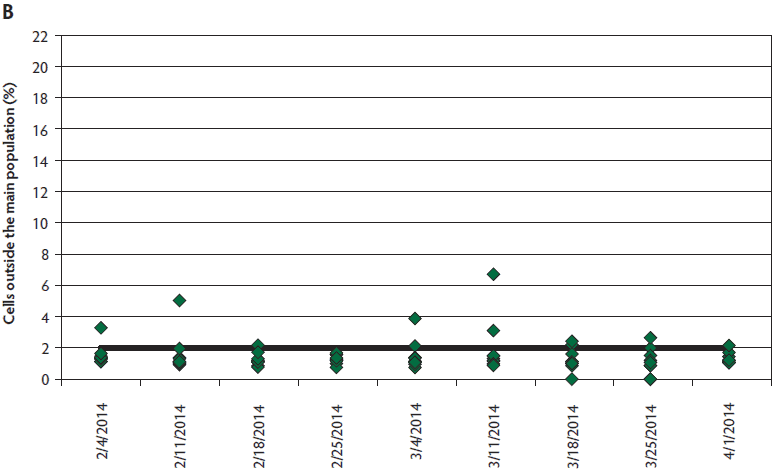
Semen quality data was not available for five observations (two infected, three controls) due to inability to obtain a sample or non-submission of collected boars. Sperm motility was significantly different between the boars housed at the PEDV-infected site and those in the control site (infected, 73%; control, 81%; P = .01), with no interaction between weeks post inoculation and location. Significant differences were not identified for normal morphology comparisons between sites (P = .09). Sperm plasma membrane viability and acrosome integrity (VIA) were measured both on fresh semen (tested on arrival) and stored semen (at expiration), with no differences at either time point by location (P > .05). While differences in VIA were also not detected for the interaction of location and WPI after storage, this interaction was significant for the fresh VIA analysis overall (P = .04). However, Tukey’s HSD test revealed no pairwise differences (P > .05). Values declined at similar rates of 5% to 8% by location between the fresh and stored readings. Differences in DNA integrity were present between the infected and control sites (P = .01) and between WPI (P < .001) (Figure 2). Pairwise comparisons revealed that DNA integrity was compromised most at 9 WPI for PEDV-infected boars. Significant differences were noted between ejaculates for individual boars for all parameters monitored, independent of PEDV exposure.
Figure 2: Comparison of sperm DNA integrity between boars (n = 9) housed at the porcine epidemic diarrhea virus- (PEDV-) infected site (panel A) and genotype and age-matched control boars (n = 10) housed at the negative control site (panel B) located approximately 14.5 km away. Sperm DNA integrity was compromised at the PEDV-infected site, while largely remaining within normal limits at the control location.
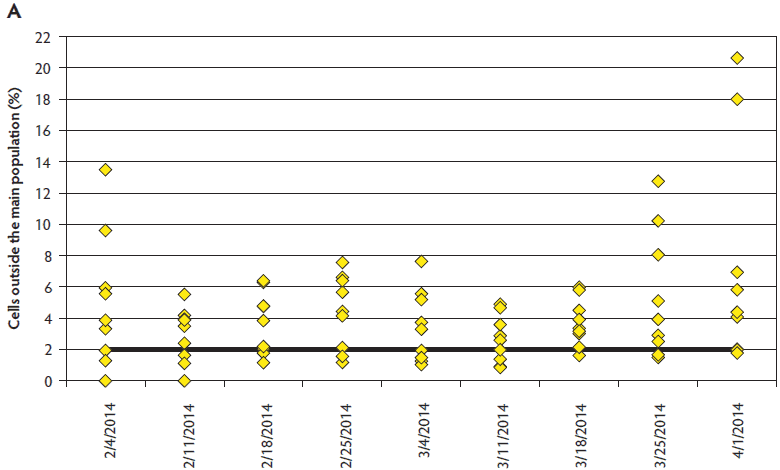
Virus was detected on three of 10 collection-dummy Swiffer pads samples collected prior to inoculation (Ct = 29.6 to 34.6). Individual dummy Swiffer pads were uniformly positive at 2 DPI (n = 5; Ct = 24.3 to 32.9) and 3 DPI (n = 6; Ct = 20.8 to 29.5). Pooled fluids obtained from Swiffer pads were PCR-positive at 6, 20, and 34 DPI with Ct values of 21.7, 31.5, and 32.5, respectively, and were PCR-negative at 48 and 55 DPI (Table 3).
Four rounds of cleaning and disinfection were performed in the isolation barn and two to three rounds of cleaning in the main barn, depending on location. Even after passing visual inspection, five of 15 samples collected from the isolation barn on the third round of cleaning were positive for PEDV by PCR, with Ct = 24.8 to 34.8. Compared to other sampled locations in the main barn, boar stalls had the highest detectable quantities of PEDV RNA. Of the laboratory and semen pick-up locations, three samples were weakly PCR-positive (20 DPI): cart (semen pick-up location), computer, and anteroom, with Ct = 33.5 to 34.4.
Sentinel animals showed no clinical signs of PED when placed in the cleaned and disinfected isolation barn or the main barn holding boars previously exposed to PEDV at 9 and 11 WPI, respectively. However, one rectal swab each from the first and second collections (11 WPI and 15 WPI) was weakly positive by PCR (Ct = 33.7 and 34.2). All other samples were PEDV-negative. Replacement boars did not develop clinical disease after being placed in the isolation and main barns at 14 and 17 WPI, respectively.
At the onset of this PEDV outbreak at the boar stud, the sow base served by this boar stud included a limited number of PEDV-positive farms, and semen shipments to these units (n = 4) resumed within 1 WPI. Semen distribution to PEDV-naive sows resumed at 13 WPI to a 2500-sow farm, and 4 weeks after that to six other PEDV-naive sow farms (17 WPI), until a total of 11 sow sites (including four PEDV-positive or exposed sow farms), with a total inventory of approximately 45,000 sows, were once again being served exclusively by this boar stud. None of the naive farms receiving semen displayed clinical signs of PEDV or produced positive PEDV diagnostic testing after resuming acceptance.
Discussion
Clinical signs in this naive farm were an early warning signal to initiate confirmatory testing and closure of the boar stud before potentially infecting sow farms, as demonstrated by no downstream infection. Similar to swine of all ages,7 individual boars varied in the timing, duration, and severity of disease. However, disease in this case may have been slightly altered by the strategic whole-herd inoculation. Rectal swab PCR testing demonstrated consistent shedding throughout this population of adult boars for at least 2 weeks, with a high proportion remaining in the suspect or positive range for nearly 6 WPI. An abrupt reduction was detected at 7 WPI. Intermittent shedding was demonstrated toward the end of the infection phase.
Environmental sampling from the collection dummy with Swiffer pads was effective at identifying PEDV in the environment throughout the outbreak. Due to the nature of the case report, dummy swab results were not available immediately prior to recognition of clinical signs, but remained positive from the time this sampling method was deployed immediately prior to oral inoculation until 20 DPI, with intermittent results from pooled samples thereafter. Use of the dummy Swiffer pads can be considered a tool to detect PEDV in the population, with the boars acting as “bio-swabs” as they become contaminated with virus from themselves, their neighbors, or the environment during normal eating, drinking, lying down, movement to the collection area, and interaction with warm-up or collection pens, then conveniently deposit the virus in a natural bottleneck (collection pen) where it is easily obtained during routine production.
Great care was taken to ensure that collected semen samples were not contaminated with fecal-associated virus. In this report, PEDV was not detected by PCR in raw semen. Both the pellet fraction and the seminal plasma were negative for all tested boars at any day post inoculation. However, testicular tissue from two euthanized boars was PCR-positive with low quantities of detectable PEDV genomic material (Ct = 29.6 to 34.3). Non-testicular reproductive organs were also sporadically PCR-positive in these boars, yet IHC for PEDV was consistently negative for all male reproductive organs and boars. Porcine epidemic diarrhea virus viremia8 or tissue contamination during the necropsy procedure are potential reasons for the tissue, but not the semen, to contain genomic material.
The semen quality monitoring suggests the possibility of a negative association between PEDV infection and the parameters of sperm motility and morphology routinely assessed at boar studs. Although weeks post inoculation did not influence motility results, a divergent trend was noted in morphology due to increased abnormalities over time at the infected site that may have gone undetected because of the few missing observations. Sperm membrane parameters were apparently unaffected. The most noteworthy finding was the difference in DNA integrity, which was challenged at the infected site, while largely remaining within normal limits for the control location. Cells outside the main population of 2% or more can put boars at risk for suboptimal reproductive performance.9-11 Of particular concern is the limitation that this assessment is not currently practical at boar studs, and may not be recognized if not evaluated at a veterinary andrology laboratory. This case report indicates the need for prospective work to further investigate the effects of PEDV infection on boar semen quality and potential fertility, especially for boar studs with the opportunity to service previously infected (immune) sow farms soon after a PEDV break.
Successful introduction of PEDV-naive stock indicates both that adequate time had passed and environmental decontamination was successful between the initial outbreak and placement of non-immune boars. Not only had remission of clinical signs occurred and viral shedding abated in inoculated boars, but the barn environment had become safe for naive stock, despite persistently positive environmental swabs. However, it should be noted that rectal swabs from sentinel animals placed in the barn showed weak PCR positivity, despite the absence of clinical signs. It is unknown if this weak positivity was true infectious virus, rogue environmental genomic material, or laboratory contamination, but this finding led to confusion regarding release of semen to naive sow farms and introducing naive boars. This highlights the sensitivity of the PEDV PCR and the reminder that presence of viral genome does not guarantee infection in the clinical setting.
The detection and closure procedure observed by this boar stud at the time of the outbreak was sufficient to prevent PEDV-negative sow farms from becoming infected. Subsequently, the interventions applied allowed semen shipments from previously infected boars to resume to sow farms that had no prior history of PEDV infection, without negative consequences. The timeline details the events of this case from infection to successful return to sow-farm service. Success in this case reinforces the possibility of returning a PEDV-infected boar stud to service and highlights the need to determine how this can be repeated safely after less down time.
Implications
• Boar reproductive organs may contain low quantities of PEDV genomic material in the acute phase of infection; however, under the conditions of this case, virus is not detectable by PCR in semen samples.
• Environmental sampling of the boar collection dummy with Swiffer pads (Proctor and Gamble, Cincinnati, Ohio) can be utilized as a PEDV environmental-monitoring tool.
• In this case, semen from boars previously exposed to PEDV could be shipped to sow farms following strategic suspension and strict collection hygiene protocols.
• Semen quality may be affected during a PEDV outbreak and should be closely monitored when ongoing service to sow farms is considered.
Acknowledgements
The authors wish to thank the Pillen Family Farms systems for accesses to facilities, boars, and staff, as well as public data presentation for the benefit of the North American swine industry. A special thanks is due to the boar stud personnel for all their assistance and hard work at cleaning and disinfection. The study was supported in part by funding from the Iowa Pork Producers Association.
Disclaimer
Scientific manuscripts published in the Journal of Swine Health and Production are peer reviewed. However, information on medications, feed, and management techniques may be specific to the research or commercial situation presented in the manuscript. It is the responsibility of the reader to use information responsibly and in accordance with the rules and regulations governing research or the practice of veterinary medicine in their country or region.
References
1. Stevenson GW, Hoang H, Schwartz KJ, Burrough ER, Sun D, Madson D, Cooper VL, Pillatzki A, Gauger P, Schmitt BJ, Koster LG, Killian ML, Yoon KJ. Emergence of Porcine epidemic diarrhea virus in the United States: clinical signs, lesions, and viral genomic sequences. J Vet Diagn Invest. 2013;25:649–654.
2. Meyers S. PEDV’s Impact: Now and Tomorrow. National Pork Board. Available at: http://m.pork.org/News/4678/PEDVsImpact.aspx. Accessed 8 July 2105.
3. Saif L, Pensaert MB, Sestak K, Yeo SG, Jung K. Coronaviruses. In: Zimmerman JJ, Karriker LA, Ramirez A, Schwartz KJ, Stevenson GW, eds. Diseases of Swine. 10th ed. Ames, Iowa: Wiley-Blackwell; 2012:501–524.
4. Madson DM, Magstadt DR, Arruda PH, Hoang H, Sun D, Bower LP, Bhandari M, Burrough ER, Gauger PC, Pillatzki AE, Stevenson GW, Wilberts BL, Brodie J, Harmon KM, Wang C, Main RG, Zhang J, Yoon KJ. Pathogenesis of porcine epidemic diarrhea virus isolate (US/Iowa/18984/2013) in 3-week-old weaned pigs. Vet Microbiol. 2014;174:60–68.
5. Althouse GC, Kuster CE, Clark SG, Weisiger RM. Field investigations of bacterial contaminants and their effects on extended porcine semen. Theriogenology. 2000;53:1167–1176.
6. Christopher-Hennings J, Nelson EA, Nelson JK, Hines RJ, Swenson SL, Hill HT, Zimmerman JJ, Katz JB, Yaeger MJ, Chase CC. Detection of porcine reproductive and respiratory syndrome virus in boar semen by PCR. J Clin Microbiol. 1995;33:1730–1734.
*7. Pospischil A, Stuedli A, Kiupel M. Update on porcine epidemic diarrhea. J Swine Health Prod. 2002;10:81–85.
8. Jung K, Wang Q, Scheuer KA, Lu Z, Zhang Y, Saif LJ. Pathology of US porcine epidemic diarrhea virus strain PC21A in gnotobiotic pigs. Emerg Infect Dis. 2014;20:668–671.
9. Boe-Hansen GB, Christensen P, Vibjerg D, Nielsen MB, Hedeboe AM. Sperm chromatin structure integrity in liquid stored boar semen and its relationships with field fertility. Theriogenology. 2008;69:728–736.
10. Broekhuijse ML, Sostaric E, Feitsma H, Gadella BM. Relationship of flow cytometric sperm integrity assessments with boar fertility performance under optimized field conditions. J Anim Sci. 2012;90:4327–4336.
11. Evenson DP, Thompson L, Jost L. Flow cytometric evaluation of boar semen by the sperm chromatin structure assay as related to cryopreservation and fertility. Theriogenology. 1994;41:637–651.
* Non-referred reference.