| Diagnostic notes | Peer reviewed |
Cite as: Burrough ER, Rotolo ML, Gauger PC, et al. Correlation of Lawsonia intracellularis semi-quantitative fecal polymerase chain reaction assay results with the presence of histologic lesions of proliferative enteropathy and positive immunohistochemical staining. J Swine Health Prod. 2015;23(4):204–207.
Also available as a PDF.
SummaryThe presence of Lawsonia intracellularis in swine feces is commonly confirmed using highly sensitive polymerase chain reaction (PCR) assays. The objective of this retrospective study was to determine, on the basis of cycle-threshold (Ct) values for a given real-time PCR assay, the likelihood of positive fecal PCR results correlating with the presence of histologic lesions and positive immunohistochemistry (IHC) in tissues from the same submission. Sixty-three cases submitted from 2012 to 2014 were selected for analysis, with Ct values ranging from 16.94 to 37.66. There was a strong negative correlation between the Ct value of a positive PCR and the quantity of L intracellularis antigen detected by IHC. On the basis of these results, PCR Ct values < 20.00 had a positive predictive value of 100% for the presence of proliferative lesions and L intracellularis antigen by IHC, and PCR Ct values > 30.00 were associated with a negative predictive value of > 95% for these variables. These data reveal a strong association between Ct values and the presence or absence of L intracellularis infection detectible by light microscopy, suggesting that specific ranges of Ct values carry strong predictive value for the presence or absence of porcine proliferative enteropathy. | ResumenLa presencia de la Lawsonia intracellularis en heces porcinas es comúnmente confirmada utilizando la prueba, altamente sensible, de ensayo de reacción en cadena de polimerasa (PCR por sus siglas en inglés). El objetivo de este estudio retrospectivo, fue determinar en base a los valores del ciclo umbral (Ct por sus siglas en inglés) para un ensayo específico de PCR en tiempo real, la posibilidad de correlacionar los resultados de PCR fecal positivo con la presencia de lesiones histológicas e inmunohistoquímica positiva (IHC por sus siglas en inglés) en tejidos del mismo envío. Se seleccionaron sesenta y tres casos enviados entre 2012 y 2014 para ser analizados, con valores de Ct oscilando de 16.94 a 37.66. Hubo una fuerte correlación negativa entre el valor del Ct de un PCR positivo y la cantidad de antígeno de L intracellularis detectado por el IHC. En base a estos resultados, los valores Ct del PCR < 20.00 tuvieron un valor predictivo positivo de 100% para la presencia de lesiones proliferativas y del antígeno L intracellularis por IHC, y los valores Ct de PCR > 30.00 fueron asociados con un valor predictivo negativo de > 95% para estas mismas variables. Estos datos revelan una fuerte asociación entre los valores Ct y la presencia o ausencia de infección de L intracellularis detectable por medio de microscopía ligera, sugiriendo que los rangos específicos de valores Ct tienen un fuerte valor predictivo para la presencia o ausencia de la enteropatía proliferativa porcina | ResuméLa présence de Lawsonia intracellularis dans les fèces de porc est généralement confirmée au moyen d’épreuves très sensibles de réaction d’amplification en chaîne par la polymérase (PCR). L’objectif de cette étude rétrospective était de déterminer, sur la base des valeurs du seuil de cycles (Ct) pour une épreuve donnée de PCR en temps réel, la probabilité de résultat positif par PCR pour un échantillon fécal ayant une corrélation avec la présence de lésions histologiques et un résultat positif par immunohistochimie (IHC) pour les tissus d’une même soumission. Soixante-trois cas soumis de 2012 à 2014 furent sélectionnés pour analyse, avec des valeurs de Ct variant de 16,94 à 37,66. Il y avait une forte corrélation négative entre la valeur de Ct d’un cas positif par PCR et la quantité d’antigène de L intracellularis détectée par IHC. Sur la base de ces résultats, des valeurs de Ct < 20,00 avaient une valeur prédictive positive de 100% pour la présence de lésions prolifératives et d’antigènes de L intracellularis par IHC, et des valeurs de Ct > 30,00 étaient associées avec une valeur prédictive négative de > 95% pour ces variables. Ces données révèlent une forte association entre les valeurs de Ct et la présence ou l’absence d’infection par L intracellularis détectable par microscopie photonique, suggérant ainsi que des écarts spécifiques de valeurs de Ct ont une forte valeur prédictive pour la présence ou l’absence d’entéropathie proliférative porcine. |
Keywords: swine, Lawsonia intracellularis, porcine proliferative enteropathy, PPE
Search the AASV web site
for pages with similar keywords.
Received: August 30, 2014
Accepted: January 20, 2015
As porcine proliferative enteropathy (PPE) typically affects growing and finishing pigs, antemortem diagnostics are often preferred over elective necropsy and tissue-based techniques. Accordingly, detection of Lawsonia intracellularis, the causative agent of PPE, is often based upon positive fecal polymerase chain reaction (PCR) assays. While PCR assays are highly specific and often very sensitive, positive PCR results alone provide limited contextual information. It is then up to the clinician to determine if a positive PCR result reflects simply detection of a potential pathogen or confirms the presence of disease associated with the detected pathogen. In some instances, such as observation of a highly specific clinical scenario or typical lesions associated with a given disease, a positive PCR result may be easily interpretable. However, in instances where the clinical scenario is vague, such as in diarrhea or soft stools often associated with various clinical manifestations of Lawsonia infection in growing-finishing pigs, a positive PCR alone may be difficult to interpret. The objective of this diagnostic note is to describe the association between positive results in a commonly used, feces-based PCR assay for detection of L intracellularis, the gold standard for detecting this pathogen, and immunohistochemistry (IHC) for antigen within proliferative intestinal lesions, the gold standard for confirmation of PPE.
Materials and methods
All samples used in this investigation were derived from routine diagnostic submissions to the Iowa State University Veterinary Diagnostic Laboratory (ISU VDL) from pigs in which L intracellularis PCR was requested on feces as part of the original diagnostic work up. Accordingly, as samples were collected for routine diagnostic purposes, the approval of an animal care committee was not required.
Samples were processed and handled under standard operating procedures for diagnostic submissions to the ISU VDL. Approximately 0.01 to 0.02 grams of feces were added to 1 mL phosphate buffered saline, and DNA was extracted using the MagMAX Pathogen RNA/DNA Kit (Life Technologies, Austin, Texas) and a KingFisher 96/Flex magnetic particle processor (Thermo Scientific, Waltham, Massachusetts), according to the manufacturers’ instructions. The PCR assay was performed using primers and probe as previously described,1 with modifications using the TaqMan Fast Virus 1-Step Master Mix (Life Technologies), and cycle parameters according to the kit insert, with inclusion of a XENO internal control (Life Technologies) to detect PCR inhibition. All samples were received between January 5, 2012, and February 11, 2014. Sixty-three cases were included in this study, with cycles-to-threshold (Ct) values ranging from 16.94 to 37.66. These samples were selected such that approximately one third of the samples had PCR Ct values < 25, another third had values ≥ 25 and ≤ 30, and the remainder had Ct values > 30. All samples came from cases in which the PCR was performed on feces from an individual animal and a corresponding tissue sample from that animal was available for histopathology and IHC. Microscopic slides from each case were reviewed by a pathologist, and any tissue section with proliferative epithelial lesions, or a section of ileum in the absence of lesions, was submitted for IHC using a mouse-monoclonal antibody specific for L intracellularis under standard operating procedures of the ISU VDL. The IHC sections were scored as follows: 0 if no L intracellularis antigen was detected; 1 if < 10% of crypt epithelial cells contained immunoreactive bacteria; 2 if 10% to 50% of crypt epithelial cells contained immunoreactive bacteria; and 3 if > 50% of crypt epithelial cells contained immunoreactive bacteria. Statistical analyses (t test and Spearman’s rho) were performed using a commercial statistical software package (JMP Pro 10; SAS Institute, Cary, North Carolina). Diagnostic sensitivity and specificity analyses for the use of PCR Ct values to detect PPE used IHC results as the gold standard for true-positive and true-negative case status.
Results
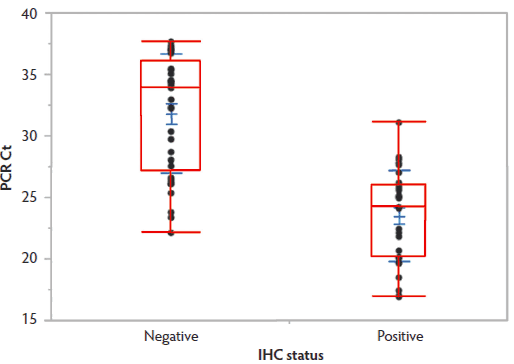
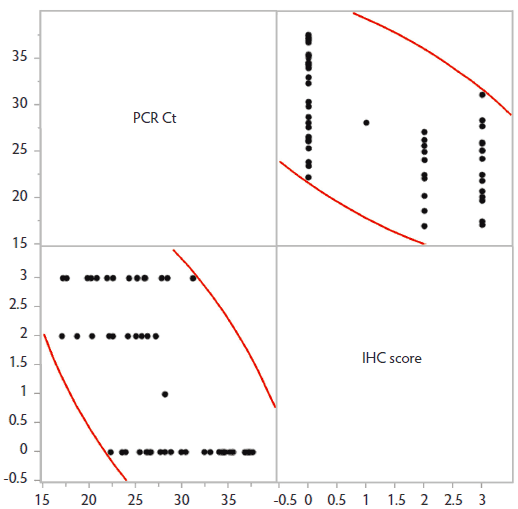
Results of IHC were positive in 30 cases versus 33 cases in which tissues were immunonegative. The mean PCR Ct value (± standard deviation) for IHC-negative pigs (31.91 ± 4.82) was higher than that for IHC-positive pigs (23.56 ± 3.70), and this difference was statistically significant (P < .001; Figure 1). Additionally, IHC scoring revealed a significant negative correlation between IHC score and PCR Ct (Spearman’s ρ = -0.6602; P < .001; Figure 2), where increasing IHC scores were associated with lower Ct values. Values of ≤ 25.08 were 90.9% to 100% specific for the presence of PPE; however, at these Ct values, diagnostic sensitivity fell below 60%. Similarly, Ct values > 28.09 were associated with diagnostic sensitivities of 90% or greater, but diagnostic specificity fell below 70%. For detection of PPE, PCR Ct values < 20 were associated with a positive predictive value of 100%, and Ct values > 30 had a negative predictive value of over 95% for the cases included in this data set.
Figure 1: Boxplots representing PCR Ct values for Lawsonia intracellularis detected in feces from 63 routine diagnostic submissions to the Iowa State University Veterinary Diagnostic Laboratory associated (n = 30) and not associated (n = 33) with concurrent detection of Lawsonia antigen within intestinal tissues by IHC. The mean PCR Ct value is indicated within each box by a solid line spanning the width of the box. The means differed significantly (P < .001; t test). PCR = polymerase chain reaction; Ct = cycle threshold; IHC = immunohistochemistry.
Discussion
In the case of common or ubiquitous infectious agents in swine populations, interpreting the clinical significance of positive PCR assays from antemortem samples (feces, oral fluids, nasal swabs, etc) can be a challenge. For disease diagnosis, the question of presence versus impact of a pathogen is critical, and without clinical context can make interpreting the positive results of assays with high analytical sensitivity quite a conundrum. A key understanding is that analytical sensitivity and specificity are not the same as diagnostic sensitivity and specificity, though many inadvertently confuse the two. Diagnostic specificity is a reflection of the positive predictive value of an assay regardless of its analytical sensitivity and specificity.2 Similarly, diagnostic sensitivity is a reflection of the negative predictive value of an assay. Therefore, an assay that has high analytical sensitivity and can detect minute quantities of nucleic acid in the laboratory is not automatically the best diagnostic assay, as detection does not necessarily correlate with predictive value for disease.
In cases where semi-quantitative data in the form of Ct values are available for a given PCR assay, it can be tempting to speculate that lower Ct values correspond to more clinically relevant colonization, infection, or disease; however, there are limited experimental data to support such an association or to determine significant threshold Ct values that may differentiate between detection of a pathogen and presence of disease. Additionally, such theoretical threshold Ct values would likely differ, depending on the specific infectious agent or assay and laboratory parameters. These parameters include template-independent factors such as severity and stage of disease, sample type and quality, sample collection method, method of nucleic acid extraction, commercial or internal PCR reagents, and PCR efficiency, as well as cycling parameters and platform.
In this report, we analyzed results of diagnostic testing of routine field cases where both fecal PCR results and IHC for L intracellularis antigen were available. Given the strong linear correlation between PCR Ct values and IHC scores, where lower Ct values are correlated with higher IHC scores, these data support the generalization that lower PCR Ct values correlate with more abundant bacteria within tissues. A similar correlation has been reported between gross intestinal lesion length and fecal shedding of L intracellularis at the time of necropsy.3 In another study,1 PCR was directly compared to IHC for detection of L intracellularis, and positive PCR results at any Ct value were associated with a diagnostic specificity of 85%. However, samples in that study were concentrated in the mid-range of Ct values (24 to 28), and only approximately 15 of 111 samples tested were in the upper range of a positive test with Ct values of 32 to 36. In the present report, the objective was to use samples more evenly distributed on the basis of their Ct values, with a similar number of cases within specified ranges to decide whether Ct values could be used to better determine detection (presence of bacteria without PPE lesions) versus disease (presence of bacteria with PPE lesions and positive IHC). This study purposely included many more PCR-positive but disease-negative pigs than in the earlier study by Lindecrona et al.1 Strong positive and negative predictive values for IHC results became apparent through evaluation of ranges of PCR Ct values, which suggests that samples with Ct results at the upper end of positive (> 30) more likely reflect detection than disease.
Figure 2: Comparison of PCR Ct values for Lawsonia intracellularis detection in pig feces with concurrent IHC scores for Lawsonia antigen within intestinal tissues from routine diagnostic submissions to the Iowa State University Veterinary Diagnostic Laboratory (n = 63 sample combinations denoted by individual black dots). Results reveal a significant negative correlation (- 0.6602) between IHC score and PCR Ct (P < .001; Spearman’s ρ), where higher IHC scores were associated with lower Ct values. IHC scores: 0, no L intracellularis antigen detected; 1, < 10% of crypt epithelial cells contained immunoreactive bacteria; 2, 10% to 50% of crypt epithelial cells contained immunoreactive bacteria; and 3, > 50% of crypt epithelial cells contained immunoreactive bacteria. PCR = polymerase chain reaction; Ct = cycle threshold; IHC = immunohistochemistry.
While the focus of this diagnostic note was to determine the potential association between PCR Ct values and the presence of histologic lesions IHC-positive for L intracellularis, it bears noting that subclinical infections with L intracellularis may still have significant production impacts. For instance, a recent study4 demonstrated that subclinically infected pigs still had a lower growth rate than non-infected controls and hence had the potential to infect other pigs in the herd. Sample pooling for PCR assays, as is common practice in swine diagnostic submissions, may impact diagnostic results and should be taken into consideration, particularly if pooling is extensive. Given the common use of modified live Lawsonia vaccine, variable immunity in herds, and the potential endemic nature of the agent, it is likely that in many instances, the agent will be detected without being associated with disease. Detection of post-vaccination shedding of Lawsonia has been demonstrated intermittently for up to 9 weeks.5 Accordingly, the significance and impact of a positive L intracellularis PCR assay depends first and foremost upon the individual production system and the health history of the affected herd.
This diagnostic note reveals that abnormal swine feces subjected to the described PCR assay at the ISU VDL with Ct values < 20 likely correlate with the presence of proliferative enteropathy, while values > 30 are likely indicative of Lawsonia infection but not necessarily disease. In the absence of concurrent tissue submission for contextual interpretation, clinicians and diagnosticians may use these data as a general guide to improve the diagnostic specificity of positive L intracellularis fecal PCR results for identifying pigs with PPE.
Implications
• When the L intracellularis PCR assay is used on fecal samples as described in this report, Ct values < 20 have a positive predictive value approaching 100% for PPE in the animal sampled.
• When the L intracellularis PCR assay is used on fecal samples as described in this report, Ct values between 20 and 30 require interpretation within clinical context and acknowledgment that other diseases may be causing clinical signs in the animal sampled.
• When the L intracellularis PCR assay is used on fecal samples as described in this report, Ct values > 30 have a negative predictive value of approximately 95% for PPE, suggesting that L intracellularis is unlikely to be the cause of observed diarrhea in the animal sampled.
Acknowledgements
This work was supported by the Boehringer Ingelheim Vetmedica Professorship in Food Animal Infectious Disease.
Conflict of interest
None reported.
Disclaimer
Scientific manuscripts published in the Journal of Swine Health and Production are peer reviewed. However, information on medications, feed, and management techniques may be specific to the research or commercial situation presented in the manuscript. It is the responsibility of the reader to use information responsibly and in accordance with the rules and regulations governing research or the practice of veterinary medicine in their country or region.
References
1. Lindecrona RH, Jensen TK, Andersen PH, Møller K. Application of a 5’ nuclease assay for detection of Lawsonia intracellularis in fecal samples from pigs. J Clin Microbiol. 2002;40:984–987.
2. Saah AJ, Hoover DR. “Sensitivity” and “specificity” reconsidered: The meaning of these terms in analytical and diagnostic settings. Ann Intern Med. 1997;126:91–94.
*3. Beckler D, Armbruster G, Rutten-Ramos S. Evaluation of fecal shedding by a high-throughput qPCR assay in a Lawsonia intracelluaris challenge. Proc 43rd AASV. Denver, Colorado. 2012;149–153.
4. Paradis MA, Gebhart CJ, Toole D, Vessie G, Winkelman NL, Bauer SA, Wilson JB, McClure CA. Subclinical ileitis: Diagnostic and performance parameters in a multi-dose mucosal homogenate challenge model. J Swine Health Prod. 2012;20:137–141.
5. Guedes RM, Gebhart CJ. Onset and duration of fecal shedding, cell-mediated and humoral immune responses in pigs after challenge with a pathogenic isolate or attenuated vaccine strain of Lawsonia intracellularis. Vet Microbiol. 2003;91:135–145.
* Non-refereed reference.