| Original research | Peer reviewed |
Cite as: Sadler LJ, Karriker LA, Johnson AK, et al. Swine respiratory disease minimally affects responses of nursery pigs to gas euthanasia. J Swine Health Prod. 2014;22(3):125–133.
Also available as a PDF.
SummaryObjectives: To assess effects of swine respiratory disease (SRD) on nursery pig responses during gas euthanasia and to compare responses to carbon dioxide (CO2) and argon (Ar) gas euthanasia in terms of efficacy and welfare. Materials and methods: Fifty-four pigs identified for euthanasia were classified as having SRD or euthanized for other reasons (OT). These pigs were distributed among three treatments: prefill CO2 (P-CO2), gradual fill CO2 (G-CO2), and prefill Ar (P-Ar). Behavioral and physiological indicators of efficacy and welfare were assessed directly and from video. Modified atmosphere CO2 and O2 concentrations (%) were collected throughout the process. Results: Respiratory disease status did not affect behavioral or physiological responses associated with efficacy or welfare with P-CO2 or G-CO2. Conversely, SRD pigs lost consciousness faster than OT pigs with P-Ar (P < .05) and duration of open-mouth breathing was shorter (P < .05), but duration of ataxia tended to be longer (P < .10). Regardless of disease status, P-CO2 was associated with superior animal welfare, with shorter latency to loss of consciousness than P-Ar, and shorter duration of ataxia and duration and intensity of righting responses. Implications: Standard operating procedures for gas euthanasia utilizing CO2 or Ar do not require adjustment for nursery pigs with respiratory disease. Minimum exposure of 10 minutes at > 70% CO2 concentration is required to reliably produce respiratory arrest in nursery pigs. Argon is not recommended as a euthanizing agent for nursery pigs. Duration of exposure to Ar required to reliably produce respiratory arrest remains unknown. | ResumenObjetivos: Evaluar los efectos de la enfermedad respiratoria porcina (SRD por sus siglas en inglés) a la respuesta de los cerdos del destete a la eutanasia por gas y comparar la respuesta a la eutanasia por gas con bióxido de carbono (CO2) y argón (Ar) en términos de eficacia y bienestar. Materiales y métodos: Se clasificaron cincuenta y cuatro cerdos identificados para eutanasia por SRD o sometidos a eutanasia por otras razones (OT por sus siglas en inglés). Estos cerdos se distribuyeron en tres tratamientos: pre-llenado CO2 (P-CO2), llenado gradual CO2 (G-CO2), y pre-llenado Ar (P-Ar). Se evaluaron los indicadores de conducta y fisiológicos de eficacia y bienestar, directamente y del video. Se recolectaron las concentraciones modificadas de O2 y CO2 de la atmósfera a lo largo del proceso. Resultados: El status de enfermedad respiratoria no afectó las respuestas fisiológicas o de conducta asociadas con la eficacia o el bienestar con el P-CO2 ó el G-CO2. Por el contrario, los cerdos con SRD perdieron conciencia más rápido que los cerdos OT con P-Ar (P < .05) y la duración de la respiración con la boca abierta fue más corta (P < .05), pero la duración de la ataxia tendió a ser más larga (P < .10). Independientemente del estatus de enfermedad, el P-CO2 fue asociado con un bienestar animal superior, con latencia más corta de pérdida de conciencia que P-Ar, y duración más corta de ataxia y duración e intensidad de respuestas de orientación. Implicaciones: Los procedimientos de operación estándar para la eutanasia de gas utilizando CO2 ó Ar no requieren ajuste para cerdos en destete con enfermedad respiratoria. Se requiere una exposición mínima de 10 minutos a una concentración de > 70% CO2 para producir de manera fiable un paro respiratorio en cerdos de lactancia. El argón no es recomendable como un agente de eutanasia para cerdos de lactancia. La duración de la exposición al Ar requerida para producir de manera fiable un paro respiratorio sigue siendo desconocida. | ResuméObjectifs: Évaluer les effets des maladies respiratoires porcines (SRD) chez les porcelets en pouponnière durant l’euthanasie au gaz et comparer les réponses au dioxyde de carbone (CO2) et à l’argon (Ar) pour l’euthanasie en terme d’efficacité et de bien-être. Matériels et méthodes: Cinquante-quatre porcs identifiés pour euthanasie ont été classés comme ayant une SRD ou euthanasiés pour d’autres raisons (OT). Ces porcs furent distribués parmi trois traitements: pré-remplissage CO2 (P-CO2), remplissage graduel CO2 (G-CO2), et pré-remplissage Ar (P-Ar). Des indicateurs comportementaux et physiologiques d’efficacité et de bien-être furent évalués directement à partir de vidéo. Les concentrations de CO2 et d’O2 (%) des atmosphères modifiées ont été mesurées durant tout le processus. Résultats: Le statut quant à une maladie respiratoire n’a pas affecté les réponses comportementales ou physiologiques associées à l’efficacité ou le bien-être avec P-CO2 ou G-CO2. À l’inverse, les porcs avec SRD perdirent conscience plus rapidement que les porcs OT avec P-Ar (P < .05) et la durée de respiration la bouche ouverte était plus courte (P < .05), mais la durée de l’ataxie avait tendance à être plus longue (P < .10). Indépendamment du statut quant à la maladie, P-CO2 était associée à un meilleur bien-être animal, une période de latence plus courte pour la perte de conscience que P-Ar, et une plus courte durée d’ataxie et durée d’intensité des réponses de redressement. Implications: Les procédures opérationnelles normalisées pour l’euthanasie au gaz utilisant le CO2 ou l’Ar ne nécessitent pas d’ajustement pour les porcs en pouponnière avec des maladies respiratoires. Un temps d’exposition minimum de 10 minutes à une concentration >70% CO2 est requis pour induire un arrêt respiratoire fiable chez les porcelets en pouponnière. L’argon n’est pas recommandé pour euthanasier les porcs en pouponnière. La durée d’exposition à l’Ar requise pour causer un arrêt respiratoire fiable demeure inconnue. |
Keywords: swine, respiratory disease, gas euthanasia, carbon dioxide, argon
Search the AASV web site
for pages with similar keywords.
Received: July 1, 2013
Accepted: September 30, 2013
Swine producers and veterinarians generally agree that euthanasia is appropriate for low-viability pigs, especially when there is suffering due to injury or illness. The National Animal Health Monitoring System reports that respiratory disease is the primary producer-identified cause of mortality in nursery pigs (44.2%).1 However, there is little empirical evidence for evaluating euthanasia techniques for pigs in this compromised state. Carbon dioxide (CO2) is the most commonly implemented gas for swine euthanasia in the United States,2 and the American Veterinary Medical Association notes “… parameters of the technique need to be optimized and published to ensure consistency and repeatability. In particular, the needs of pigs with low tidal volume must be explored.”3 A pig suffering from swine respiratory disease differs from a healthy pig in several physiological parameters that may be important when utilizing gas as a euthanizing agent. Perhaps most importantly, the damaged lung likely reduces gas exchange rates.
With CO2 as the method of euthanasia, loss of consciousness and death result from hypercapnia when pigs are gradually exposed to the gas (such as gradual fill at 20% box-volume exchange rate [BVR] per minute) or from a combination of hypercapnia and hypoxia when pigs are placed in a prefilled box at 80% concentration.4 Carbon dioxide is mildly acidic, which may cause irritation to the mucus membranes.5 At 10% CO2 concentrations, human subjects report experiencing breathlessness, described as being unpleasant, and the majority of subjects report 50% CO2 concentration as being very pungent and painful.6 This has led to questions about whether CO2 is appropriate for pig euthanasia.7 Argon (Ar) has been proposed as an alternative gas euthanasia method.8 The European Food Safety Authority recommends stunning pigs with a 30:60 ratio of CO2 to Ar or a 90:10 ratio of Ar to air.9 Argon is a noble gas, and as such is likely unreactive throughout the physiological systems.10 Loss of consciousness and death are produced through hypoxia, creating the physiological state of hypocapnic anoxia.11 As the mechanisms of CO2 and Ar are different, it is important that both be examined in the compromised pig.
Euthanasia is composed of two stages: first, induction of unconsciousness (insensibility) and second, death. The induction phase is critical to ensure the welfare of the pigs. The entire process, including death, is important to ensure practical implementation. The primary objective of this research was to examine the welfare implications of CO2 and Ar for euthanasia of nursery pigs suffering from swine respiratory disease. A secondary objective was to compare welfare implications of CO2 and Ar for euthanasia of nursery pigs regardless of disease status.
Materials and methods
The protocol for this experiment was approved by the Iowa State University Institutional Animal Care and Use Committee.
Experimental design
The experiment was conducted over 4 days in July 2012. Pigs identified for euthanasia were allocated to two disease-status categories: swine respiratory disease (SRD) and other (OT). Pigs of each disease status were enrolled in three gas treatments. The first treatment was a 100% CO2 prefilled box (P-CO2), followed by a 20% BVR per minute. The second treatment was 100% CO2 at 20% BVR per minute (G-CO2), and the third was a 100% Ar prefilled box (P-Ar) followed by 50% BVR per minute. Eleven SRD-OT pig pairs were enrolled in each CO2 treatment, and five SRD-OT pig pairs were enrolled in the Ar treatment for a total of 54 pigs (two disease statuses × two CO2 gas treatments × 11 replicates per CO2 treatment plus five replicates of Ar treatment). Pigs from both the SRD and OT categories were arbitrarily selected and paired. Gas treatments were applied to the pig pairs in a randomized order created with a random number generator. The original protocol called for the exchange rate for G-CO2 to be 35% BVR per minute, and the P-CO2 treatment followed by 50% BVR per minute. However, due to technical difficulties during the trial, only a 20% BVR per minute was achieved in the system.
Study animals and enrollment criteria
Pigs were housed in and sourced from a commercial nursery farm located in north central Missouri. Genetics were a custom Landrace × Yorkshire cross × Duroc sire performance line. Pigs were eligible for enrollment if they were weaned and 3 to 10 weeks of age. Enrolled pigs were chosen from a pool of pigs identified by farm staff as candidates for euthanasia and placed in a cull pen. These pigs were then assigned a disease status, SRD or OT, based on the Guidance for industry: Recommended study design and evaluation of effectiveness studies for swine respiratory disease claims.12 This document provides guidance for indications of SRD in live pigs, based on the parameters of rectal temperature and four-point scoring systems for both respiration and depression. Briefly, a respiration score of 0 denotes a normal respiration rate and pattern; 1 denotes mild, slightly increased respiratory rate; 2 denotes a moderate increase in respiratory rate indicated by some abdominal breathing; and 3 denotes severe respiratory distress indicated by increased respiratory rate with abnormal effort. A depression score of 0 denotes a normal, alert, active pig, well-hydrated and with a normal coat and appetite. A depression score of 1 denotes mild depression, indicated by the pig moving more slowly than normal, with a slightly rough coat; the pig appears lethargic, but upon stimulation appears normal. A depression score of 2 denotes moderate depression, indicated by a pig that that may be recumbent but is able to stand, is gaunt, and may be dehydrated. A score of 3 denotes severe depression, indicated by a down pig or a pig reluctant to get up and gaunt and dehydrated. These scores were collected under both normal and stressed conditions. First, a respiratory score was assigned while the pigs were minimally disturbed in the cull pen; second, assessment was conducted while each pig was restrained by a technician and was presumably in a stressed state. The pigs were also assigned a depression score while in the cull pen, concurrent with the respiration score. Pigs were enrolled as SRD if rectal temperature was ≥ 40.00°C, respiratory score was ≥ 2, and depression score was ≥ 2. Pigs were enrolled as OT if rectal temperature was < 39.72°C, respiratory score was 0, and depression score was ≤ 1. Pigs with respiration score 1 or temperatures ranging between 39.72°C and 39.99°C were not enrolled.
Euthanasia equipment
Gas was administered to the pigs via a modified Euthanex AgPro system (Value-Added Science and Technology, Mason City, Iowa). This gas delivery apparatus was designed by Euthanex Corporation (Palmer, Pennsylvania), a manufacturer of gas delivery systems for rodents and small animals. The system allows for variable administration of gas types, mixtures, flow rates, and delivery times, and once set, ensures precise and controlled administration of gases to the box.
To facilitate behavioral observations, the box’s top and front panel were constructed of clear plastic. The top panel was hinged for placing pigs in the box. A foam gasket created an airtight seal. The remaining four panels were constructed of opaque plastic (Figure 1). The gas flowed through 3.25 m of 0.64-cm diameter rubber hoses prior to entering the box. The floor was fitted with a custom foam mat (1.3 cm thick) overlaid with a thin rubber mat (0.16 cm thick) and a layer of wood sawdust (approximately 1 cm deep; TLC Premium Horse Bedding, Centerville, Arizona) to aid in traction and comfort for the pigs.
Figure 1: Diagram showing the dimensions of a plastic box for administration of euthanasia gases to nursery pigs 3 to 10 weeks of age. The front and top panels were transparent and the top panel was hinged at the front. The inlet valve (diameter 0.64 cm) was located on a side panel, 7.6 cm from the back panel and 7.6 cm from the top of the box. The exhaust valve (diameter 0.64 cm) was located on the same side panel, 44 cm from the back panel and 3.8 cm from the top of the box. 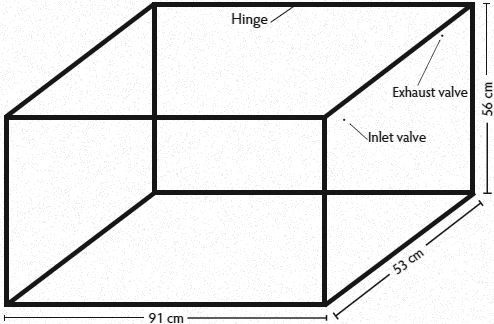 |
Constant and precise gas flow was provided by compressed gas cylinders equipped with compressed gas regulators and meters. The CO2 gas was industrial grade (99% pure), and the Ar gas had a guaranteed analysis of 99.99% pure. Prior to each treatment, sawdust was removed from the box by a vacuum (5.24 m3 per minute), and the rubber mat and box were then cleaned (Windex; S. C. Johnson, Racine, Wisconsin) and disinfected (Roccal; Pfizer Animal Health, New York, New York), and fresh sawdust was added. The vacuum was also utilized to remove gas traces, pulling air from the bottom of the box for a minimum of 3 minutes.
Environmental conditions
A HOBO data logger (U23-001; Onset Computer Corporation, Cape Cod, Massachusetts) was used to record temperature (°C) and relative humidity (%) within the box. The data logger was set to record every 10 seconds. Oxygen concentrations (%) were collected with an oxygen sensor (TR25OZ; CO2Meter.com, Ormond Beach, Florida) attached to a HOBO data logger (U12; Onset Computer Corporation), which collected the oxygen concentration every second. Data were collected continuously throughout the treatment day and exported into Microsoft Office Excel (version 2007; Redmond, Washington). A CO2 meter (CO2IR-WR 100%; CO2Meter.com) monitored concentrations (%) every 1.25 seconds. All sensors were placed at the head level of the standing pig. Over all days, the average temperature in the box was 32.0°C, ranging from 25.7°C to 38.5°C. Relative humidity averaged 41.7%, ranging from 12.9% to 73.3%.
Euthanasia procedure and confirmation of insensibility and death
For identification during behavior observations, pigs were marked with an animal-safe marker (LA-CO Industries Inc, Elk Grove, Illinois). The testing area provided isolation, minimizing noise and distractions. A 10-second respiration rate, 10-second pulse rate, rectal temperature, and body weight were recorded for each pig prior to placement in the box. During this assessment, pigs were held by a technician. To achieve a prefilled environment, CO2 was supplied to the box at 20% BVR for at least 13 minutes and Ar gas at 50% BVR for at least 5 minutes. Upon placement of the SRD-OT piglet pair into the box, gas was immediately started or restarted (gradual or prefill, respectively) and delivery was continued until the pigs were confirmed dead. Two minutes after the last movement (respiratory arrest), pigs were removed individually from the box and examined for signs of insensibility.13-16
Three insensibility tests were conducted: first, a corneal reflex response, in which the cornea of the eye was touched with the tip of a finger for absence of an eye blink or withdrawal response; second, a pupillary reflex, in which a light-beam (Mini MAGLite; Mag Instrument, Inc, Ontario, California) was shone into the eye for absence of pupil constriction; and third, a nose prick, in which a 20-gauge needle was touched to the snout distal to the rostral bone for absence of a withdrawal response. After insensibility was confirmed, cardiac arrest was confirmed by auscultation with a stethoscope. If the pig showed signs of sensibility or cardiac activity, it was placed back into the box for an additional minute of gas exposure. This process was repeated until confirmation of cardiac arrest, allowing us to establish duration of exposure required for death to occur after maximum change in gas concentration (dwell time).
For ethical and practical reasons, the protocol was terminated if pigs displayed signs of consciousness (regained posture, made righting attempts or vocalizations, or had not transitioned to gasping) after 10 minutes of gas exposure. Additionally, a maximum value of 10 minutes was allowed for death (cardiac arrest) after loss of consciousness. For pigs that did not achieve these outcomes within the designated times, captive bolt was utilized as a secondary euthanasia method, in accordance with the American Veterinary Medical Association’s guidelines.3
Assessment of lungs
Immediately upon confirmation of death, necropsy was performed. Lungs were removed and a single technician, blinded to disease status, scored the lungs for total macroscopic lesions as described by Opriessnig, et al.17 This scoring system was based on gross visible damage and the approximate volume each lung lobe contributes to the whole lung. The right cranial lobe, right middle lobe, cranial part of the left cranial lobe, and caudal part of the left cranial lobe contribute 10% each to total lung volume; the accessory lobe contributes 5%; and the right and left caudal lobes contribute 27.5% each. Each lobe was scored as follows: 0% indicating no gross damage; 50% indicating > 0 to ≤ 50% of the lobe grossly affected; 100% indicating > 50% grossly affected. These lobe scores were aggregated for a total lung-damage score, ranging from 0% to 100%. Four samples of the lung tissue were collected, with diseased tissue sampled when grossly visible. If no gross lesions were visible, two samples were collected from each of the left and right middle lobes.
Samples were fixed in 10% buffered formalin until scored. Histological examination was performed by pathologists at the Iowa State University Veterinary Diagnostic Laboratory, who were blind to disease status and gas treatments. Sections of formalin-fixed lung were embedded in paraffin, processed routinely, and stained with hematoxylin and eosin. To confirm gross observations as lesions, a pathologist examined lung sections for evidence of antemortem hemorrhage or atelectasis and also characterized the lesions of pneumonia as nonsuppurative interstitial pneumonia or suppurative bronchopneumonia. Pleuritis, when present, was also noted.
Behavioral observations
Behavioral data were collected by direct observation and via video recording. For direct observation, one observer per pig stood approximately 1.5 m from the box and recorded behavioral indicators of welfare, physiological responses (Table 1), and insensibility. Videos were created utilizing a Noldus Portable Lab (Noldus Information Technology, Wageningen, The Netherlands). Two color cameras (WV-CP484; Panasonic, Kadoma, Japan) were connected to a multiplexer, allowing the image to be recorded onto a personal computer using HandiAvi (version 4.3; Anderson’s AZcendant Software, Tempe, Arizona) at 30 frames per second. Behavioral data were collected from video recordings by a single trained observer, blinded to disease status and gas treatments, using Observer software (version 10.1.548; Noldus Information Technology). Data were collected for the individual pig for behavioral and physiological indicators of efficacy and welfare of the euthanasia process (Table 1). Latencies for all behaviors were determined from the point when each pig was placed into the box.
Table 1: Ethogram developed for investigating latency (L), duration (D), prevalence (P), and frequency (F) of behavioral indicators of welfare or sensation during gas euthanasia of swine*
* Ethogram applied to 54 nursery pigs (3 to 10 weeks of age) classified as having swine respiratory disease (SRD; 15.4 ± 1.4 kg) or euthanized for other reasons (OT; 10.0 ± 1.4 kg) during three gas euthanasia treatments: prefilled carbon dioxide (CO2), gradual CO2 (20% box volume exchange rate per minute), or prefilled argon (Ar). Gas administered via a modified Euthanex AgPro system (Value-Added Science and Technology, Mason City, Iowa). To facilitate behavioral observations, the box top and front panels were constructed of clear plastic (Figure 1). Behavioral data collected by direct observation and via video recordings. † Adapted from Velarde et al.18 ‡ Adapted from Johnson et al.19 § Adapted from Blood et al.20 ¶ Adapted from Hurnik et al.21 ** Adapted from Raj and Gregory.8 |
Statistical analysis
Behaviors were quantified as latency, duration, and frequency of occurrence, or percent of pigs displaying the behavior as indicated for the parameter. Data were analyzed using linear mixed models fitted with the GLIMMIX procedure (duration, number, prevalence; SAS Institute Inc, Cary, North Carolina) or with a Cox proportional hazard model (latency) fitted with the PHREG procedure of SAS. Individual pig was the measurement unit for SRD versus OT pigs, while pig pair served as the experimental unit for gas type. Least squares means estimates for each treatment group and the corresponding standard error (SE) are reported. The linear model included the fixed effect of disease status (SRD, OT) and gas treatment (P-CO2, G-CO2, P-Ar) and all two-way interactions. A random blocking effect of pig pair was included. The Kenward-Rogers method was utilized for determining the denominator degrees of freedom. Statistical significance was established at P < .05 and a trend at P < .10. The GLIMMIX procedure of SAS was utilized to establish correlations between latency to behaviors and total lung damage, with the fixed effect of gas treatment and a random blocking effect of pig pair.
Results
Rectal temperature, respiration rate, and weight were greater in SRD pigs than in OT pigs (Table 2). Pulse rate did not differ by disease status (P > .05). Lung damage was greater in SRD pigs than in OT pigs (Table 2). Grossly scored lung damage was confirmed by histological examination, with 100% agreement between gross and histological damage scores. Total lung damage was a predictor for loss of posture (P < .05), associated with approximately 0.5-second shorter latency for every 10% of identified damage. Differences were not observed (P > .05) between gas treatments for the pigs’ parameters of rectal temperature, respiration rate, weight, pulse rate, or lung damage.
Table 2: Means and standard errors by disease status for descriptive parameters of pigs identified as in need of euthanasia, data collected prior to gas application*
* Nursery pigs (described in Table 1) were identified for euthanasia for either SRD or OT and assigned into a disease status category by a single technician in accordance with the document Guidance for industry: Recommended study design and evaluation of effectiveness studies for swine respiratory disease claims.12 † Linear mixed model; statistical significance established at P < .05 and a trend at P < .10. SE = standard error; SRD = swine respiratory disease; OT = pigs identified for euthanasia for reasons other than SRD; NA = not applicable. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Within a gas treatment, O2 and CO2 concentrations in the box at the time of loss of consciousness did not differ for SRD and OT pigs. Oxygen concentrations at loss of consciousness (means ± SE) were 5% ± 5%, 17% ± 1%, and 3% ± 3% for P-CO2, G-CO2, and P-Ar, respectively. Carbon dioxide concentrations at loss of consciousness were 63% ± 4%, 46% ± 2%, and 0% ± 0% for P-CO2, G-CO2, and P-Ar, respectively.
In P-Ar, latency to loss of consciousness was shorter for SRD pigs than for OT pigs, but did not differ in P-CO2 or G-CO2 (Table 3). Comparing gas treatments independent of disease status, latency to loss of consciousness was shortest in P-CO2 (P-CO2 versus G-CO2, P < .001; P-CO2 versus P-Ar, P < .001), whereas latency to loss of consciousness did not differ between G-CO2 and P-Ar (P > .05). Latency to last limb movement and respiratory arrest did not differ between SRD and OT pigs in any gas treatment (P > .05). Comparing gas treatments independent of disease status, latency to last limb movement was shorter in P-CO2 than in G-CO2 (P < .001). There was a trend for latency to last limb movement to be shorter in P-CO2 than in P-Ar (P < .10), whereas a difference was not observed between G-CO2 and P-Ar (P > .05). Latency to respiratory arrest did not differ between gas treatments regardless of disease status. In P-CO2, latency to cardiac arrest was shorter for SRD than for OT pigs (Table 3). However, differences by disease status were not observed for G-CO2 or P-Ar. Comparing gas treatments independent of disease status, latency to cardiac arrest was shortest in P-CO2 (P-CO2 versus G-CO2, P < .05; P-CO2 versus P-Ar, P < .05), but did not differ (P > .05) between G-CO2 and P-Ar. Two OT pigs in P-Ar required secondary euthanasia procedures; one did not achieve loss of consciousness and one did not achieve cardiac arrest in the allotted time. All pigs displayed open-mouth breathing and ataxia. In P-CO2 and G-CO2, duration of open-mouth breathing did not differ between SRD and OT pigs (P > .05). However, in P-Ar, duration was greater for OT pigs than for SRD pigs (Table 4). Independent of disease status, duration of open-mouth breathing was shorter in P-CO2 than in G-CO2 (P < .05), but did not differ between P-CO2 and P-Ar (P > .05). Duration of ataxia did not differ between SRD and OT in P-CO2 or G-CO2 (P > .05). In P-Ar, there was a trend for greater duration of ataxia in SRD versus OT pigs (P < .10). Independent of disease status, duration of ataxia was shorter in P-CO2 than in either G-CO2 or P-Ar (P-CO2 versus G-CO2, P < .05; P-CO2 versus P-Ar, P < .05), but did not differ between G-CO2 and P-Ar. In P-CO2, 46% of both SRD and OT pigs displayed a righting response. In G-CO2, 82% of SRD pigs and 64% of OT pigs displayed a righting response. In P-Ar, all pigs displayed a righting response. When examining intensity of the righting response (number of efforts per pig), differences were not observed (P > .05) between SRD and OT pigs within any gas treatment: mean efforts were one for SRD in P-CO2, one for OT in P-CO2, two for SRD in G-CO2, one for OT in G-CO2, three for SRD in P-Ar, and four for OT in P-Ar. Independent of disease status, duration of righting response was shorter in P-CO2 and G-CO2 than in P-Ar (P-CO2 versus P-Ar, P < .01; G-CO2 versus P-Ar, P < .05). Duration did not differ between P-CO2 and G-CO2. When examining intensity of righting response, P-Ar showed greater intensity than P-CO2 or G-CO2 (P-CO2 versus P-Ar, P < .001; G-CO2 versus P-Ar, P < .01), whereas P-CO2 and G-CO2 did not differ (P > .05).
Table 3: Mean latencies (± SE) in seconds for parameters of gas euthanasia efficacy comparing disease status of nursery pigs within gas treatments*
* Means are for non-zero values. Study described in Table 1. Pigs were assigned into a disease status category by a single technician in accordance with the document Guidance for industry: recommended study design and evaluation of effectiveness studies for swine respiratory disease claims.12 † Box (described in Figure 1) was filled with CO2, pigs placed within, and then CO2 supplied at 20% box-volume exchange rate (BVR)/minute. ‡ Pigs placed within, and then CO2 supplied at 20% BVR/minute. § Box was filled with argon, pigs placed within, and then argon supplied at 50% BVR/minute. ¶ Cox proportional hazards model; statistical significance established at P < .05 and a trend at P < .10. SE = standard error; CO2 = carbon dioxide; Ar = argon; SRD = nursery pigs identified for euthanasia suffering from swine respiratory disease; OT = pigs identified for euthanasia for reasons other than SRD. |
Table 4. Mean durations (±SE) in seconds for welfare behavioral measures of gas euthanasia comparing disease status within gas treatments*
* Study described in Table 1. A single technician assigned pigs to a disease-status category (SRD or OT) that was based on the document Guidance for industry: Recommended study design and evaluation of effectiveness studies for swine respiratory disease claims.12 † Box (described in Figure 1) was filled with CO2, pigs placed within, and then gas supplied at 20% box-volume exchange rate (BVR)/minute. ‡ Pigs placed within and then CO2 supplied at 20% BVR/minute. § Box was filled with argon, pigs placed within, and then gas supplied at 50% BVR/minute. ¶ Cox proportional hazards model; statistical significance established at P < .05 and a trend at P < .10. |
|||||||||||||||||||||||||||||||||||||||||||||||||
Prevalence of escape attempts did not differ (P > .05) for disease status or gas type, with 45% of SRD pigs in P-CO2, 36% of OT pigs in P-CO2, 55% of SRD pigs in G-CO2, 9% of OT pigs in G-CO2, 20% of SRD pigs in P-Ar, and 40% of OT pigs in P-Ar displaying this behavior, nor did the range of number of attempts per individual pig differ (zero to three). Oral discharge was a rare event, observed in six pigs: one SRD pig in P-CO2, one OT pig in P-CO2, one SRD pig in G-CO2, and three OT pigs in G-CO2. Of these, three occurred prior to gas treatment application. Ocular and nasal discharges were each displayed by one pig, both in G-CO2. Blood was never visible in the discharges. Sneezing, coughing, and vomiting were not observed in this study.
Prefill conditions required the box to be filled with the designated gas and then the lid opened for placement of the pigs, allowing atmospheric air to enter and quickly changing conditions within the box. Over all trials, O2 concentrations in the box, after pig placement and with the lid closed, were 5% to 8%, 20% to 21%, and 5% to 7% for P-CO2, G-CO2, and P-Ar, respectively. The protocol utilized in the present study required the lid to be opened for confirmation of death, making it difficult to maintain continuous O2 and CO2 concentrations throughout each run. Opening the lid resulted in increased O2 concentrations (Ar and CO2 treatments; < 7%) and decreased CO2 concentrations (CO2 treatments; > 55%). Gas concentrations were regained (< 60 seconds) as gas flow was maintained throughout the procedure.
Discussion
The objectives of this study were to examine and assess the efficacy of gas euthanasia and welfare of nursery pigs suffering from SRD during euthanasia with either CO2 or Ar, and to compare efficacy and welfare, regardless of disease status, of gas euthanasia with either CO2 or Ar. It was hypothesized that SRD pigs would have less respiratory membrane available for gas exchange than pigs not suffering from a respiratory ailment, resulting in greater latency to measures of efficacy and inferior welfare during gas euthanasia. Contrary to our hypothesis, disease status did not affect behavioral or physiological responses associated with efficacy or welfare when euthanizing with P-CO2 or G-CO2. However, when utilizing Ar, minimal differences were observed between disease statuses, with a greater time spent conscious for the OT pigs than for the SRD pigs. Also in Ar, minimal differences were observed in measures of welfare between SRD and OT pigs, with SRD pigs displaying shorter open-mouth breathing but greater ataxia. When comparing prefilled conditions, CO2 resulted in better welfare than Ar by shorter latency to loss of consciousness, shorter duration of ataxia, and shorter duration and lower intensity of righting response, whereas differences were not observed in the other measures of welfare that were collected. Differences between disease statuses were small enough to not warrant changes to gas euthanasia procedures.
Weights of the SRD pigs were greater than those of the OT pigs. This is likely due to variability in disease processes in these two groups. Pigs with swine respiratory disease develop clinical signs gradually, and often are not identified nor warrant euthanasia until late in the nursery phase. Conversely, OT pigs were identified for euthanasia for multiple reasons, including acute reasons such as injury, and thus OT pigs regularly occur and are identified over the entire nursery phase. Previous research has indicated that weight is not a significant factor in gas euthanasia of healthy nursery-age pigs.22 Additionally, in the current study, differences were observed between disease statuses only in the Ar treatment, thus it is unlikely that differences in weight account for differences in responses by SRD and OT pigs.
In this study, the euthanasia process was evaluated in two phases: conscious and unconscious. There is a transition phase prior to loss of consciousness during which a number of behaviors are typically observed, including open-mouth breathing, ataxia, and righting response. The level of awareness, hence capacity of animals to suffer during this transition, is unclear, and we chose a conservative estimate by including all measures up to the point of loss of consciousness to ensure appropriate pig welfare. Behaviors chosen for welfare assessment included physiological distress such as open-mouth breathing, and psychological distress such as escape attempts and righting response.15,23-30 Although more invasive methods to assess efficacy and welfare, such as EEG or ECG monitoring, can provide robust data in the laboratory, they are not practical on farm and cannot be used in tandem with measurement of naturally occurring behaviors that are induced during gas euthanasia procedures. Behavior was chosen as the primary outcome of interest for welfare since behavioral observations provide more sensitive measures of the animal’s experience than physiologic responses, particularly since euthanasia with inhalant gases can produce confounding effects on physiologic responses.31
When CO2 was utilized at either flow rate, disease status did not affect any welfare parameters measured. Open-mouth breathing is a physiological reaction associated with breathlessness, and has been identified as an indicator of compromised welfare in the pig.27 When pigs were exposed to CO2, duration of open-mouth breathing was similar to that previously observed in nursery pigs for both prefill and gradual conditions (12 ± 2 seconds and 34 ± 2 seconds, respectively).22 In P-Ar, duration of open-mouth breathing was approximately four times greater for OT pigs than for SRD pigs. To the authors’ knowledge, the duration of open-mouth breathing in P-Ar has not been previously reported in nursery pigs, though observed values in this trial are approximately three times less than that reported in suckling pigs (110 ± 21 seconds).32
Ataxia is likely an indicator of impaired function of the cerebellum; however, it is unclear how this correlates with impaired cortical function. If ataxia indicates that the pig is aware of its surroundings, but is unable to react in a coordinated manner, this could be distressing to the pig. In this study, we defined ataxia as a potential stressor for the pig, and hence, a shorter duration of this behavior would correlate with improved welfare. In P-Ar, duration of ataxia was approximately four times greater in SRD pigs than in OT pigs. This longer display of ataxia may be attributed to the general health status of the SRD pigs.33,34 With a greater depression score, they may have been more likely to display ataxia even without application of gas. Regardless of disease status, inferior welfare was observed with the use of Ar and the gradual flow rate compared to that in P-CO2. The lack of righting response has been cited as a critical indicator that a pig is successfully rendered unconscious prior to slaughter.13,27 Hence, duration and intensity of the righting response (number of efforts) were used as indicators of welfare in this study. Righting response was not affected by disease status in any gas treatment. In the prefilled gas treatments, inferior welfare was observed with the use of Ar, as indicated by a six-fold greater duration of righting attempts and four-fold greater number of attempts than for CO2. The inferior welfare observed in the gradual flow rate was not surprising, since it is consistent with previous research in our laboratory in which welfare was superior with the use of prefill or a faster flow rate (50% BVR per minute).22 Other flow rates not examined in this study may be advantageous to the pig. Given that disease status did not affect pig responses in the two extreme flow rates tested with CO2, it is likely SRD disease status would not be a factor at any rate between these extremes.
In addition to minimizing the potential distress caused by the gases, an important goal for euthanasia includes minimizing latency to loss of consciousness to ensure the most humane process is achieved. In Ar, pigs in the OT category took more than twice as long to lose consciousness, being conscious for nearly 4.5 minutes. Latency to loss of consciousness was greater with Ar and the G-CO2 than with P-CO2. This is similar to what was observed in suckling pigs.32 During the gas euthanasia process in pigs, once regular breathing (including open-mouth breathing) controlled by the respiratory center of a mammal’s brain fails, gasping is recruited, thus indicating a loss of brain function coordinating with loss of consiousness.35,36 Respiratory arrest (cessation of gasping) represents the point at which gases can no longer be introduced into the pig’s respiratory system. This point is critical to the euthanasia process, because the pig will not recover without intervention. During gas euthanasia, gasping will become slower and shallower until breathing finally ceases. In this study, respiratory arrest was the last observed movement by the pig, and this is consistent with observations of suckling pigs undergoing gas euthanasia.32 Current recommendations for CO2 advise exposure for > 5 minutes.3,15 In the present study, the longest observed latency to respiratory arrest, 585 seconds, was observed in CO2, suggesting that a minimum of 10 minutes exposure to high CO2 concentrations is indicated for euthanasia. Current recommendations for Ar advise exposure for > 7 minutes.3 In the present study, one Ar pig was still conscious after 10 minutes of exposure and thus a longer, unknown duration would need to be implemented when using this gas. Surprisingly, despite the difference in diseased lung tissue between SRD and OT pigs, the only observed difference occurred in latency to cardiac arrest when CO2 was the euthanizing agent. Since cardiac arrest occurs post loss of consciousness and respiratory arrest, it is likely this difference is not of consequence to either welfare or practical implementation, because the pig is insensible and gases can no longer be introduced into the pig’s system.
Pigs that had been clinically identified as SRD were confirmed to have severely diseased lungs, almost three times more damage than the OT pigs. The visible assessment of the lungs was confirmed through histology, with 100% agreement on identification of gross lesions. During respiratory disease, the pulmonary membrane becomes inflamed and highly porous, allowing fluid to leak into the alveoli, effectively decreasing functional respiratory membrane. Additionally, respiratory disease causes inflammation and decreased diameter or blockage of infected airways. This obstruction makes expiration difficult, trapping air which may be reabsorbed, leading to collapse of the affected lung sections. The consequences of less functional respiratory membrane include hypoxemia and hypercapnia.37 To compensate for the hypoxic and hypercapnic state, the SRD pigs displayed tachypnea. Pigs were assessed for a respiratory score as part of the selection process. It is interesting to note that the physiological and compensatory effects of lung damage were observed in both normal and stressed conditions. Assessment of respiratory rate under stressed conditions is the likely cause of this value being greater for both SRD and OT pigs than the expected values (25 to 40 breaths per minute in a normal nursery pig versus SRD 96 and OT 78 breaths per minute).38 Although total lung damage significantly affected loss of posture, the effects were minor (statistically modeled: 5 seconds difference between 0% and 100% lung damage) and not substantial enough to merit modifications of standard operating protocols for euthanasia.
Implications
• Under the conditions of this study, with respect to efficacy and pig welfare, a successful gas euthanasia protocol that utilizes CO2 does not need to be adjusted for pigs with respiratory disease.
• A minimum exposure of 10 minutes at > 70% CO2 concentration is required to reliably produce respiratory arrest in nursery pigs.
• Producing O2 concentrations necessary for euthanasia with Ar is difficult with current on-farm equipment.
• Duration of exposure to Ar required to reliably produce respiratory arrest remains unknown.
• Under the conditions of this study, Ar results in lower efficacy and inferior welfare compared to CO2 and is not recommended as a euthanizing agent for nursery pigs.
Acknowledgements
This material is based upon work supported by the National Institute of Food and Agriculture, US Department of Agriculture, under Award No. 2012-67021-19363. Pigs enrolled in the study were kindly provided by Murphy Brown LLC Western Division. The authors gratefully acknowledge technical assistance provided by Elizabeth Beilke, Lourdes Maldonado-Galarza, Rebecca Parsons, and Natalie White.
Conflict of interest
The authors report no conflict of interest.
References
1. National Animal Health Monitoring System. Swine 2006. Part III: Reference of swine health, productivity, and general management in the United States, 2006. United State Department of Agriculture. 2008. Available at: http://www.aphis.usda.gov/animal_health/nahms/swine/downloads/swine2006/Swine2006_dr_PartIII.pdf. Accessed 14 January 2014.
*2. Daniels CS. Gas euthanasia methods in swine: process and physiology. Proc AASV. Omaha, Nebraska. 2010;447–450.
3. American Veterinary Medical Association. Guidelines for the Euthanasia of Animals: 2013 Edition. Schaumburg, Illinois: American Veterinary Medical Association; 2013. Available at: https://www.avma.org/KB/Policies/Documents/euthanasia.pdf. Accessed 14 January 2014.
4. Raj ABM, Johnson SP, Wotton SB, McInstry JL. Welfare implications of gas stunning pigs: 3. the time to loss of somatosensory evoked potential and spontaneous electrocorticogram of pigs during exposure to gases. Vet J. 1997;153:329–339.
5. Danneman PJ, Stein S, Walshaw SO. Humane and practical implications of using carbon dioxide mixed with oxygen for anesthesia or euthanasia of rats. Lab Anim Sci. 1997;47:376–385.
6. Gregory N, Raj M, Audsey A, Daly C. Effects of CO2 on man. Fleischwirtschart. 1990;70:7–9.
*7. Wright AJ, Whiting M, Taylor A. Letter to the Editor on the surgical castration of piglets. Anim Int J Anim Biosci. 2009;3:1474–1475.
8. Raj ABM, Gregory NG. Welfare implications of the gas stunning of pigs 2. Stress of induction of anaesthesia. Anim Welf. 1996;5:71–78.
9. EFSA (European Food Safety Authority). Opinion of the Scientific Panel on Animal Health and Welfare on a request from the Commission related to welfare aspects of the main systems of stunning and killing the main commercial species of animals. EFSA J. 2004;45:1–29. doi.10.2903/j.efsa.2004.45.
10. Mann C, Boccara G, Grevy V, Navarro F, Fabre JM, Colson P. Argon pneumoperitoneum is more dangerous than CO2 pneumoperitoneum during venous gas embolism. Anesth Analg. 1997;85:1367–1371.
11. Raj ABM. Behaviour of pigs exposed to mixtures of gases and the time required to stun and kill them: welfare implications. Vet Rec. 1999;144:165–168.
12. FDA (US Department of Health and Human Services Food and Drug Administration Center for Veterinary Medicine). Guidance for industry: Recommended study design and evaluation of effectiveness studies for swine respiratory disease claims. Final Guidance. US Department of Health and Human Services Food and Drug Administration Center for Veterinary Medicine. 2007. Available at: http://www.fda.gov/downloads/AnimalVeterinary/GuidanceComplianceEnforcement/GuidanceforIndustry/ucm052473.pdf. Accessed 14 January 2014.
13. Grandin T. Recommended Animal Handling Guidelines & Audit Guide: A Systematic Approach to Animal Welfare. American Meat Institute Foundation. 2010. Available at: http://www.meatami.com/ht/a/GetDocumentAction/i/61388. Accessed 14 January 2014.
14. Kissin I. Depth of anesthesia and bispectral index monitoring. Anesth Analg. 2000;90:1114–1117.
15. American Association of Swine Veterinarians and National Pork Board. On-Farm Euthanasia of Swine: Recommendations for the Producer. Des Moines, Iowa: National Pork Board. 2009.Available at: https://www.aasv.org/aasv/documents/SwineEuthanasia.pdf. Accessed 14 January 2014.
16. Whelan G, Flecknell PA. The assessment of depth of anaesthesia in animals and man. Lab Anim. 1992;26:153–162.
17. Opriessnig T, Thacker EL, Yu S, Fenaux M, Meng XJ, Halbur PG. Experimental reproduction of postweaning multisystemic wasting syndrome in pigs by dual infection with Mycoplasma hyopneumoniae and porcine circovirus type 2. Vet Pathol. 2004;41:624–640.
18. Velarde A, Cruz J, Gispert M, Carrion D, Torre R, Diestre A, Manteca X. Aversion to carbon dioxide stunning in pigs: effect of carbon dioxide concentration and halothane genotype. Anim Welf. 2007;16:513–522.
19. Johnson AK, Sadler LJ, Gesing LM, Feuerbach C, Hill H, Faga M, Bailey R, Stalder KJ, Ritter MJ. Effects of facility system design on the stress responses and market losses of market weight pigs during loading and unloading. Prof Anim Sci. 2010;26:9–17.
20. Blood DC, Studdert VP, Gay CC. Saunders Comprehensive Veterinary Dictionary. 3rd ed. Philadelphia, Pennsylvania: WB Saunders; 2007:150.
21. Hurnik JF, Webster AB, Siegel PB. Dictionary of Farm Animal Behaviour. Guelph, Ontario, Canada: University of Guelph. 1985:168
22. Sadler LJ, Hagen CD, Wang C, Widowski TM, Johnson AK, Millman ST. Effects of flow rate and gas mixture on the welfare of weaned and neonate pigs during gas euthanasia. J Anim Sci. 2014;92:793-805. doi: 10.2527/jas.2013-6598.
23. Forslid A. Pre-slaughter CO2-anaesthesia in swine: influence upon cerebral electrical activity, acid/base balance, blood oxygen tension and stress hormones [doctoral thesis]. Uppsala, Sweden: Swedish University of Agricultural Sciences, Faculty of Veterinary Medicine, Department of Physiology; 1987.
24. Martoft L, Kolthoff C, Rodriguez BE, Jensen EW, Jorgensen PF, Pedersen HD, Forslid A. Effects of CO2 anaesthesia on central nervous system activity in swine. Lab Anim. 2002;36:115–126.
25. Mota-Rojas D, Bolanos-Lopez D, Concepcion-Mendez M, Ramirez-Telles J, Roldan-Santiago P, Flores-Peinado S, Mora-Medina P. Stunning swine with CO2 gas: controversies related to animal welfare. Int J Pharmacol. 2012;8:141–151.
26. Kohler I, Meier R, Busato A, Neiger- Aeschbacher G, Schatzmann U. Is carbon dioxide (CO2) a useful short acting anaesthetic for small laboratory animals? Lab Anim. 1999;33:155–161.
27. Grandin T. Objective scoring of animal handling and stunning practices at slaughter plants. JAVMA. 1998;212:36–39.
28. Sandström V. Development of a monitoring system for the assessment of cattle welfare in abattoirs. Sveriges lantbruksuniversitet. Skara, Sweden: Swedish University of Agricultural Sciences. 2009. Available at: http://ex-epsilon.slu.se:8080/archive/00003176/01/pdf_VS_Epsilon.pdf. Accessed 14 January 2014.
29. Anil MH. Studies on the return of physical reflexes in pigs following electrical stunning. Meat Sci. 1991;30:13–21.
30. American Veterinary Medical Association. Guidelines on Euthanasia (Formerly Report of the AVMA Panel on Euthanasia). Schaumburg, Illinois: American Veterinary Medical Association; 2007. Available at: http://grants.nih.gov/grants/olaw/Euthanasia2007.pdf. Accessed 14 January 2014.
31. Burkholder TH, Niel L, Weed JL, Brinster LR, Bacher JD, Foltz CJ. Comparison of carbon dioxide and argon euthanasia: effects on behavior, heart rate, and respiratory lesions in rats. J Am Assoc Lab Anim Sci. 2010;49:448.
*32. Sadler L, Karriker L, Johnson A, Wang C, Widowski T, Millman S. Effects of depression score on welfare implications of CO2 and Argon gas euthanasia of suckling piglets. Proc 47th Cong Int Soc Appl Etho. Florianopolis, Brazil. 2013;44.
33. Cutler RS, Anthony V, Cronin GM, Spicer EM. Preweaning mortality. In: Straw BE, D’Allaire S, Mengeling WL, Taylor DJ. Diseases of Swine. 9th ed. Victoria, Australia: Blackwell Publishing. 2006:993-1009
34. Fernandez NJ, Barton J, Spotswood T. Hypoglycemia in a dog. Can Vet J. 2009;50:423–426.
35. Guyton AC, Hall JE. Guyton and Hall Textbook of Medical Physiology. 11th ed. Philadelphia, Pennsylvania: Elsevier Saunders; 2010:514-523.
36. St John WM. Noeud vital for breathing in the brainstem: gasping—yes, eupnoea—doubtful. Philos Trans R Soc London (Biol). 2009;364:2625–2633.
37. Guyton AC, Hall JE. Guyton and Hall Textbook of Medical Physiology. 11th ed. Philadelphia, Pennsylvania: Elsevier Saunders; 2010:524-533.
38. Dewey CE, Straw BE. Herd examination. In: Straw BE, D’Allaire S, Mengeling WL, Taylor DJ. Diseases of Swine. 9th ed. Victoria, Australia: Blackwell Publishing. 2006:3-14.
* Non-refereed references.