| Brief communication | Peer reviewed |
Cite as: Seo HW, Lee J, Park C, et al. Comparison of commercial one-dose and two-dose baculovirus-expressed porcine circovirus type 2 subunit vaccines. J Swine Health Prod. 2014;22(6):291–295.
Also available as a PDF.
SummaryOne- and two-dose recombinant porcine circovirus type 2 (PCV2) vaccines did not differ significantly in terms of immunological testing (neutralizing antibody titers, number of interferon-γ-secreting cells), virological testing (number of PCV2 genomic copies per mL serum), and pathological evidence of infection (lymphoid lesions scores and PCV2 antigen-positive cells). | ResumenLas vacunas de una y dos dosis del circovirus porcino tipo 2 recombinante (PCV2 por sus siglas en inglés) no difirieron significativamente en términos de pruebas inmunológicas (títulos de anticuerpos neutralizantes, número de células secretoras de interferón-γ), pruebas virológicas (número de copias genómicas del PCV2 por mL de suero), y evidencia patológica de infección (puntajes de lesiones linfoides y células positivas al antígeno de PCV2). | ResuméDes vaccins recombinants une- et deux-doses contre le circovirus porcin de type 2 (PCV2) ne différaient pas significativement en terme de réponse immunologique (titres d’anticorps neutralisant, nombre de cellules secrétant de l’interféron-γ), d’analyses virologiques (nombre de copies du génome de PCV2 par mL de sérum), et d’évidence pathologique de l’infection (pointage des lésions lymphoïdes et cellules positives pour l’antigène PCV2). |
Keywords: swine, porcine circovirus type 2, porcine circovirus type 2 vaccine, PCV2
Search the AASV web site
for pages with similar keywords.
Received: January 1, 2014
Accepted: May 20, 2014
Porcine circovirus type 2 (PCV2) is a small, non-enveloped, single-stranded DNA virus in the genus Circovirus within the family Circoviridae.1 It has been incriminated as a major causative agent of postweaning multisystemic wasting syndrome (PMWS), which is known as PCV2-associated diseases (PCVAD);2,3 PCVAD is considered to be an economically important global issue. Most Korean swine farms (95.5%) use a PCV2 vaccine for control of PCVAD because the vaccine is highly efficacious.4
Currently, five commercial PCV2 vaccines are available in Korea.5 These include three subunit vaccines that are based on the capsid protein expressed in a baculovirus system, and two inactivated vaccines based on PCV2 or on chimeric PCV1–2.4,6 The baculovirus-expressed subunit vaccines, requiring one- or two-dose administration, are most commonly used in Korean herds.5 Among them, Porcilis PCV (one dose; MSD Animal Health, Summit, New Jersey) and Circumvent PCV (two doses; MSD Animal Health) are different preparations of the same core antigen.4 Both vaccines are available in Korea, but in other countries, only one or the other is available.4 For example, Circumvent PCV alone is available in North America, while Porcilis PCV alone is available in Europe.4
Under field conditions, some swine producers prefer a one-dose PCV2 vaccine because of less labor and stress to animals, while others prefer a two-dose vaccine that generates an immunological booster response. To the knowledge of the authors, there are no reports comparing commercial one- and two-dose recombinant PCV2 vaccines having the same core PCV2 antigen. Hence, the objective of this study was to compare the immune response, virus levels, and lesions in pigs vaccinated with one- and two-dose PCV2 subunit vaccines.
Materials and methods
All animal protocols were approved by the Seoul National University Institutional Animal Care and Use Committee.
Thirty colostrum-fed, cross-bred, conventional piglets were purchased at 14 days of age from a commercial farm. At arrival, all piglets were negative for porcine reproductive and respiratory syndrome virus (PRRSV) and Mycoplasma hyopneumoniae when tested with the PRRS X3 Ab test (Idexx Laboratories Inc, Westbrook, Maine) and the Idexx M. hyo. Ab test (Idexx Laboratories, Inc), respectively. All piglets were negative for PCV2 viremia when tested by real-time polymerase chain reaction (PCR), and all were seronegative against PCV2 when blood samples collected when the pigs were 3 weeks old and again on the day of challenge were tested using a commercial enzyme-linked immunosorbent assay (ELISA; SERELISA PCV2 Ab Mono Blocking, Synbiotics, Lyon, France).
A total of 30 piglets were randomly assigned to six groups (five pigs per group) using the random number generation function in Excel (Microsoft Corporation, Redmond, Washington) (Table 1). Sample size was calculated assuming a 90% power (1- β = .90) of detecting a difference at the 5% level of significance (α = .05).7 The treatment timeline is shown in Table 1. In Group 1 and Group 2 pigs, one 2.0-mL dose of Porcilis PCV was administered intramuscularly (IM) at 3 weeks of age in the right side of the neck. In Group 3 and Group 4 pigs, two 2-mL doses of Circumvent PCV were administered IM at 3 and 6 weeks of age, one on each side of the neck. At 28 days post vaccination, each pig in Group 1 (49 days of age), Group 3 (70 days of age), and Group 5 (70 days of age) was inoculated intra-nasally with 2 mL of PCV2b (strain SNUVR000463; 5th passage; 1.0 × 105 median tissue culture infectious doses per mL). Group 5 pigs served as the positive control group (challenged but not vaccinated). Group 6 pigs were unchallenged and unvaccinated (no product administration) and served as the negative control group. Groups were housed in separate rooms within the facility. Blood samples were collected at study days -28, 0 (day of challenge), 7, 14, 21, and 42.
Table 1: Means (standard deviation) of lymphoid lesion score and numbers of lymphoid porcine circovirus type 2 (PCV2) antigen-positive cells in pigs vaccinated with a one-dose or a two-dose PCV2 vaccine and challenged with PCV2*
| Group | Vaccine | Vaccination | Challenge | Lymphoid lesion score† | No. of PCV2+ lymphoid cells‡ | ||
|---|---|---|---|---|---|---|---|
| 3 weeks | 6 weeks | 7 weeks | 10 weeks | ||||
| 1 | Porcilis PCV | 2 mL | None | Yes | No | 0.8 (0.44)a | 7.6 (4.22)a |
| 2 | Porcilis PCV | 2 mL | None | No | No | 0 (0)b | 0 (0)b |
| 3 | Circumvent PCV | 2 mL | 2 mL | No | Yes | 0.6 (0.45)a | 6.0 (5.29)a |
| 4 | Circumvent PCV | 2 mL | 2 mL | No | No | 0 (0)b | 0 (0)b |
| 5 | None | NA | NA | No | Yes | 1.2 (0.54)a | 18.0 (6.82)c |
| 6 | None | NA | NA | No | No | 0 (0)b | 0 (0)b |
* Group 1 and 2 pigs were vaccinated with a one-dose PCV2 vaccine (Porcilis PCV; MSD Animal Health, Summit, New Jersey) at 3 weeks of age, and Group 3 and 4 pigs were vaccinated with a two-dose PCV2 vaccine (Circumvent PCV; MSD Animal Health) at 3 and 6 weeks of age. Group 1 pigs were inoculated with PCV2b strain SNUVR000463 at 7 weeks of age and Group 3 and Group 5 pigs were inoculated with the same PCV2b strain at 10 weeks of age. For each group, n = 5 pigs.
† Pigs in all six groups were euthanized for necropsy at 42 days post challenge. Superficial inguinal lymph nodes were collected for histopathology and immunohistochemistry. Lymphoid lesion score ranged from 0 to 3: 0 = no lymphoid depletion or granulomatous replacement; 1 = mild lymphoid depletion; 2 = moderate lymphoid depletion; and 3 = severe lymphoid depletion and histiocytic replacement. Scores were compared among groups using Fisher’s exact test.
‡ The number of lymphoid PCV2 antigen-positive cells per unit area (0.25 mm2) was counted using an NIH Image J 1.45s program (http://imagej.nih.gov/ij/download.html). Numbers of positive cells were compared among groups using Tukey’s test.
abc Within a column, different letters indicate a significant difference among groups (P < .05).
PCV2+ = PCV2 antigen-positive; NA = not applicable.
Extraction of DNA from serum samples was performed using the QIAamp DNA Mini Kit (Qiagen Inc, Valencia, California). The DNA extracts were used to quantify PCV2 DNA copy numbers by real-time PCR as previously described.8 The number of genomic copies of PCV2 genomic DNA per mL of serum was transformed to log10 for analysis.
All pigs were euthanized for necropsy at Day 42. Superficial inguinal lymph nodes were collected for histopathology and immunohistochemistry.
Serum samples were tested using a commercial PCV2 ELISA IgG (Synbiotics) and virus neutralization.9 Serum samples were considered positive for PCV2 IgG antibody if the titer was greater than 350, according to the manufacturer’s instructions. The neutralizing antibody (NA) data were transformed to log2 for analysis. The numbers of PCV2-specific interferon-γ-secreting cells (IFN-γ-SCs) were determined in peripheral blood mononuclear cells (PBMCs) as previously described.10 Whole PCV2b (the same strain used for challenge) at a multiplicity of infection of 0.01 was used as stimulant of PBMCs. Phytohemagglutinin (10 µg per mL; Roche Diagnostics GmbH, Mannheim, Germany) and phosphate buffered saline were used as a positive and negative control, respectively.
For the morphometric analysis of histopathological lesion score and number of PCV2-positive cells in lymph nodes, three superficial inguinal lymph-node sections were examined blindly as previously described.11,12 Lymphoid lesions were scored on a scale from 0 to 3: 0, no lymphoid depletion or granulomatous replacement; 1, mild lymphoid depletion; 2, moderate lymphoid depletion; and 3, severe lymphoid depletion and histiocytic replacement.11 The number of lymphoid PCV2 antigen-positive cells per unit area (0.25 mm2) were counted using an NIH Image J 1.45s program (http://imagej.nih.gov/ij/download.html).12
Continuous data (PCV2 DNA, PCV2 serological results, PCV2-specific IFN-γ-SCs, and lymphoid PCV2 antigen-positive cells) were analyzed using a one-way analysis of variance (ANOVA). If the ANOVA showed a significant effect, Tukey’s test for multiple comparisons was performed at each time point. Discrete data (lymphoid lesion score) were analyzed by Fisher’s exact test. A value of P < .05 was considered significant.
Results
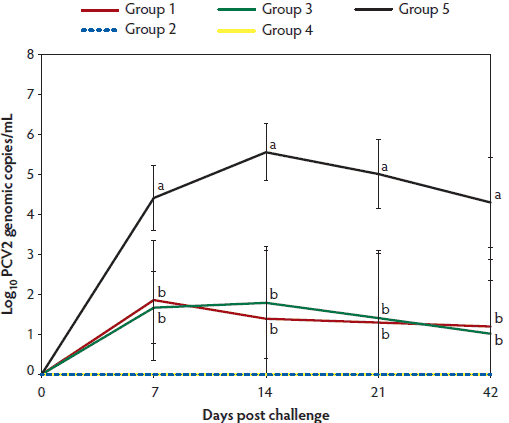
At Day 0, PCV2 DNA was not detected in the serum of any pigs. In Group 1 and Group 3 (vaccinated challenged animals), the number of genomic copies of PCV2 in serum was significantly lower (P < .05) on days 7 to 42 than in the unvaccinated challenged animals in Group 5. However, number of genomic copies of PCV2 in serum did not differ between Group 1 (immunized with one-dose PCV2 vaccine) and Group 3 (immunized with two-dose PCV2 vaccine) (Figure 1). No PCV2 DNA was detected in serum of pigs in groups 2, 4, and 6 throughout the experiment.
Figure 1: Means (with standard deviation) of the log10 transformed number of genomic copies of PCV2 DNA in serum of pigs in the study described in Table 1. Different letters indicate significant differences among groups (P < .05: one-way ANOVA).
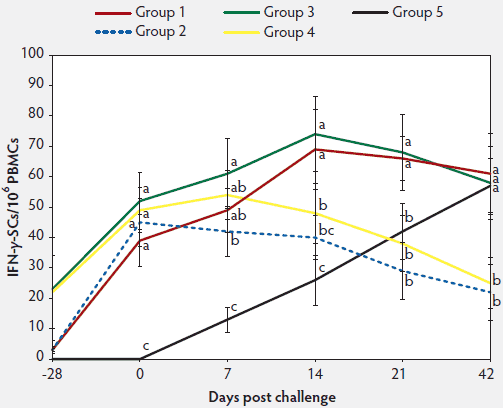
In vaccinated animals (Group 1, 2, 3, and 4), anti-PCV2 IgG antibody titers and geometric mean NA titers were significantly higher (P < .05) on days 0 to 21 than in unvaccinated challenged animals (Group 5) (Figure 2A and 2B). In vaccinated animals (Group 1, 2, 3, and 4), numbers of PCV2-specific IFN-γ-SCs, were significantly higher (P < .05) than in unvaccinated challenged animals (Group 5) at days 0 and 7 (Figure 2C). In animals vaccinated with the two-dose PCV2 vaccine (Group 3 and 4), titers of anti-PCV2 IgG antibodies were significantly higher (P < .05) on Day 0 than in animals immunized with the one-dose PCV2 vaccine (Group 1 and 2) (Figure 2A). Anti-PCV2 IgG antibody titers, geometric mean NA titers, and numbers of PCV2-specific IFN-γ-SCs did not differ on days 7 to 42 between the vaccinated challenged animals in Group 1 (one-dose vaccine) and Group 3 (two-dose vaccine) or between the vaccinated unchallenged animals in Group 2 (one-dose vaccine) and Group 4 (two-dose vaccine) (Figure 2). No anti-PCV2 IgG antibodies or PCV2-specific NA or IFN-γ-SCs were detected in Group 6, the negative control animals.
Figure 2A: Means (with standard deviation) for anti-PCV2-IgG antibody titers in the study described in Table 1. Different letters indicate significant differences among groups (P < .05; one-way ANOVA).
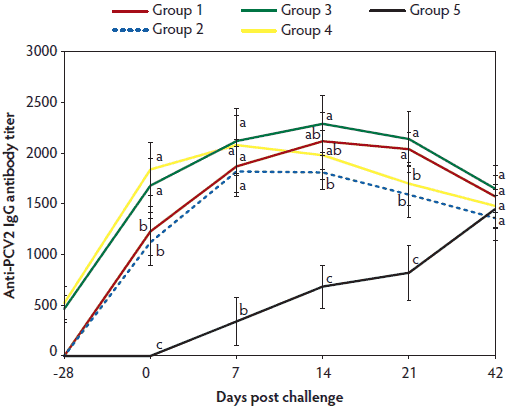
Figure 2B: Log2 transformed group means (with standard deviation) for neutralizing antibody (NA) titers. Treatments described in Table 1. Different letters indicate significant differences among groups (P < .05; one-way ANOVA).
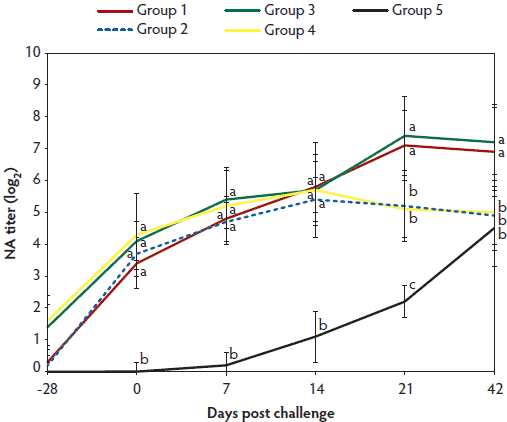
Figure 2C: Mean numbers (with standard deviation) of PCV2-specific interferon-γ-secreting cells (IFN-γ-SCs) in peripheral blood mononuclear cells (PBMCs). Treatments described in Table 1. Different letters indicate significant differences among groups (P < .05; one-way ANOVA).
The number of lymphoid PCV2 antigen-positive cells was significantly lower (P < .05) in the vaccinated groups (Group 1, 2, 3, and 4) than in the positive control group (Group 5) (Table 1). However, lymphoid lesion scores and the number of lymphoid PCV2 antigen-positive cells did not differ between one-dose (Group 1) and two-dose (Group 3) vaccinated challenged animals.
Discussion
It is reasonable to determine the parameters for PCV2 vaccine efficacy on the basis of induction of protective immunity, the number of copies of PCV2 genomic DNA per mL of serum, and the presence of PCV2-associated lesions and PCV2 antigen within these lesions.4 Induction by the vaccine of protective immunity such as PCV2-specific neutralizing antibody and IFN-γ-SCs plays a critical role in reducing the PCV2 load in the blood.13,14 Although differences in serological parameters were apparent between two-dose and one-dose products at the time of challenge, this did not result in significant differences in PCV2 viremia or PCV2-associated lesions after challenge. These observations are further supported by a study15 in which the reduction of PCV2 DNA in the serum did not differ between pigs given a one-dose chimeric PCV1-2 vaccine (2 mL) and a two-dose vaccine (ie, the same one-dose product administered twice, 1 mL per dose).
In the current study, in pigs vaccinated with either the one- or two-dose product, the number of copies of PCV2 genomic DNA per mL of serum was lower than the number observed in the unvaccinated, unchallenged group. However, mean lymphoid lesion scores did not differ between vaccinated groups of pigs and the group that was not challenged. These data suggest that PCV2-associated microscopic lesions were not prominent in the present study, as the pigs were challenged with PCV2 alone. Co-infections may be necessary and crucial for the full development of typical pathological lesions related to PCVAD.15,16
Single-dose PCV2 vaccines are more popular because less labor is required of the workers and there is less stress to the animals. Swine producers are more likely to be compliant with a one-dose vaccine than with a two-dose regimen. Compliance cannot be monitored as easily with one-dose baculovirus-expressed PCV2 vaccines because there is no reliable baculovirus antibody ELISA test.17,18 Although the small number of animals tested is a limitation of this study, there is no serological evidence that it makes any difference whether one or two doses of PCV2 vaccine are administered. These results will greatly facilitate swine practitioners in providing information to producers on whether to use a one-dose or two-dose PCV2 vaccine.
Implications
• Under the conditions of this study, it makes no difference to protection whether a one-dose or two-dose PCV2 vaccine is used.
• Using a one-dose instead of a two-dose PCV2 vaccine creates less stress for both the pigs and animal-care workers.
Acknowledgements
This research was supported by contract research funds of the Research Institute for Veterinary Science (RIVS) from the College of Veterinary Medicine and by the Brain Korea 21 PLUS Program for Creative Veterinary Science Research in the Republic of Korea. The first two authors contributed equally to this work.
Conflict of interest
None reported.
References
1. Meehan BM, McNeilly F, Todd D, Kennedy S, Jewhurst VA, Ellis JA, Hassard LE, Clark EG, Haines DM, Allan GM. Characterization of novel circovirus DNAs associated with wasting syndromes in pigs. J Gen Virol. 1998;79:2171–2179.
2. Chae C. A review of porcine circovirus 2-associated syndromes and diseases. Vet J. 2005;169:326–336.
3. Chae C. Postweaning multisystemic wasting syndrome: a review of aetiology, diagnosis and pathology. Vet J. 2004;168:41–49.
4. Chae C. Commercial porcine circovirus type 2 vaccines: Efficacy and clinical application. Vet J. 2012;194:151–157.
5. Chae C. Porcine circovirus type 2 and its associated disease in Korea. Virus Res. 2012;164:107–113.
6. Fenaux M, Opriessnig T, Halbur PG, Elvinger F, Meng XJ. A chimeric porcine circovirus (PCV) with the immunogenic capsid gene of the pathogenic PCV type 2 (PCV2) cloned into the genomic backbone of the nonpathogenic PCV1 induces protective immunity against PCV2 infection in pigs. J Virol. 2004;78:6297–6303.
7. Chow SC, Wang H, Shao J. Comparing means. In: Chow SC, Wang H, Shao J, eds. Sample Size Calculations in Clinical Research. 2nd ed. New York: Chapman and Hall/CRC; 2008:60–92.
8. Gagnon CA, del Castillo JR, Music N, Fontaine G, Harel J, Tremblay D. Development and use of a multiplex real-time quantitative polymerase chain reaction assay for detection and differentiation of Porcine circovirus-2 genotypes 2a and 2b in an epidemiological survey. J Vet Diagn Invest. 2008;20:545–558.
9. Pogranichniy RM, Yoon KJ, Harms PA, Swenson SL, Zimmerman JJ, Sorden SD. Characterization of immune response of young pigs to porcine circovirus type 2 infection. Viral Immunol. 2000;13:143–153.
10. Seo HW, Han K, Oh Y, Park C, Chae C. Efficacy of a reformulated inactivated chimeric PCV1-2 vaccine based on clinical, virological, pathological and immunological examination under field conditions. Vaccine. 2012;30:6671–6677.
11. Fenaux M, Halbur PG, Haqshenas G, Royer P, Thomas P, Nawagitgul P, Gill M, Toth TE, Meng XJ. Cloned genomic DNA of type 2 porcine circovirus is infectious when injected directly into the liver and lymph nodes of pigs: Characterization of clinical disease, virus distribution, and pathologic lesions. J Virol. 2002;76:541–551.
12. Kim D, Ha Y, Lee YH, Chae S, Lee K, Han K, Kim J, Lee JH, Kim SH, Hwang KK, Chae C. Comparative study of in situ hybridization and immunohistochemistry for the detection of porcine circovirus 2 in formalin-fixed, paraffin-embedded tissues. J Vet Med Sci. 2009;71:1001–1004.
13. Meerts P, van Gucht S, Cox E, Vandebosch A, Nauwynck HJ. Correlation between type of adaptive immune response against porcine circovirus type 2 and level of virus replication. Viral Immunol. 2005;18:333–341.
14. Meerts P, Misinzo G, Lefebvre D, Nielsen J, Botner A, Kristensen CS, Nauwynck HJ. Correlation between the presence of neutralizing antibodies against porcine circovirus 2 (PCV2) and protection against replication of the virus and development of PCV2-associated disease. BMC Vet Res. 2006;2:6–16.
15. Harms PA, Sorden SD, Halbur PG, Bolin SR, Lager KM, Morozov I, Paul PS. Experimental reproduction of severe disease in CD/CD pigs concurrently infected with type 2 porcine circovirus and porcine reproductive and respiratory syndrome virus. Vet Pathol. 2001;38:528–539.
16. Opriessnig T, Thacker EL, Yu S, Fenaux M, Meng XJ, Halbur PG. Experimental reproduction of postweaning multisystemic wasting syndrome in pigs by dual infection with Mycoplasma hyopneumoniae and porcine circovirus type 2. Vet Pathol. 2004;41:624–640.
*17. Kuehn T, Sondermeijer P, Eggen A, Fiebig K, Von Rueden S. Development of a new marker ELISA (BacuCheck™) for compliance testing following the vaccination of piglets against PCV2. Proc 6th Int Symp Emerg Re-emerg Pig Dis. 2011:42.
18. Gerber PF, Johnson J, Shen H, Striegel D, Xiao CT, Halbur PG, Opriessnig T. Association of concurrent porcine circovirus (PCV) 2a and 2b infection with PCV associated disease in vaccinated pigs. Res Vet Sci. 2013;95:775–781.
* Non-refereed reference.