| Literature review | Peer reviewed |
Cite as: Al Masri S, Hünigen H, Al Aiyan A, et al. Influence of age at weaning and feeding regimes on the postnatal morphology of the porcine small intestine. J Swine Health Prod. 2015;23(4):186–203.
Also available as a PDF.
SummaryThe small intestinal mucosal epithelium is the interface between ingested nutrients and their distribution networks in the underlying vasculature and lymphatics. This review reports on the small intestinal mucosal surface changes in the piglet from birth to the time of natural weaning (> 54 days). Despite numerous publications on the morphological characteristics of the gastrointestinal tract, there is limited comparability among these due to substantial methodological differences. The comparability of the methodological designs used in this review was achieved by relativizing the data to the day of weaning. Weaning at 35 days or later had little to no effect on the intestinal mucosa. Early weaning at 28, 21, 14, 5, 3, and 1 day after birth was associated with dramatic structural changes in the mucosa. A frequent observation after early weaning was prominent villus atrophy. While the crypt epithelium responds to redress these dramatic changes, villus recovery to near preweaning status may be slow. The earlier a piglet is weaned, the greater the villus atrophy and the longer the time to recovery. A causal relationship between reduced feed intake in the first days after weaning, independent of the diet, and the morphological alterations of the intestine is apparent. | ResumenEl epitelio mucoso del intestino delgado es la interfase entre los nutrientes ingeridos y su red de distribución en la vasculatura subyacente y linfáticos. Esta revisión reporta los cambios en la superficie de la mucosa del intestino delgado en el lechón desde el nacimiento hasta el destete natural (> 54 días). A pesar de numerosas publicaciones sobre las características morfológicas del tracto gastrointestinal, la posibilidad de comparación entre ellas es limitada debido a diferencias metodológicas sustanciales. La posibilidad de comparación de los diseños metodológicos utilizados en este análisis se logró al relativizar los datos hasta el día del destete. El destete a los 35 días o después tuvo poco o ningún efecto sobre la mucosa intestinal. El destete temprano a los 28, 21, 14, 5, 3, y 1 días después del nacimiento fue asociado con cambios estructurales dramáticos en la mucosa. Una observación frecuente después del destete temprano fue la atrofia prominente de la vellosidad intestinal. Aunque el epitelio críptico responde para reparar estos cambios dramáticos, la recuperación de la vellosidad intestinal a su estado pre-destete puede ser lenta. Cuanto más se adelanta el destete del lechón, mayor es la atrofia de la vellosidad intestinal, y más largo el tiempo de recuperación. Es aparente una relación causal entre el consumo reducido de alimento en los primeros días después del destete, independientemente de la dieta, y las alteraciones morfológicas del intestino. | ResuméL’épithélium de la muqueuse du petit intestin est l’interface entre les nutriments ingérés et leurs réseaux de distribution dans les vaisseaux sanguins et lymphatiques sous-jacents. La présente revue fait état des changements qui surviennent à la surface de la muqueuse du petit intestin chez les porcelets de la naissance jusqu’au moment du sevrage naturel (> 54 jours). Malgré de nombreuses publications sur les caractéristiques morphologiques du tractus gastro-intestinal, la comparabilité entre les études est limitée étant donné les différences méthodologiques marquées. La comparabilité des designs méthodologiques utilisés dans la présente revue fut obtenue en relativisant les données au jour du sevrage. Un sevrage à 35 jours ou plus avait peu ou pas d’effet sur la muqueuse intestinale. Un sevrage hâtif à 28, 21, 14, 5, 3, et 1 jour après la naissance était associé à des changements structuraux dramatiques dans la muqueuse. Une observation fréquente après un sevrage hâtif était une atrophie marquée des villosités. Alors que l’épithélium des cryptes répond pour renverser ces changements dramatiques, la récupération des villosités à un statut pré-sevrage peut être lente. Plus un porcelet est sevré tôt, plus l’atrophie des villosités est marquée et plus le temps de récupération est long. Une relation causale entre une diminution de l’ingestion de nourriture dans les premiers jours qui suivent le sevrage, indépendamment de la diète, et les altérations morphologiques de l’intestin est apparente. |
Keywords: swine, intestine, villus, crypt, morphometry
Search the AASV web site
for pages with similar keywords.
Received: July 14, 2014
Accepted: December 11, 2014
With ongoing pressure to improve production efficiency, coupled with legislative requirements to reduce the use of antibiotics in the pig industry, it is imperative to identify, qualify, and quantify factors affecting nutrient utilization and intestinal function. Additionally, the pig is an important model for many studies of human intestinal physiology and pathology.1-4 Piglets are used as models to study enteric infections because the piglet gastrointestinal tract, particularly around birth and at weaning, closely resembles that of humans.
The most important function of the small intestine is degradation and absorption of nutrients.5-8 Careful qualitative and precise quantitative investigations are critical to measure the effects of nutrients over time on intestinal morphological parameters.9 The small intestine tunica mucosa’s surface epithelium is the principal interface where nutrient degradation and absorption take place.10 The mucosal functional surface area is increased by specializations, such as folds, villi, and crypts. The mucosal columnar epithelium consists of many different cell types, most of them having prominent microvilli in the form of a brush border at the luminal surface. In total, the specialised architecture of the mucosa increases the surface area by a factor of 600.11,12
As growth performance in pig production is an important parameter that is dependent on optimal intestinal function, morphometric analysis of normal and pathologically affected mucosa, particularly villi and crypts, is widely used in intestinal research. Because much of the enzymatic processing of the dietary components, as well as absorption within the small intestine, occurs near and around the villi and crypts, postweaning villus atrophy and crypt hyperplasia and the subsequent reconstructional processes cause a temporary decrease in digestive and absorptive capacity.13 A reduction in small intestinal villus height after weaning is associated with a reduction in brush-border enzyme activity. Therefore, postweaning weight gain is correlated with villus height.14 Positive correlations of villus height to daily weight gain have been demonstrated.15 Villus atrophy, therefore, impairs pig growth performance by reducing nutrient absorption.14-17
Morphometry involves a quantitative assessment of intestinal architecture and is more reliable and reproducible than any subjective assessment. It may also be important in assisting in the diagnosis of many pathological conditions, such as discriminating different types of inflammatory diseases of the small intestine not readily apparent during routine assessment.18,19 In addition, morphometry has been used to evaluate the condition of the intestinal mucosa after antibiotic treatment in human patients with small intestinal bacterial overgrowth.20 It has been suggested that, in pigs, a reduction in digestion and absorption would encourage development of an osmotic diarrhea, while unabsorbed dietary material could act as a substrate for enterotoxigenic Escherichia coli in the gut.21 However caution should be taken when evaluating morphology alone as a measure of gut development or health. For example, in humans it was found that diarrheal diseases like cholera or norovirus infection may be without histological changes in the intestine, despite substantial rates of net fluid loss, electrolyte secretion, and altered barrier function.22 Moreover, it is not known whether the presence of pathogens in the small intestine is a cause or effect of changes in small intestinal morphology.13
This review aims to survey the current literature on morphometric evaluation of the postnatal development of the porcine small intestine, focusing on the influences of age, weaning, and feeding regimes. Only data from researchers who presented their results in numerical form and over an observation period of more than 1 day were evaluated in the review. Pig breeds used in the research reviewed and evaluated were Landrace, Large White, and their hybrids. Due to differences in study design in the publications being reviewed, and to allow meaningful comparisons, the data were converted to percentages of the parameters measured at the time of weaning. The parameters measured in the studies reviewed include intestinal weight and length, villus height and width, crypt depth, and villus:crypt ratio.
Challenges in defining the small intestinal segments and artifacts associated with tissue processing as well as morphometry
The gross anatomy of the pig small intestine has been described previously in textbooks.23-26 As in all mammals, the pig small intestine has three structurally and functionally different regions: the duodenum, the jejunum, and the ileum. The three regions of the small intestine are less clearly defined microscopically in the pig than in humans.27-29 In contrast to most mammals, the submucosal glands of Brunner in adult pigs extend not only the full length of the duodenum, but also into the proximal jejunum, thus extending along approximately 4 m of the small intestine.11,30 A further porcine characteristic is that the lymphatic aggregations are much more extensive in their distribution. Components of the gut-associated lymphatic tissue (GALT) are found along the whole length of the porcine intestine.31,32 The aggregated lymphatic nodules in the tunica mucosa and tela submucosa, known as Peyer’s patches, which occur in different forms and locations in the pig, are not restricted to the ileum.27-29
Sample sites used in the morphometric studies reviewed vary considerably. Some researchers provided little or no information regarding the exact areas from which their samples were taken. This is of particular importance when considering the elongate jejunum. To minimize the effects of this problem, wherever possible, the data on the small intestine were classified into three categories: the proximal, middle, and distal thirds. The intestinal segments studied by the various research groups are indicated as closely as possible. In cases where researchers used “duodenum, jejunum, and ileum,” for example Makkink et al,33 we classified these as “proximal, middle, and distal” small intestine. Identification of sample sites used in the morphometric studies was not the only problem in evaluating morphometric data. Many artefacts associated with tissue processing and evaluation for villus height measurements may confound the results of studies and make them difficult to compare. As Greeson and Jan34 point out, in diverse examples in human anatomy, many challenges are associated with evaluation of morphometry. For example, the duodenum is constantly assaulted by damaging peptic juices that often cause gastric surface cell metaplasia, irregular villous architecture, and Brunner’s gland hyperplasia. Villus morphology may also appear markedly different in the presence of large lymphoid aggregations, such as Peyer’s patches in the terminal ileum. The orientation of the tissue when the section is cut can cause distortion and apparent shortening of the villi. Probably the most important artifact associated with tissue orientation is that of tangential positioning. This is the single most common cause of errors in small intestine biopsy interpretation because it results in an illusion of shorter villi that appear to be associated with an increased number of lamina propria cells. The effect of crushing tissues both during sample collection and in subsequent processing is another artefact. Some degree of crush artefact may be present at the edges of biopsy specimens, but the central portions should be intact if the tissue is oriented correctly. Even with perfect orientation, one rarely encounters many normal villi in a row, all perpendicular to the lumen. More often villi are bent in different directions, and the crypts have varying angulations. Consequently, a diagnosis of “normal” requires examination of many sections and overall familiarity with the tissues being examined.34,35 Use of different protocols for tissue processing is another factor influencing morphological parameters. For example, Rieger et al9 showed that different fixatives can have a huge influence on porcine intestinal tissue shrinkage, and consequently studies using different protocols are difficult to compare.
Tables 1 and 2 provide overviews of the experimental designs taken from the literature included in this review. The treatments of each study analyzed (weaning age, days of sampling, studied small intestinal segments, parameters measured, and number of animals examined) are summarized here.
Table 1: Analyzed references regarding effects of weaning age of pigs and time of sampling
| Reference | Weaning age (days) | Time of sampling | |
|---|---|---|---|
| Days after weaning | Age (days) | ||
| Efird et al36 | 21 | 7, 14, 21 | NP |
| Hampson37 | 21 | 0-5, 8, 11 | NP |
| Unweaned | NA | 21-26, 29, 32 | |
| Cera et al10 | 21 | 0, 3, 7, 14, 21, 28 | NP |
| 35 | 3, 7 | NP | |
| Unweaned | NA | 2, 10, 21, 28, 35 | |
| Kelly et al38 | 14 | 0, 3, 5, 7 | NP |
| Unweaned | NA | 14, 21, 22 | |
| Kelly et al39 | 14 | 5 | NP |
| Makkink et al33 | 28 | 0, 3, 6, 10 | NP |
| Pluske et al16 | 28 | 0, 5 | NP |
| Nunez et al40 | 5 | 0, 30 | NP |
| van Beers-Schreurs et al41 | 28 | 0, 4, 7 | NP |
| Tang et al42 | 12 | 0, 3, 22 | NP |
| Spreeuwenberg et al43 | 26 | 0, 1, 2, 4 | NP |
| Conour et al44 | 1 | 0, 3, 7 | NP |
| Gu et al45 | 17 | 0, 3, 7, 14 | NP |
| 21, 28, 35 | 0, 7, 14, 21 | NP | |
| Marion et al46 | 7 | 0, 3, 7, 14 | NP |
| Unweaned | NA | 7, 21 | |
| Vente-Spreeuwenberg et al47 | 27 | 0, 4, 7, 14 | NP |
| Skrzypek et al48 | 35 | 3 | NP |
| Unweaned | NA | 0, 3, 7, 21 | |
| Brown et al49 | 19 | 1, 3, 11, 25 | NP |
| Verdonk50 | 28 | 0, 1, 2, 4 | NP |
| Montagne et al51 | 21 | 0, 2, 5, 8, 15 | NP |
| Verdonk et al52 | 26 | 0, 4, 7 | NP |
| Moeser et al53 | 21 | 0, 4 | NP |
NP = not provided; NA = not applicable.
Table 2: Analyzed references, small intestinal segment (SI), parameters measured, and number of animals examined
| Reference | Small intestinal segment | Parameters measured | No. of pigs |
|---|---|---|---|
| Efird et al36 | Entire length of SI | SI length, SI weight | 69 |
| Hampson37 | 2%, 25%, 50%, 75%, and 98% along SI | SI length, villus height, crypt depth | 112 |
| Cera et al10 | From the intestinal midpoint | SI weight, villus height, microvillus height | 195 |
| Kelly et al38 | 10%, 30%, 50%, 70%, and 90% along SI | SI weight, villus height, crypt depth | 20 |
| Kelly et al39 | 10%, 30%, 50%, 70%, and 90% along SI | SI weight, villus height, crypt depth | 36 |
| Makkink et al33 | Duodenum, jejunum, ileum | SI weight, villus height, crypt depth | 70 |
| Pluske et al16 | 25%, 50%, and 75% along SI | Villus height, crypt depth | 18 |
| Nunez et al40 | Proximal, middle, and distal SI | SI weight, villus height, villus width, crypt depth | 19 |
| van Beers-Schreurs et al41 | 10%, 25%, 50%, 75%, and 95% along SI | SI weight, villus height, crypt depth | 54 |
| Tang et al42 | Proximal, middle, and distal SI | Villus height, crypt depth | 15 |
| Spreeuwenberg et al43 | Proximal, middle, and distal SI | SI weight, villus height, crypt depth | 66 |
| Conour et al44 | Jejunum, ileum | SI length, SI weight, villus height, villus width, crypt depth | 38 |
| Gu et al45 | Proximal duodenum, proximal jejunum, distal jejunum, and middle ileum | Villus height, crypt depth | 54 |
| Marion et al46 | Proximal, middle, and distal SI | SI length, SI weight, villus height, villus width, crypt depth | 56 |
| Vente-Spreeuwenberg et al47 | Proximal, middle, and distal SI | Villus height, crypt depth | 108 |
| Skrzypek et al48 | Duodenum, jejunum, ileum | Villus height | 10 |
| Brown et al49 | Duodenum, jejunum, ileum | Villus height, villus width, crypt depth | 88 |
| Verdonk50 | Three jejunal sites* | Villus height, crypt depth | 48 |
| Montagne et al51 | Proximal jejunum, distal ileum | SI length, SI weight, villus height, villus width, crypt depth, crypt width | 60 |
| Verdonk et al52 | Proximal and distal small intestine | Villus height, crypt depth | 48 |
| Moeser AJ et al53 | Mid-jejunum and distal ileum | Villus height, crypt depth | 36 |
* 0.5 m and 3.5 m distal to the ligament of Treitz and 0.5 m proximal to the ileocaecal ligament.
Influence of age on postnatal development and morphometry of the small intestine: intestinal weight and length
Weight and length have been used as indicators of the digestive and absorptive capacity of the small intestine.10,36,46,51 In the adult pig, the overall length of the small intestine is reported to be between 16 and 21 m, of which the duodenum ranges from 0.70 to 0.95 m, the jejunum from14 to 19 m, and the ileum from 0.70 to 1 m.23 In the growing pig, these parameters are constantly changing. In the first postnatal week, the small intestine of piglets increases up to 70% in total tissue weight, 115% in mucosal tissue weight, 24% in length, and 15% in diameter.46,54 However, Wijtten et al55 reported that the small intestine’s relative total tissue weight and the small intestinal mucosa’s relative weight (tissue weight per kg body weight) decreased over the period immediately after birth until about 21 days of age. After this time, the relative weight of the small intestine started to increase in suckling pigs, but not in piglets that had been weaned. These differing findings, notably of the mucosal development in the growing piglet, highlight the problem of comparing relative and absolute measurements, since they can lead to completely contradictory interpretations.
Weaning is a stressful process for piglets, with severe consequences on the intestinal tract. This was seen at 2 to 3 days post weaning when the relative small intestinal weight was about 80% of the preweaning weight as a consequence of low feed intake during this period.10,51 The small intestine’s relative weight recovers rapidly to preweaning level by 7 days post weaning and continues to rise to about 200% of the preweaning weight by 21 days post weaning. Wijtten et al55 suggest that this later rapid increase in small intestinal weight is probably related to consumption of solid food. Presumably, consumption of solid food stimulates increases in cell numbers and dimensions in the tunica muscularis, tela submucosa, and tunica mucosa due to development of full mechanical, degradative, absorptive, and immunological functions resulting in a dramatic rise in intestinal and mucosal weights.55
In contrast to intestinal weight, intestinal length has a more constant development (Figure 1). Efird et al36 showed that there was a significant linear effect (P < .05) between age and the relative length of the small intestine. According to Efird et al,36 relative small intestinal length tended to decrease with age (significant linear effect), with the difference being greatest at 42 days, when it reached statistical significance. This seems to be a natural developmental process and is in line with the data of Marion et al,46 who weaned piglets at 7 days of age and found that at 21 days of age, in unweaned piglets, as well as in those weaned, relative small intestinal length was 35% shorter (P < .001) than at 7 days of age.
Figure 1: Length of the small intestine (SI) per kg body weight (BW) (panel A) and length of the SI per kg BW post weaning expressed as a percentage of length of the SI per kg BW at weaning (panel B) in pigs receiving different diets (treatments). Each line represents a trial: the specific treatment in each trial is indicated via the combination of numbers and letters following the reference: Numbers immediately after references refer to age of weaning in days. The first letter refers to the physical form of the diet: d, dry; dm, dry milk powder; ds, dry soybean; f, fasted; i, total parenteral feeding; li, liquid; m, mash; PEN, 80% parenteral and 20% enteral feeding; u, unweaned. The second letter refers to the feed intake category: a, adequate; h, high; l, low. The dotted line (panel B) represents baseline value at weaning.
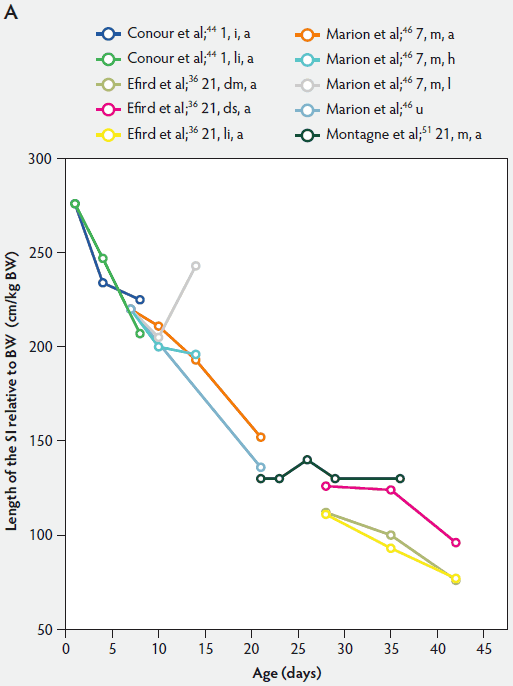
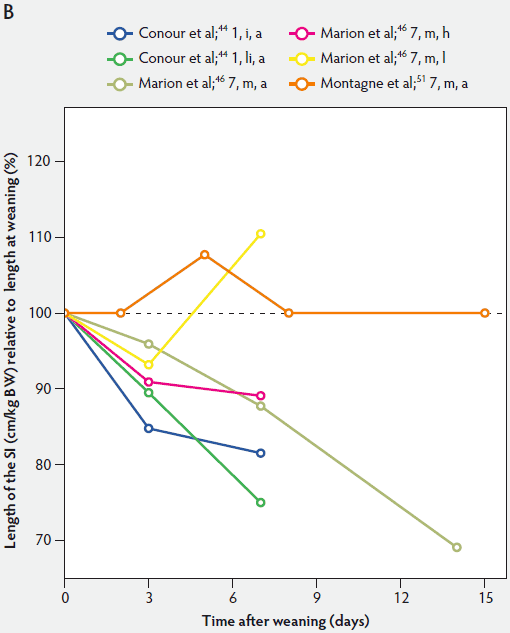
Relative intestinal length appears to be related to feed intake and feed composition after weaning (Figure 1).36,46 Marion et al46 suggested that the length of the small intestine was correlated with the metabolizable energy intake after weaning. Between 3 and 7 days post weaning, relative length of the small intestine remained unchanged in piglets with a high feed intake, whereas in those on a low feed intake, length of the small intestine increased approximately 118%.
Efird et al36 investigated the specific effects of various protein sources, ie, pigs weaned at 21 days of age to a 24% cow’s milk protein diet fed dry ad libitum, a 24% cow’s milk protein diet fed as a liquid hourly, and a 24% corn-soybean protein meal diet fed dry ad libitum. They found that pigs fed the dry corn-soybean protein diet tended to have a greater relative intestinal length than pigs fed either dry or liquid protein from cow’s milk (P < .05; Figure 1A). It can be assumed that the cow’s milk protein is more readily digested.16,56
In conclusion, in keeping with the data presented in Figure 1, the relative length of the small intestine in both weaned and suckling piglets decreased with age.
Influence of age on postnatal development and morphometry of the small intestine in unweaned piglets: villus height and width
Skrzypek et al48 reported that at birth, the surface of the mucosa in the small intestine is folded and covered by finger-shaped villi ranging in height from 289 µm in the duodenum to over 746 µm in the mid jejunum and 537 µm in the ileum. However, by day 38 (3 days post weaning) the heights of the villi were quite different, ranging from 350 µm in the duodenum to 314 µm in the mid jejunum and 282 µm in the ileum.48 Skrzypek et al48 used scanning electron microscopy to investigate the changes in villus shape following birth. They noted that at birth the villi were uniformly finger-like in shape. The density of villi was high throughout the entire small intestine. The villus surface was irregular and had many transverse furrows. Over time (examined at 3, 7, and 21 days of age), villus shape changed gradually to become leaf- or tongue-like, and villus forms became more irregular, with many becoming branched and divided. The surface of the villi became progressively smoother, and the transverse furrows were less numerous, narrower, and shallower, but still present at 21 days of age.
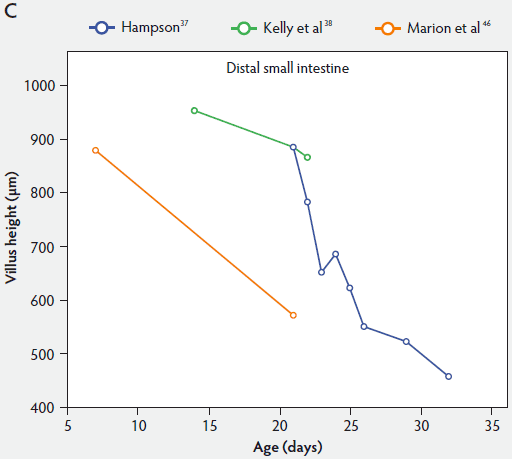
In general, after birth there is an initial elongation of the villi, then a gradual shortening over time, depending on age and location in the various intestinal segments. For example, comparing the small intestinal segments with each other, Marion et al46 reported that, associated with longer villi proximally, the reduction of villus height was more marked in the proximal than in the distal small intestine. They found that villus height in the proximal small intestine was 17% greater (P < .05) at 7 days of age than in the middle and distal small intestine. In contrast, at 21 days, location in the various intestinal segments had no effect on villus height. Gu et al45 reported that age of piglets had a significant effect on villus height in the duodenum, distal jejunum, and ileum, with the shortest villi occurring on day 29, while villus height in the proximal jejunum was unaffected by age of piglets.
From Figure 2, one can see that several histomorphometric studies report inconsistent changes in villus height in the proximal small intestine (Figure 2A), but villus heights in the mid and distal small intestine decreased (Figure 2B and 2C).
Figure 2: Absolute villus height in the small intestine of unweaned piglets (proximal, panel A; mid, panel B; and distal, panel C).
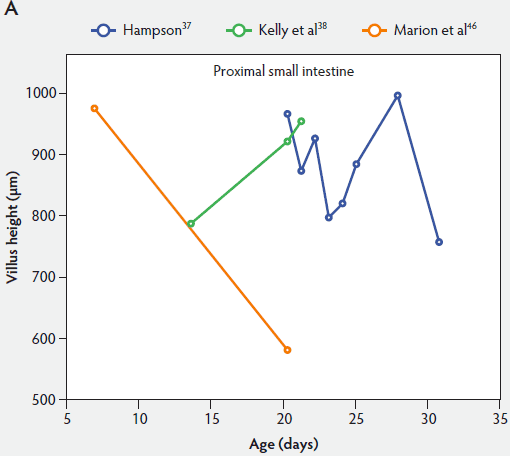
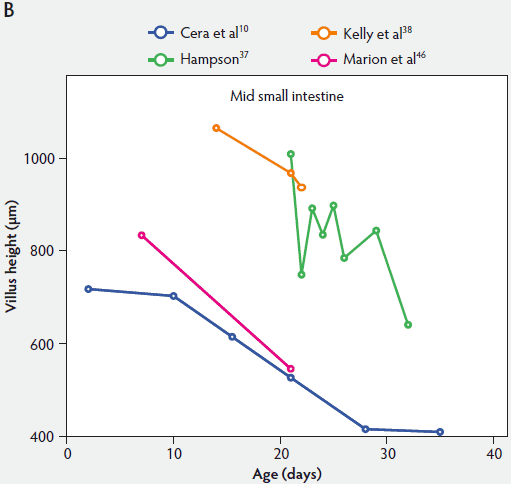
Marion et al46 found that at day 7, villus width in unweaned piglets was 127 µm, 128 µm, and 139 µm in the proximal, middle, and distal small intestine, respectively. Between 7 days and 21 days of age, villus width in the proximal and middle small intestine increased in unweaned piglets (115% and 108%, respectively), but decreased in the distal small intestine (87%). However, these data were not statistically significant.
Influence of age on postnatal development and morphometry of the small intestine in unweaned piglets: crypt development
In unweaned pigs, crypt depth is an indicator of the rate of crypt cell production, as well as an indicator of the functional maturity of villous enterocytes.37 An increase in crypt cell production is usually a response to a higher rate of cell loss on the villi and leads to greater crypt depth.10,37,57,58 A method to estimate crypt cell production is to determine the villus or crypt cell populations by counting epithelial cell nuclei.37,58,59 Another approach is to determine the mitotic index. Kenworthy58 accomplished this by counting the total number of crypt cells and the number of crypt cells in mitosis. The mitotic index is the number of cells in mitosis per 100 crypt cells.
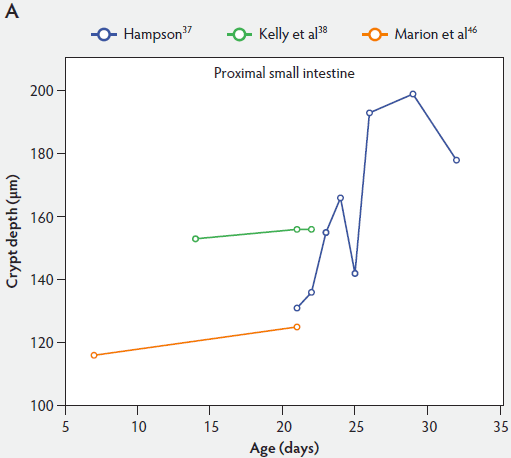
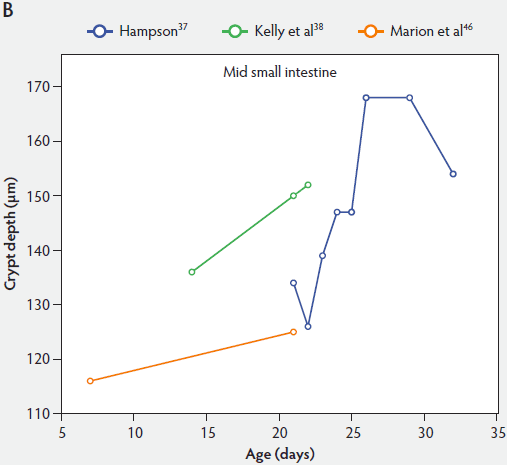
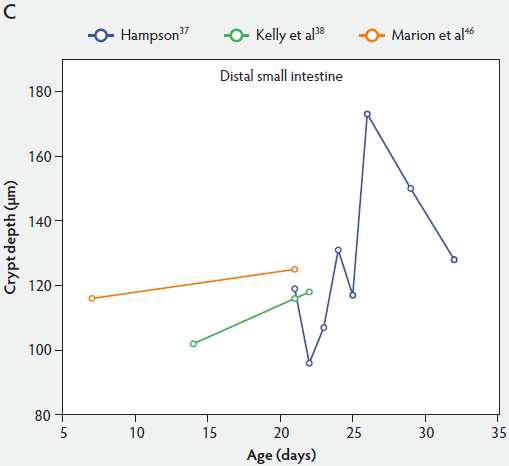
Crypt depth along the length of the small intestine increases with the age of the piglets (Figure 3). According to the studies of Hampson,37 crypt depth ranged between 131 µm and 199 µm in the proximal small intestine, between 126 and 168 µm in the mid small intestine, and between 96 and 173 µm in the distal small intestine. Kelly et al38 found a significant decrease (P < .05) in crypt depth from proximal to distal small intestine (Figure 3). In contrast, Marion et al46 reported that site along the small intestine had no significant effect on crypt depth in suckling piglets.
Figure 3: Absolute crypt depth in the small intestine of unweaned piglets (proximal, panel A; mid, panel B; and distal, panel C).
Villus:crypt ratio is the relationship of villus height to crypt depth. A low villus:crypt ratio may indicate villus atrophy associated with an increased rate of cell loss from the villus apex, concurrent with increased crypt cell production and hence greater crypt depth. A higher villus:crypt ratio suggests a more differentiated state of the gut.10,37,42,57
Because in unweaned piglets the villi shorten and the crypts deepen with age, the villus:crypt ratio becomes smaller. Hampson37 found that the villus:crypt ratio in suckling piglets gradually decreases by approximately 50% between 21 and 32 days of age (ratios 8:1 and 4:1, respectively). He suggested that the gradual reduction of the villus:crypt ratio may have resulted from a corresponding decline in the nutrient content of the sow’s milk.
As villus height, crypt depth, and villus:crypt ratio show remarkable developmental changes in the young piglet, their morphometric measurements should not be considered individually, but should be seen as an entity, forming an overall picture.
Influence of weaning on postnatal development of the small intestine: villus development
Weaning is a taxing process for piglets because it involves complex social changes that result in stress (eg, separation from the sow, moving, and mixing with unfamiliar piglets) as well as physiological and morphological changes associated with the changes in feed regimen, especially diet composition. A typical result of weaning is a decrease in feed intake and an increase in the number of intestinal infections in the days immediately after weaning.56,60-65 In Figure 4, a rapid reduction in villus height immediately after weaning is clearly demonstrated for all small intestinal segments. For instance, Hampson37 showed that villus height along the small intestine was reduced to approximately 75% of preweaning values within just 1 day post weaning. The maximal atrophy of small intestinal villi occurred between 3 and 5 days after weaning (Figure 4). At this time, villus height in the proximal and mid small intestine had dropped, in extreme cases, to less than 40% of the values found on the day of weaning. This is most likely due to stressors at weaning that lead to low feed intake and increased microbial challenges that occur after weaning. Immediately after weaning, the milieu of the small intestinal lumen is drastically altered because of the change from highly digestible sow’s milk to less readily digestible solid food, mainly of plant origin.66 The homeostatic control provided by milk bioactive substances, such as epidermal growth factor, polyamines, insulin, and insulin-like growth factors,57 is no longer present, and the intestinal tract has to rapidly adapt its motility and secretions to the altered conditions. After 5 days post weaning, villus height slowly increases, but does not reach the values found at weaning (Figure 4). This correlates with the decrease in villus height with age reported in unweaned piglets as part of the small intestine’s normal development (Figure 2).37,38,46
Figure 4: Villus height post weaning expressed as a percentage of villus height at weaning in the proximal, mid, and distal small intestine (panels A, B, and C, respectively) in pigs receiving different treatments as described in Figure 1. The dotted line represents baseline value at weaning. NP = not provided.
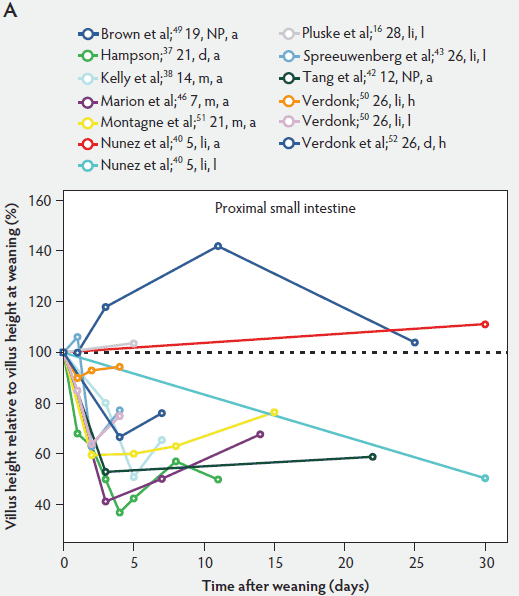
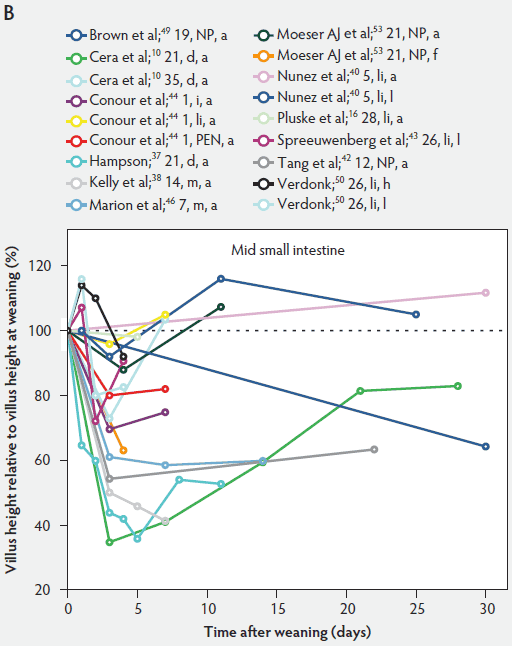
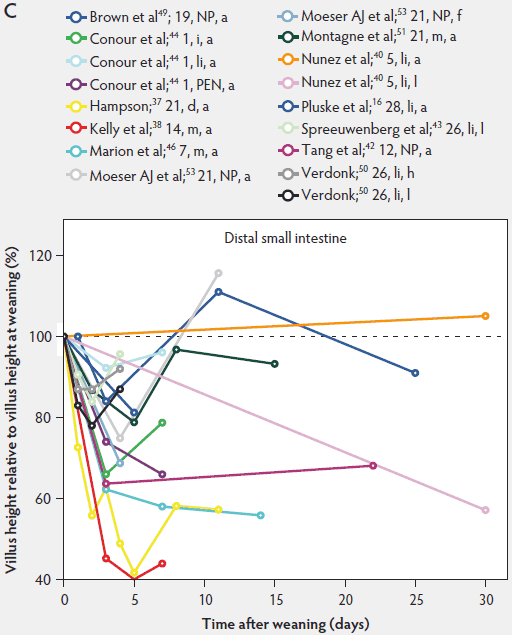
Villus atrophy after weaning is caused by either an increased rate of cell loss or a reduced rate of cell renewal.57 Several authors have reported that the villus atrophy that occurs immediately post weaning is more pronounced in the proximal small intestine than more distally.37,46,51 In addition, Marion et al46 provided evidence that recovery from villus atrophy by 14 days post weaning was more pronounced in the proximal than in other parts of the small intestine. They found that by 3 days post weaning, after the initial decrease, both villus height and width increased linearly (P < .05) from the proximal to the distal part of the small intestine. Contrarily, by 14 days post weaning, villus height decreased linearly (P < .05) from the proximal to the distal small intestine.
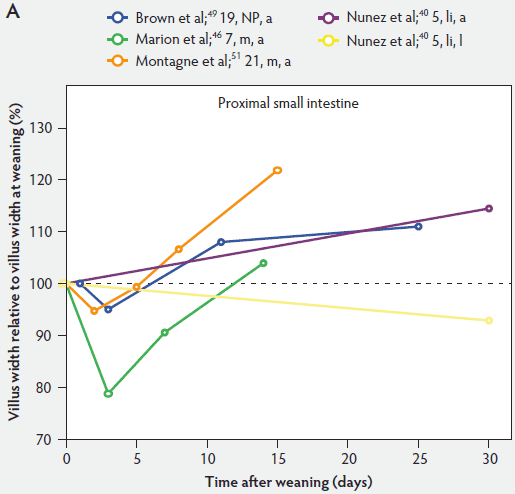
Figure 5 shows that immediately after weaning, villus width also decreases. In subsequent studies, Marion et al46 found that regardless of small intestinal site, villus height and width were reduced on day 3 post weaning by 41% and 15% of the values measured before weaning, respectively. Only the reduction in villus height was significant (P < .001). In contrast to the slow recovery time of villus height, recovery of villus width was rapid, as reported by Nunez et al,40 Brown et al,49 and Montagne et al51 (Figure 5). For example, Montagne et al51 noted that at weaning (21 days), villus width in the proximal jejunum was 151 µm, and after a marginal decrease, villus width reached 150 µm only 5 days later and was still increasing (P < .05) by day 8 (161 µm) and day 15 (184 µm) post weaning.
Figure 5: Villus width post weaning as a percentage of villus width at weaning in the proximal, mid, and distal small intestine (panels A, B, and C, respectively), in pigs receiving different treatments as described in Figure 1. The dotted line represents baseline value at weaning. NP = not provided.
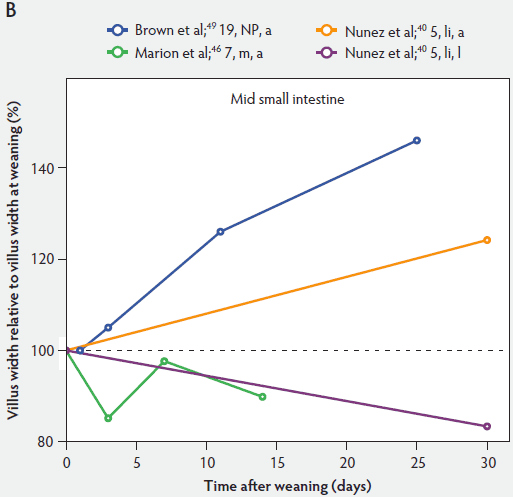
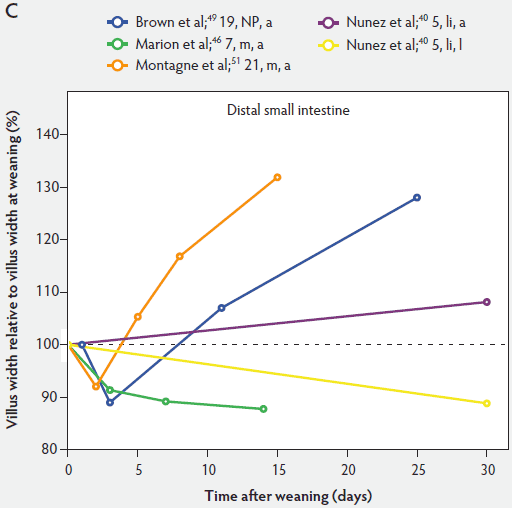
Influence of weaning on postnatal development of the small intestine: crypt development
In contrast to villus height, crypt depth shows no clear indications of change immediately after weaning (Figure 6). Several research groups report increases in crypt depth ranging from 10% to 50% in the first 4 to 5 days post weaning.10,37,38,42,52 Hampson37 has made a major contribution to this research area, reporting a steady increase in crypt depths from 21 until 32 days of age in both weaned and unweaned piglets. However, the increase was much greater and statistically significant (P < .01) in the weaned piglets, especially in the distal half of the small intestine. When he counted cell columns, he found that villus atrophy was associated with a reduction in the number of enterocytes lining the villus due to either an increased rate of cell loss from the villus apex or a reduction in the rate of cell production in the crypts. Furthermore, he suggested that the increase in crypt depth over the postweaning period was due to increased crypt cell production. The increased crypt cell production counteracted the rate of reduction in villus height and eventually equalled the rate of cell loss from the villi. Kelly et al38 reported that crypt depth was similar in sow-reared pigs at 14 and 22 days of age, but tended to increase in the weaned groups at all sites along the small intestine. This effect was significant (P < .05) at 7 days post weaning. On the other hand, Hall and Byrne67 determined the crypt cell production rate by counting the number of crypt epithelial cells arrested in metaphase and expressed it as cells produced per crypt per hour, calculated from a regression line of the accumulated metaphase-blocked cells against time. They found that a decrease in crypt cell production rate was associated with villus atrophy. Since crypt depth was reduced at 3 days after weaning, they suggested that villus shortening was caused by a lower rate of cell renewal.67 An initial transient decrease in crypt depth or only a marginal effect of weaning on crypt depth was found by Beers-Schreurs et al,41 Spreeuwenberg et al,43 Marion et al,46 Verdonk,50 and McCracken et al.63 A large decrease in duodenal, jejunal, and ileal crypt depth (P < .05) between days 1 and 3 after weaning was observed by Brown et al,49 and crypt depth did not return to the initial values found at weaning over the subsequent 25 days post weaning. Both Marion et al46 and Brown et al49 interpreted the rapid decline and slow recovery in crypt depth as reduced antigenic stimulation of the villus epithelium in their experiments. This is supported by the earlier finding of Miller et al,61 who reported shorter crypts in pigs weaned into an environment with lower antigenic load than in pigs weaned into an environment having a higher antigenic load, suggesting that rate of epithelial renewal may be dependent on the level of pathogen exposure. Studies by Hampson et al60 have shown that the weaning process is accompanied by significant increases in the numbers of pathogens such as hemolytic Escherichia coli and rotaviruses, as well as by a reduction in favorable lactobacilli in the small intestine of piglets. Invasion of the intestine by pathogens leads to epithelial cell damage.49,68 The intestine may respond to this by increasing its rate of epithelial renewal,68 thus impacting villus and crypt architecture.
Figure 6: Crypt depth after weaning as a percentage of crypt depth at weaning in the proximal, mid, and distal small intestine (panels A, B, and C, respectively), in pigs receiving different treatments as described in Figure 1. The dotted line represents baseline value at weaning. NP = not provided.
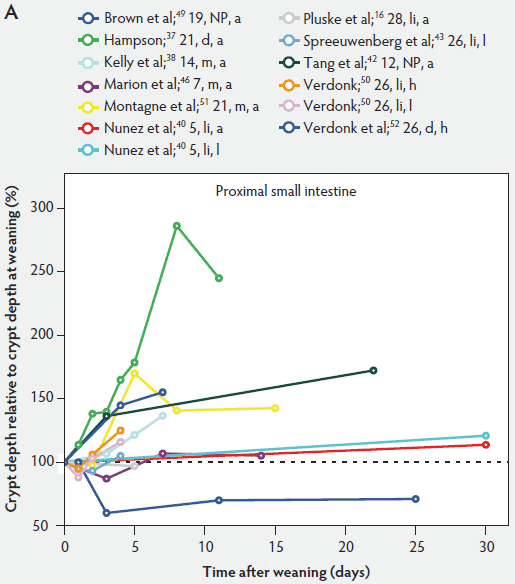
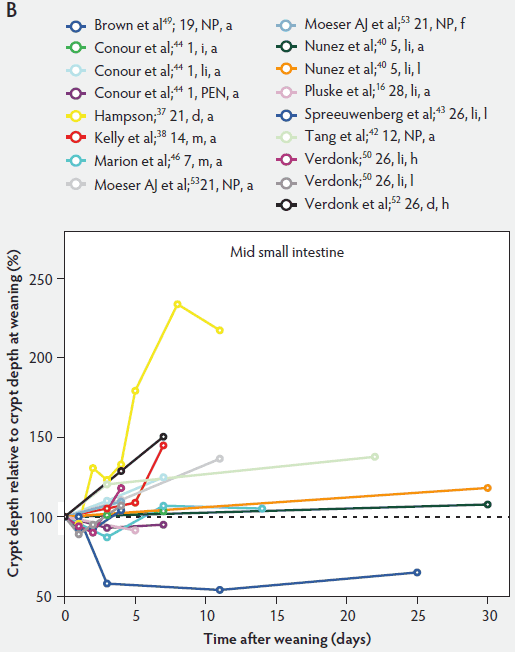
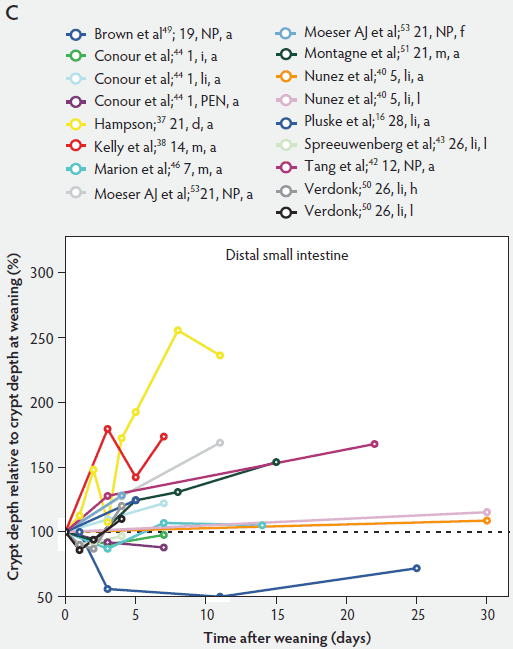
As a consequence of postweaning changes in villus height and crypt depth, the villus:crypt ratio is significantly lower in weaned piglets than in unweaned piglets. Villus atrophy seems to be associated with an increased rate of villus cell loss or decreased crypt cell production or both. These have the greatest effect on villus and crypt architecture.57 Kelly et al38 weaned pigs at 14 days of age and found that the villus:crypt ratio was significantly lower (P < .001) in weaned pigs than in sow-reared animals at 21 days of age (ratios being 2.44 and 6.62, respectively).
Hampson37 reported that the lowest values of the villus:crypt ratio (1.5 to 2.0 along the small intestine) occurred approximately 5 days after weaning and remained the same until at least 11 days post weaning. He suggested that, following this short period, there was a dynamic relationship between cell production and cell loss along the small intestine to establish an optimal ratio of villus height and crypt depth, linked to the animal’s diet. This required at least 5 weeks post weaning.
Evaluating the data sets from the literature indicates no clear signs of change of crypt depth, as demonstrated in Figure 6, which shows that in the first 5 days after weaning, crypt depth may increase or decrease depending on factors such as age at weaning, diet, and genetic background of animals. However, in most studies, crypt depth on day 4 to day 5 post weaning was greater than the initial values found at weaning. It increases steadily thereafter, ie, for 30 days as reported by Nunez et al.40
Effect of age at weaning
Natural weaning occurs around week 17. This was determined over a 3-year period from 37 lactations in 16 free-ranging domestic pigs.69 The normal process is for villi to undergo shortening before natural weaning.37 The management processes of weaning earlier than week 17 can have a significant influence on the morphology of the villus and crypt epithelial cells. Weaning stress can cause morphological changes, such as villus atrophy and crypt hypertrophy, that may last up to 12 days.37,58,70 It has been proven that the age of piglets at weaning influenced the period of recovery from villus atrophy.10,45 Cera et al10 reported a dramatic decline of jejunal villus height within 3 days in groups weaned at both 21 and 35 days. Thereafter, villus height subsequently increased (Figure 4B). Within 7 days of weaning at 35 days, the villi had changed their shape from finger-like to tongue-shaped, and their height had returned to preweaning levels. In contrast, in pigs weaned at 21 days, villus recovery was much slower. The longer villi, clearly evident by 14 days post weaning, did not have the characteristic long, narrow, finger-like morphological structure present during the preweaning period. Instead, they were elongate and flattened. Villi subsequently changed to have a tongue-shaped appearance by 28 days post weaning.
The morphological adaptation responses to weaning in the small intestine, characterized by transformation from a finger-like villus population to compact tongue-shaped villi, is associated with an increase in the luminal surface area. This process occurs more rapidly in piglets weaned after 28 days of age.10
Hall et al71 reported that late weaning at 56 days of age had little effect on the post weaning structure and function of piglet small intestine. Gu et al45 examined the influence of age at weaning on changes in intestinal development. They weaned piglets at four ages: 17, 21, 28, and 35 days, respectively, and their experiment ended on day 50. They found that the morphology of the small intestine changed more post weaning when weaning age was earlier. Villus height in the proximal jejunum of piglets weaned at day 17 decreased and was shortest on day 5 post weaning. It required 11 days post weaning for villus heights to return to normal, much longer than in piglets weaned at later times. When piglets were weaned at 28 days of age, proximal jejunal villus height did not decrease, and by 15 days post weaning it had increased to 111% of the weaning height. Gu et al45 also found that, in piglets weaned at 35 days, proximal jejunal villus height increased steadily over time, ie, until the experiment finished at 50 days of age. In contrast, Marion et al46 showed that early weaning at 7 days caused an unrecoverable villus atrophy in the small intestine. They found that villus height had decreased to 60% of preweaning values 3 days after weaning and remained at this level for up to 14 days (Figure 4). Thus, it appears that pigs weaned before 28 days of age do not completely recover from villus atrophy, whereas pigs weaned at 28 days or later recover readily.55 However, reduction of villus height in unweaned piglets of comparable age (Figure 2) supports the hypothesis of Wijtten et al55 that the severity of villus atrophy after weaning is similar for pigs weaned at 1 to 4 weeks of age, taking into account that a natural reduction of villus height also occurs in unweaned pigs up to 4 week of age.
Recent studies have shown that, in pigs weaned on day 21, villus height and villus:crypt ratio on days 3 and 7 post weaning were lower than at the preweaning stage.72,73 The shorter villi and deeper crypts confirm the deterioration of intestinal structure induced by weaning. Even so, villus height and crypt depth returned to their preweaning values by day 14 post weaning. However, recovery of intestinal barrier function was slower than recovery of intestinal mucosal morphology.72,73 Results of Hu et al72 indicated that early weaning induced sustained impairment in the intestinal barrier, as measured by decreased mRNA expression of tight-junction proteins and upregulated expression of proinflammatory cytokines.
Influence of feed regimens on postnatal development of the small intestine
Several studies have reported that major postweaning changes, notably villus atrophy, seen in small intestinal structure and function are a consequence of the low voluntary food intake occurring at this time. The effects of the psychological stressors of weaning, such as separation from the sow, moving, and mixing with others in the cohort, are less substantial .38,40,41,46,56
Likewise, total parenteral nutrition (TPN) causes villus atrophy in pigs.16,44,53,74 Park et al74 found that, on day 7, body weights were similar in unweaned piglets receiving TPN starting at day 1 post partum and those being fed orally. However, in TPN piglets, small intestinal weight, jejunal and ileal villus height, and surface area were all approximately 50% less.
In 2002, Conour et al44 assigned 38 one-day-old weanlings to three dietary treatment groups: 100% enterally fed (TEN), 100% parenterally fed (TPN), and 80% parenterally and 20% enterally fed (PEN) over 7 days. Body-weight gain was similar for all piglets throughout the experiment. Small intestinal weight (g per kg) was greater (P < .05) in TEN piglets than in TPN and PEN piglets both on day 3 and day 7. A trend of decreased villus height was seen in the jejunum and ileum of both parenteral groups, compared with TEN values across time (Figure 4B and 4C). On day 3, ileal crypt depth was lower (P < .05) in both parenteral groups than in the TEN groups (P < .05). At day 7, ileal and jejunal crypt depths were significantly lower in both parenteral groups than in the TEN groups (Figure 6B and 6C). Conour et al,44 using proliferating cell nuclear antigen (PCNA) techniques, reported that TPN in pigs decreased enterocyte proliferation and migration rates. They found that PCNA-positive epithelial cells were localized in the intestinal crypts, and their numbers were not affected by mode of nutrition during the first 3 days of treatment. By day 7, the number of PCNA-positive crypt cells was significantly elevated in the ilea of the TEN piglets relative to baseline (day 0). For TPN, a progression towards reduced epithelial proliferation was noted, in that the number of PCNA crypt cells was significantly reduced at day 3 and at day 7, relative to baseline (day 0).44
Moeser et al53 fasted piglets weaned at 21 days for 4 days and found that villus height in the jejunum was lower in the fasted group than in the control group on day 4 (P < .05). No differences between the two groups were observed in villus height in the ileum or crypt depth in the jejunum or ileum (Figure 4B and 4C, and Figure 6B and 6C). While there is only limited data for pigs, Goodlad and Wright,59 using 24-hour fasted mice, counted the number of crypt epithelial cells arrested in metaphase in animals killed at timed intervals. Comparing the fasted group to a time-matched control group, in the fasted group, there was a marked fall in crypt cell production rate along the entire length of the small intestine after 24 hours of fasting. This remained low until 9 hours after re-feeding. The crypt cell production rate of all sites then returned to control values.
It seems that the main effect of starvation and re-feeding is to increase and decrease the duration of the cell-cycle time.57 It is likely that luminal nutrition plays a major role in the integrity and maturation of the structure and function of the small intestine after weaning, and that the physical presence of food in the gastrointestinal tract per se is necessary for structural and functional maintenance of the intestinal mucosa. This suggests that the rapid decrease in mucosal weight and villus height in piglets immediately after weaning is most likely due to starvation or low feed intake at this time.
Recent studies showed that feed supplementation of early-weaned pigs with zinc oxide (ZnO)75,76 or diosmectite-ZnO composite (DS-ZnO)77 can alleviate weaning-related intestinal disorders. Their results show that supplemental ZnO or DS-ZnO improved daily gain and feed intake and improved intestinal morphology, as indicated by increased villus height, villus height:crypt depth ratio, and intestinal barrier function. This emphasizes the importance of an optimal nutrient supply in this period of life for optimal small intestinal development
Conclusion
The morphology of the small intestine of the pig is subject to dynamic changes in the postnatal period. Despite numerous publications on morphological characteristics of the gastrointestinal tract, there is limited comparability of different studies because of substantial methodological differences. This review underlines that villus height and crypt depth show remarkably interdependent developmental changes. Therefore, their morphometric measurement cannot be considered individually. Instead, the villus:crypt ratio should be evaluated.
Age drives the maturation process, but exogenous factors, especially the change of diet at weaning, are important modulators. A critical evaluation of the available data shows that weaning piglets under the age of 28 days has a major effect on the structure of the intestinal epithelium, especially that of the villi and crypts. Thus, a morphologically mature and stable gastrointestinal tract is age dependent, and weaning piglets at 28 days or later should allow a safe transition from milk to solid feed.
Implications
• Villus:crypt ratio, rather than villus height or crypt depth, should be considered as a single measure for evaluation of small intestine maturity and health in swine.
• Additional studies or meta-analyses may be necessary to determine an optimal range of villus:crypt ratio for morphological maturity and health of the small intestine in piglets.
• A morphologically mature and stable gastrointestinal tract is age dependent.
• Independent of the starter diet, weaning under the age of 28 days has a major effect on the structure of the intestinal epithelium, while later weaning is likely to maintain a favourable mucosal structure.
Acknowledgements
We particularly wish to thank Dr Kathrin Dietze and Dr Abdelnasser Al Awad for help with manuscript preparation and acknowledge the kind editorial assistance by Ms Wiebke Gentner.
Conflict of interest
None reported.
Disclaimer
Scientific manuscripts published in the Journal of Swine Health and Production are peer reviewed. However, information on medications, feed, and management techniques may be specific to the research or commercial situation presented in the manuscript. It is the responsibility of the reader to use information responsibly and in accordance with the rules and regulations governing research or the practice of veterinary medicine in their country or regions.
References
1. Aigner B, Renner S, Kessler B, Klymiuk N, Kurome M, Wünsch A, Wolf E. Transgenic pigs as models for translational biomedical research. J Mol Med. 2010;88:653–664.
2. Zhang Q, Widmer G, Tzipori S. A pig model of the human gastrointestinal tract. Gut Microbes. 2013;4:193–200.
3. Llanos JC, Bakonyi Neto A, Lerco MM, Clark RM, Polachini do Valle A, Sousa MM. Induction of short gut syndrome and transplantation in a porcine model. Transplant Proc. 2006;38:1855–1856.
4. Sodhi C, Richardson W, Gribar S, Hackam DJ. The development of animal models for the study of necrotizing enterocolitis. Dis Model Mech. 2008;1:94–98.
5. Rothkötter H, Pabst R, Bailey M. Lymphocyte migration in the intestinal mucosa: entry, transit and emigration of lymphoid cells and the influence of antigen. Vet Immunol Immunopathol. 1999;72:157–165.
6. Williams BA, Verstegen MW, Tamminga S. Fermentation in the large intestine of single-stomached animals and its relationship to animal health. Nutr Res Rev. 2001;14:207–228.
7. Lallès JP, Boudry G, Favier C, Le Floc’h N, Luron I, Montagne L, Oswald IP, Pié S, Piel C, Sève B. Gut function and dysfunction in young pigs: physiology. Anim Res. 2004;53:301–316.
8. Rosenthal R, Günzel D, Finger C, Krug SM, Richter JF, Schulzke JD, Fromm M, Amasheh S. The effect of chitosan on transcellular and paracellular mechanisms in the intestinal epithelial barrier. Biomaterials. 2012;33:2791–2800.
9. Rieger J, Twardziok S, Huenigen H, Hirschberg RM, Plendl J. Porcine intestinal mast cells. Evaluation of different fixatives for histochemical staining techniques considering tissue shrinkage. Europ J Histochem. 2013. doi.10.4081/ejh.2013.e21.
10. Cera KR, Mahan DC, Cross RF, Reinhart GA, Whitmoyer RE. Effect of age, weaning and postweaning diet on small intestinal growth and jejunal morphology in young swine. J Anim Sci. 1988;66:574–584.
11. Banks WJ. Applied Veterinary Histology. 3rd ed. Oxford: Elsevier LTD; 1993:350–354.
12. Smollich A, Michel G. Mikroskopische Anatomie der Haustiere [Microscopic Anatomy of Domestic Animals (in German)]. Stuttgart, Germany: Gustav Fischer Verlag; 1992:125–141.
*13. Kitt SJ, Miller PS, Lewis A. Factors affecting small intestine development in weanling pigs. Nebraska Swine Reports. 2001;99.
14. Tsukahara T, Kishino E, Inoue R, Nakanishi N, Nakayama K, Ito T, Ushida K. Correlation between villous height and the disaccharidase activity in the small intestine of piglets from nursing to growing. Anim Sci J. 2013;84:54–59.
15. Zijlstra RT, Whang KY, Easter RA, Odle J. Effect of feeding a milk replacer to early-weaned pigs on growth, body composition, and small intestinal morphology, compared with suckled littermates. J Anim Sci. 1996;74:2948–2959.
16. Pluske JR, Thompson MJ, Atwood CS, Bird PH, Williams IH, Hartmann PE. Maintenance of villus height and crypt depth, and enhancement of disaccharide digestion and monosaccharide absorption, in piglets fed on cows’ whole milk after weaning. Br J Nutr. 1996;76:409–422.
17. Chwen LT, Foo HL, Thanh NT, Choe DW. Growth performance, plasma fatty acids, villous height and crypt depth of preweaning piglets fed with medium chain triacylglycerol. Asian-Australasian J Anim Sci. 2013;26:700–704.
18. Kuitunen P, Kosnai I, Savilahti E. Morphometric study of the jejunal mucosa in various childhood enteropathies with special reference to intraepithelial lymphocytes. J Pediatr Gastroenterol Nutr. 1982;1:525–531.
19. Gulbinowicz M, Berdel B, Wojcik S, Dziewiatkowski J, Oikarinen S, Mutanen M, Kosma VM, Mykkanen H, Morys J. Morphometric analysis of the small intestine in wild type mice C57BL/6L – a developmental study. Folia Morphol. 2004;63:423–430.
20. Haboubi NY, Lee GS, Montgomery RD. Duodenal mucosal morphometry of elderly patients with small intestinal bacterial overgrowth: response to antibiotic treatment. Age Ageing. 1991;20:29–32.
21. Hampson DJ. Postweaning Escherichia coli diarrhoea in pigs. In: Gyles CL, ed. Escherichia coli in Domestic Animals and Humans. Wallingford, UK: CAB International; 1994:171–191.
22. Petri WA, Miller M, Binder HJ, Levine MM, Dillingham R, Guerrant RL. Enteric infections, diarrhea, and their impact on function and development. J Clin Invest. 2008;118:1277–1290.
23. Schwarze E. Kompendium der Veterinär-Anatomie: Eingeweidesystem [Compendium of Veterinary Anatomy: The Visceral System (in German)]. Jena, Germany: Gustav Fischer Verlag; 1962:83–107.
24. Cranwell PD. Development of the neonatal gut and enzyme systems. In: Varley MA, ed. The Neonatal Pig: Development and Survival. Wallingford, UK: CAB International; 1995:99–154.
25. Yen JT. Anatomy of the digestive system and nutritional physiology. In: Lewis AJ, Southern LL, eds. Swine Nutrition. 2nd ed. Boca Raton, Florida: CRC Press; 2001:31–63.
26. Nickel R, Schummer A, Seiferle E, Seck WO, The Viscera of Domestic Mammals. 2nd rev ed. Berlin GmbH: Springer-Verlag; 1979:139–145.
27. Binns R, Pabst R. Lymphoid tissue structure and lymphocyte trafficking in the pig. Vet Immunol Immunopathol. 1994;43:79–87.
28. Barman N, Bianchi A, Zwart R, Pabst R, Rothkötter H. Jejunal and ileal Peyer’s patches in pigs differ in their postnatal development. Anat Embryol (Berl). 1996;195:41–50.
29. Charerntantanakul W, Roth JA. Biology of porcine T lymphocytes. Anim Health Res Rev. 2006;7:81–96.
30. Van Ginneken C, Van Meir F, Weyns A. Stereologic characteristics of pig small intestine during normal development. Dig Dis Sci. 2002;47:868–878.
31. Lackovic G, Tomaskovic M, Njari B, Vrbanac I, Krsnik B, Rode B, Valpotic I. Distribution of immune cells expressing CD3a, CD21 and S-100 protein markers in the porcine gut-associated lymphoid tissues. Eur J Histochem. 1999;43:39–46.
32. Helm RM, Golden C, McMahon M, Thampi P, Badger TM, Nagarajan S. Diet regulates the development of gut-associated lymphoid tissue in neonatal piglets. Neonatology. 2007;91:248–255.
33. Makkink CA, Negulescu GP, Qin G, Verstegen MW. Effect of dietary protein source on feed intake, growth, pancreatic enzyme activities and jejunal morphology in newly-weaned piglets. Br J Nutr. 1994;72:353–368.
34. Greeson JK, Jan D. Small bowel mucosal diseases. In: Riddell R, Jain D, Lewin KJ, eds. 2nd ed. Weinstein and Riddell’s Gastrointestinal Pathology and its Clinical Implications. Philadelphia, Pennsylvania: Lippincott, Williams and Wilkins; 2014:929–983.
35. Pekas JC. Morphometry of the intestine of the pig. I. A method for complete circumsection analysis. Dig Dis Sci. 1986;31:79–89.
36. Efird RC, Armstrong WD, Herman DL. The development of digestive capacity in young pigs: effects of weaning regimen and dietary treatment. J Anim Sci. 1982;55:1370–1379.
37. Hampson DJ. Alterations in piglet small intestinal structure at weaning. Res Vet Sci. 1986;40:32–40.
38. Kelly D, Smyth JA, McCracken KJ. Digestive development of the early-weaned pig. 1. Effect of continuous nutrient supply on the development of the digestive tract and on changes in digestive enzyme activity during the first week post-weaning. Br J Nutr. 1991;65:169–180.
39. Kelly D, Smyth JA, McCracken KJ. Digestive development of the early-weaned pig. 2. Effect of level of food intake on digestive enzyme activity during the immediate post-weaning period. Br J Nutr. 1991;65:181–188.
40. Nunez MC, Bueno JD, Ayudarte MV, Almendros A, Rios A, Suarez MD, Gil A. Dietary restriction induces biochemical and morphometric changes in the small intestine of nursing piglets. J Nutr. 1996;126:933–944.
41. van Beers-Schreurs HM, Nabuurs MJ, Vellenga L, Kalsbeek-van der Valk HJ, Wensing T, Breukink HJ. Weaning and the weanling diet influence the villous height and crypt depth in the small intestine of pigs and alter the concentrations of short-chain fatty acids in the large intestine and blood. J Nutr. 1998;128:947–953.
42. Tang M, Laarveld B, Van Kessel AG, Hamilton DL, Estrada A, Patience JF. Effect of segregated early weaning on postweaning small intestinal development in pigs. J Anim Sci. 1999;77:3191–3200.
43. Spreeuwenberg MA, Verdonk JM, Gaskins HR, Verstegen MW. Small intestine epithelial barrier function is compromised in pigs with low feed intake at weaning. J Nutr. 2001;131:1520–1527.
44. Conour JE, Ganessunker D, Tappenden KA, Donovan SM, Gaskins HR. Acidomucin goblet cell expansion induced by parenteral nutrition in the small intestine of piglets. Am J Physiol Gastrointest Liver Physiol. 2002;283:G1185–G1196.
45. Gu X, Li D, She R. Effect of weaning on small intestinal structure and function in the piglet. Arch Tierernahr. 2002;56:275–286.
46. Marion J, Biernat M, Thomas F, Savary G, Le Breton Y, Zabielski R, Le Huërou-Luron I, Le Dividich J. Small intestine growth and morphometry in piglets weaned at 7 days of age. Effects of level of energy intake. Reprod Nutr Dev. 2002;42:339–354.
47. Vente-Spreeuwenberg M, Verdonk J, Bakker G, Beynen A, Verstegen M. Effect of dietary protein source on feed intake and small intestinal morphology in newly weaned piglets. Livest Prod Sci. 2004;86:169–177.
48. Skrzypek T, Piedra JV, Skrzypek H, Wolinski J, Kazimierczak W, Szymanczyk S, Pawlowska M, Zabielsk R. Light and scanning electron microscopy evaluation of the postnatal small intestinal mucosa development in pigs. J Physiol Pharmacol. 2005;56:71–87.
49. Brown DC, Maxwell CV, Erf GF, Davis ME, Singh S, Johnson ZB. The influence of different management systems and age on intestinal morphology, immune cell numbers and mucin production from goblet cells in post-weaning pigs. Vet Immunol Immunopathol. 2006;111:187–198.
50. Verdonk JMAS. Nutritional strategy affects gut wall integrity in weaned piglets. PhD thesis, Wageningen Universiteit, Wageningen, the Netherlands; 2006.
51. Montagne L, Boudry G, Favier C, Le Huërou-Luron I, Lalles JP, Seve B. Main intestinal markers associated with the changes in gut architecture and function in piglets after weaning. Br J Nutr. 2007; 97:45–57.
52. Verdonk J, Bruininx E, Van Der Meulen J, Verstegen M. Post-weaning feed intake level modulates gut morphology but not gut permeability in weaned piglets. Livest Sci. 2007;108:146–149.
53. Moeser AJ, Borst LB, Overman BL, Pittman JS. Defects in small intestinal epithelial barrier function and morphology associated with peri-weaning failure to thrive syndrome (PFTS) in swine. Res Vet Sci. 2012;93:975–982.
54. Xu R, Mellor D, Tungthanathanich P, Birtles M, Reynolds G, Simpson H. Growth and morphological changes in the small and the large intestine in piglets during the first three days after birth. J Dev Physiol. 1992;18:161–172.
55. Wijtten P, Langhout D, Verstegen M. Small intestine development in chicks after hatch and in pigs around the time of weaning and its relation with nutrition: A review. Acta Agr Scand Sec A-An Sci. 2012;62:1–12.
56. Pluske J, Williams I, Aherne F. Villous height and crypt depth in piglets in response to increases in the intake of cows’ milk after weaning. Anim Sci. 1996;62:145–158.
57. Pluske JR, Hampson DJ, Williams IH. Factors influencing the structure and function of the small intestine in the weaned pig: a review. Livest Prod Sci. 1997;51:215–236.
58. Kenworthy R. Observations on the effects of weaning in the young pig. Clinical and histopathological studies of intestinal function and morphology. Res Vet Sci. 1976;21:69–75.
59. Goodlad R, Wright N. The effects of starvation and refeeding on intestinal cell proliferation in the mouse. Virchows Arch [Cell Pathol]. 1984;45:63–73.
60. Hampson D, Hinton M, Kidder D. Coliform numbers in the stomach and small intestine of healthy pigs following weaning at three weeks of age. J Comp Pathol. 1985;95:353–362.
61. Miller B, James P, Smith M, Bourne F. Effect of weaning on the capacity of pig intestinal villi to digest and absorb nutrients. J Agr Sci. 1986;107:579–589.
62. McCracken BA, Gaskins HR, Ruwe-Kaiser PJ, Klasing KC, Jewell DE. Diet-dependent and diet-independent metabolic responses underlie growth stasis of pigs at weaning. J Nutr. 1995;125:2838–2845.
63. McCracken BA, Spurlock ME, Roos MA, Zuckermann FA, Gaskins HR. Weaning anorexia may contribute to local inflammation in the piglet small intestine. J Nutr. 1999;129:613–619.
64. Pluske J, Williams I, Aherne F. Maintenance of villous height and crypt depth in piglets by providing continuous nutrition after weaning. Anim Sci. 1996;62:131–144.
65. Hampson DJ, Hopwood DE. Interactions between the intestinal microflora, diet and diarrhoea, and their influences on piglet health in the immediate post-weaning period. In: Pluske JR, Le Dividich J, Verstegen MWA, eds. Weaning the Pig: Concepts and Consequences. Wageningen, the Netherlands: Wageningen Academic Publishers; 2003:199–218.
66. Zabielski R, Barej W, Leniewska V, Pierzynowski SG. Pancreas and upper gut dysfunctions around weaning in pigs and calves. In: Wensing TH, ed. Production Diseases in Farm Animals. Wageningen, the Netherlands: Wageningen Academic Publishers; 1999:134–144.
67. Hall GA, Byrne TF. Effects of age and diet on small intestinal structure and function in gnotobiotic piglets. Res Vet Sci. 1989;47:387–392.
68. Gaskins H. Immunological aspects of host/microbiota interactions at the intestinal epithelium. In: Mackie RI, White BA, Isaacson RE, eds. Gastrointestinal Microbiology. New York, New York: International Thomson Publishing; 1997:537–587.
69. Jensen P, Recén B. When to wean – observations from free-ranging domestic pigs. Appl Anim Behav Sci. 1989;23:49–60.
70. Li DF, Nelssen JL, Reddy PG, Blecha F, Klemm RD, Giesting DW, Hancock JD, Allee GL, Goodband RD. Measuring suitability of soybean products for early-weaned pigs with immunological criteria. J Anim Sci. 1991;69:3299–3307.
71. Hall G, Parsons K, Waxler G, Bunch K, Batt R. Effects of dietary change and rotavirus infection on small intestinal structure and function in gnotobiotic piglets. Res Vet Sci. 1989;47:219–224.
72. Hu CH, Xiao K, Luan ZS, Song J. Early weaning increases intestinal permeability, alters expression of cytokine and tight junction proteins, and activates mitogen-activated protein kinases in pigs. J Anim Sci. 2013;9:1094–1101.
73. Xiao K, Song ZH, Jiao LF, Ke YL, Hu CH. Developmental changes of TGF-β1 and Smad signaling pathway in intestinal adaption of weaned pigs. PloS one. 2014;9:e104589.
74. Park YK, Monaco MM, Donovan SM. Delivery of total parenteral nutrition (TPN) via umbilical catheterization: development of a piglet model to investigate therapies to improve gastrointestinal structure and enzyme activity during TPN. Biol Neonate. 1998;73:295–305.
75. Hu CH, Song ZH, Xiao K, Song J, Jiao LF, Ke YL. Zinc oxide influences intestinal integrity, the expressions of genes associated with inflammation and TLR4-myeloid differentiation factor 88 signaling pathways in weanling pigs. Innate Immunity. 2013;20:478–486. doi:10.1177/1753425913499947.
76. Song ZH, Xiao K, Ke YL, Jiao LF, Hu CH. Zinc oxide influences mitogen-activated protein kinase and TGF-β1 signaling pathways, and enhances intestinal barrier integrity in weaned pigs. Innate Immunity. 2014:1–8. doi:10.1177/1753425914536450.
77. Hu C, Song J, Li Y, Luan Z, Zhu K. Diosmectite-zinc oxide composite improves intestinal barrier function, modulates expression of pro-inflammatory cytokines and tight junction protein in early weaned pigs. Br J Nutr. 2013;110:681–688.
*Non-refereed reference.