| Original research | Peer reviewed |
Cite as: Schmidt M, Lahrmann KH, Ammon C, et al. Assessment of body temperature in sows by two infrared thermography methods at various body surface locations. J Swine Health Prod. 2013;21(4):203–209.
Also available as a PDF.
SummaryObjectives: To investigate whether measurement of core body temperature by rectal thermometer can be replaced by infrared techniques and to determine whether it is possible to record changes in body temperature at eight anatomical sites in sows using an infrared camera (IRC) or an infrared thermometer (IRT). Materials and methods: The study was conducted in a farrow-to-finish farm using 45 multiparous Large White × German Landrace sows of first to ninth parity. Sows were between 3 days ante partum and 7 days post partum. In Phase 1 of the study, body temperatures of 15 sows were measured by IRC and IRT at eight anatomical sites twice daily for 4 days. In Phase 2, body temperatures of 30 sows were measured once daily for 4 days at four of the previously selected sites. Infrared and rectal temperatures (RT) were measured simultaneously. Results: The eye (areas near the medial and lateral canthus) and back of the ear (between the transition of the pinna and the dorsal margo of the M cutaneous colli) were promising locations in terms of practicability for IRC and IRT measurement of body temperature. Vulva and mammary gland generated acceptable results, but are less practical. The mean range of the difference between infrared and rectal thermometer temperatures was 4.21 for IRT and 6.67 for IRC. Implication: Infrared techniques appear promising for continuous temperature monitoring where changes in temperature are more important than individual temperature values. | ResumenObjetivos: Investigar si la medición de la temperatura corporal con termómetro rectal puede ser remplazada por técnicas infrarrojas y determinar si es posible registrar los cambios en la temperatura corporal en ocho sitios anatómicos en hembras utilizando una cámara infrarroja (IRC por sus siglas en inglés) o un termómetro infrarrojo (IRT por sus siglas en inglés). Materiales y métodos: El estudio se condujo en una granja de ciclo completo utilizando 45 hembras multíparas Large White × Landrace Alemanas de primera a novena paridad. Las hembras se encontraban entre 3 días antes del parto y 7 días después del parto. En la Fase 1 del estudio, se midieron las temperaturas corporales de 15 hembras con IRC e IRT en ocho sitios anatómicos dos veces al día por 4 días. En la Fase 2, se midieron las temperaturas corporales de 30 hembras una vez al día por 4 días en cuatro de los sitios previamente seleccionados. Se midieron las temperaturas rectales (RT por sus siglas en inglés) e infrarrojas simultáneamente. Resultados: El ojo (áreas cerca del canto medial y lateral) y parte de atrás de la oreja (entre la transición del pabellón de la oreja y el margo dorsal del M cutaneous colli) fueron sitios prometedores en términos de viabilidad para medición de temperatura corporal IRC e IRT. La vulva y las glándulas mamarias generaron resultados aceptables, pero menos prácticos. El rango promedio de la diferencia entre las temperaturas del termómetro rectal e infrarrojo fue de 4.21 para el IRT y 6.67 para el IRC. Implicación: Las técnicas infrarrojas parecen promisorias para el monitoreo de temperatura continuo donde los cambios en temperatura son más importantes que los valores de temperatura individual. | ResuméObjectifs: Afin d’évaluer si la prise de température corporelle par un thermomètre rectal peut être remplacée par des techniques infrarouges et de déterminer s’il est possible d’enregistrer les changements de température corporelle à huit sites anatomiques chez des truies en utilisant une caméra à infrarouge (IRC) ou un thermomètre à infrarouge (IRT). Matériels et méthodes: Cette étude a été menée dans une ferme de naisseurs-finisseurs sur 45 truies croisées Large White × Landrace Allemand multipares de une à neuf parités. Les truies étaient entre 3 jours ante-partum et 7 jours post-partum. Dans la Phase 1 de l’étude, les températures corporelles de 15 truies étaient mesurées par IRC et IRT à huit sites anatomiques deux fois par jour pendant 4 jours. Dans la Phase 2, les températures corporelles de 30 truies ont été mesurées une fois par jour pendant 4 jours à quatre des sites sélectionnés précédemment. Les températures infrarouges et rectales (RT) ont été mesurées simultanément. Résultats: Les yeux (régions près des canthus médial et latéral) et le derrière de l’oreille (entre la transition du pavillon de l’oreille et de la bordure dorsale du muscle cutané du cou) étaient des régions prometteuses en terme pratiques pour la mesure de la température corporelle par IRC et IRT. La vulve et la glande mammaire ont donné des résultats acceptables, mais sont moins pratiques. L’écart moyen de la différence entre les températures infrarouges et par thermomètre rectal était de 4,21 pour IRT et de 6,67 pour IRC. Implication: Les techniques infrarouges apparaissent prometteuses pour le suivi continu de la température où des changements de température sont plus importants que les valeurs individuelles de température. |
Keywords: swine, infrared thermography, temperature, non-invasive
Search the AASV web site
for pages with similar keywords.
Received: July 30, 2012
Accepted: January 8, 2013
Continuous monitoring of the body temperature of a sow in a farrowing room remains an issue of major importance. Keeping sows healthy is important not only for high profit and performance, but also for animal welfare. Fever is the earliest and one of the main clinical signs of many diseases, for example, the mastitis-metritis-agalactia complex.1 On average, it requires 15 seconds to measure the rectal temperature (RT) of a sow (personal communication, K. H. Lahrmann, Clinic for Ruminants and Swine, Freie Universität Berlin; 2012). Therefore, a non-contact method would save time and reduce stress on the animals. Traulsen et al2 measured body surface temperature in swine at various anatomical sites (eye, mammary gland, back of the ear, vulva, and inner part of the ear) with an infrared camera (IRC) and concluded that infrared thermography allows routine measurements of body surface temperatures that can be used for early disease detection. Röhlinger et al3 measured surface temperatures at the eye, mammary gland, forehead, and vulva using an IRC and an infrared thermometer (IRT) and described the possibility of using body surface temperatures for early disease detection in various animals, including swine. Johnson et al4 concluded that it is possible to detect fever in ponies using an IRC near the caruncula lacrimalis at the medial canthus of the eyelid, with a test sensitivity of 74.6% and using RT as the gold standard. Schaefer et al5 used an IRC in calves to demonstrate that the temperature of the orbital area, including the eyeball and its surroundings, varied less than other body surface areas. Furthermore, Scolari et al6 used an IRC to detect the rise and fall of the vulvar skin temperature of sows during estrus. Knizkova et al7 concluded that infrared thermography (IRT and IRC) can be used to predict and detect illnesses when taking into account the limitations of these techniques, such as environmental conditions, circadian rhythms, and dirt or foreign material on the animals, which may negatively influence the measurements and the usability of the acquired data. In contrast, studies performed by Chen and White8 using IRC in rabbits and by Dewulf et al9 and Wendt et al10 using IRC in swine concluded that the infrared method is not suited to detect fever.
A recent review by Stewart et al11 described changes in peripheral blood flow, resulting in alterations of skin temperature, that can be detected with infrared thermography in livestock. Areas innervated by the sympathetic nervous system, which has many capillary beds, are particularly sensitive to changes in blood flow. The sympathetic system also responds to stress and fever,12,13 both of which may be associated with farrowing.
Swine have an unusual means of regulating their core temperature. Changes in infrared measurements occur early during the course of disease.5 Because they lack sweat glands,14,15 swine reduce their core temperature by increasing the rate of peripheral blood flow through the skin, by panting, or by moistening the skin with water. However, panting and evaporation are less efficient regulation systems under some environmental conditions and are not always possible in confinement pig housing. The results of Godynicki et al16 and Ingram and Weaver17 regarding the skin of swine and the way that it is organized to emit heat also support the idea of using infrared images to detect body temperature.
The aim of this study was to determine the method of infrared thermography (IRC or IRT) and the anatomical sites best suited to detect fever under practical conditions in sows near farrowing, using digital rectal thermometry as the gold standard.
Materials and methods
The authors declare that all experiments comply with the current laws of Germany,18 where this study was performed. The animals were humanely treated during their day-to-day care by the owner and during our study.
Animals and housing system
The study was conducted on a farrow-to-finish farm with 340 breeding sows (Large White × German Landrace) and 17,000 finishing pigs. Measurements of body temperature by infrared methods took place in the farrowing room, where sows were housed starting at 1 week ante partum. Each sow was housed in a farrowing crate inside a farrowing pen during her time in the farrowing room. Farrowing pens were 1.80 m wide and 2.40 m long. Maximum length of the crate was 2.25 m. Piglets were able to move about freely in the pen. The piglet nest, on the side of the pen closest to the wall, could be heated with a heater located in the lid of the nest so that there was no effect on the sow. Some piglet nests were heated during measurements. Temperatures of all sows were measured within 3.5 days of farrowing.
During the study, sows were kept on partially slatted plastic floors without straw bedding, had ad libitum access to fresh water by nipple drinkers located in the trough at the front of the farrowing crate, and received manual feeding twice a day. The feed was a farm mixture containing barley, wheat, soy, and minerals. Feed allowance was individually adjusted for each sow after farrowing, taking into account litter size and body condition of the sow.
Some temperature data were excluded from the study because sows showed signs of illness, and some IRC images could not be analyzed.
Study design
Phase 1. Phase 1 was conducted in early summer using 15 multiparous sows, including one with reduced appetite and another with diarrhea. For 4 consecutive days, body temperatures of 10 sows confined in farrowing crates were measured twice daily, avoiding feeding times, for a total of eight measurements per sow and infrared target site. On the second and third days, body temperatures of another five sows were measured twice daily, again avoiding feeding times, for a total of 10 measurements per sow and infrared target site. Infrared temperature was measured by IRC and IRT in the following eight body surface target sites shown in Figure 1: the eye, the forehead, the back of the ear, the back (sacral and lumbar areas), the mammary gland, the lateral thigh, and the vulva.
Figure 1: Overall view of target anatomical sites for measuring body temperature in sows using infrared technology. Sites are identified as follows: 1 = back of the ear (between the transition of the pinna and the dorsal margo of the M cutaneous colli); 2 = lumbar area of the back (at the transition between the thoracic and lumbar vertebrae); 3 = sacral area of the back (between the last lumbar vertebra and the sacrum); 4 = vulva (halfway along the length of the vulva and 1 cm lateral to the labia pudenda); 5 = lateral thigh (15 cm below the hip and 5 cm caudal to the stifle joint); 6 = mammary gland (between the last two gland complexes); 7 = eye area; 8 = forehead (2 to 3 cm above the eye). 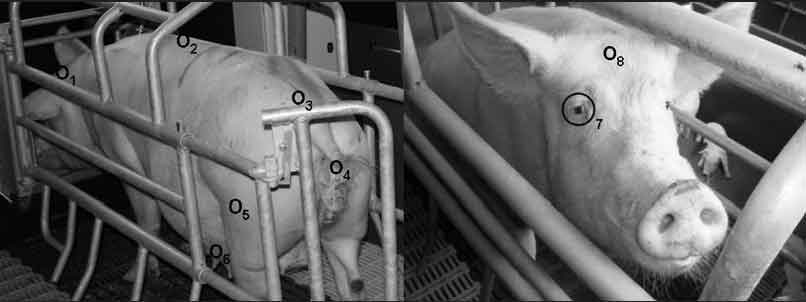 |
Phase 2. Phase 2 was conducted in autumn using 30 multiparous sows, including one with a vaginal discharge, one that was lame, and two with mastitis. The body temperature of each sow was measured once every second day, avoiding feeding times, resulting in a total of two measurements per sow and infrared target site. For Phase 2, the four target sites with the lowest levels of variation in Phase 1 were selected: the eye, the back of the ear, the mammary gland, and the vulva.
Techniques and methods of body temperature measurement
The following measuring procedures were applied to each sow: RT with a digital thermometer (ApoNorm; Hillscheid, Germany), body surface temperatures with an IRC (OPTRIS PI; Optris, Berlin, Germany), and body surface temperatures with an IRT (RAYMX4PTDG; Raytek, Berlin, Germany). Temperature measurements took approximately 10 minutes per sow in Phase 1 and approximately 6 minutes per sow in Phase 2. For infrared measurements, distance between the body surface and the device varied from 30 to 40 cm.
The emissivity for human skin, 0.985, was used for both infrared devices. The IRC functioned within a temperature range of -20°C to 900°C, with a sensitivity of 80 milliKelvins and a spectral range of 7.5 to 13 µm. The images had an optical resolution of 160 × 120 pixels. Each video was recorded with the IRC at the same distance and using the same order of target sites for every sow. The digital images were analyzed with Optris software (Optris PI Connect 2.0.2009.0). By determining narrower measurement areas, it was possible to focus more on the target area and to collect data regarding the maximal and average temperatures of each target area. Measurements were made with the IRT for approximately 5 seconds per site, and the mean for this time was recorded. The IRT was focused at the measuring area. The target site diameter was approximately 2 cm at a distance of 30 to 40 cm.
For the eye location, measurements were taken directly at the eye. Using the IRC, the mean temperature of a target site (2-cm diameter) and the maximal temperature of this measuring area were taken. The measuring point for the forehead location was 2 to 3 cm above the eye. Measurements for the back of the ear were made between the transition of the pinna and the dorsal margo of the M cutaneous colli. Measurements for the sacral location on the back were made between the last lumbar vertebra and the sacrum. Measurements for the lumbar location on the back were made at the transition between the thoracic and lumbar spine. Measurements for the mammary gland were made between the last two gland complexes. Measurements for the lateral thigh were made 15 cm below the hip bone and 5 cm caudal to the stifle joint. Measurements for the vulva were made halfway along the length of the vulva and 1 cm lateral to the labia pudenda.
Statistical analysis
Statistical analysis was performed using SAS 9.2 (SAS Institute, Inc, Cary, North Carolina). Phase 1 of the study was used to obtain a general idea about the variability of the temperature measurements at different anatomical sites using the IRC and IRT methods. For both Phase 1 and Phase 2 of the study, data were evaluated graphically by method and anatomical site using box-and-whisker plots. The goal was to identify sites that provided body temperatures with small interquartile ranges; minimizing variation from the reference temperature was a secondary concern. Median difference between the RT and each infrared temperature was calculated for each site. Phase 2 data were evaluated graphically according to the method of Bland and Altman,19 recommended by Grouven et al20 for comparison of the IRC and IRT methods. This statistical procedure is recommended for comparing two methods, since it not only considers the average difference (bias), but also puts emphasis on the variation of differences (d) between pairs of measurement values. The differences between measurements with both methods are plotted against the arithmetic mean of the two methods. Bias is expressed as mean difference and the 95% limits of agreement are expressed as d ± 2s, where s is the standard deviation of the differences. The range of the differences between the RT and each infrared temperature was tested for effect of infrared methods and locations with a two-factorial ANOVA model without interactions at a significance level of .05.
Results
Phase 1
Although sows were housed in crates for all temperature measurements, they were capable of considerable movement during the procedures. Infrared temperatures (IRC and IRT) measured at all eight body surface locations were lower and varied more than the rectal temperatures. At all sites except the thigh, the median infrared temperatures were higher using the IRC than the IRT. Among the eight target sites, variances were smaller for the eye, back of the ear, mammary gland, and vulva with both infrared methods (Figure 2); therefore, these four sites were used in Phase 2 of the study.
Figure 2: Phase 1 of study: body temperatures of 15 sows measured by digital rectal thermometer (rectal temperature; RT), infrared camera (IRC), and infrared thermometer (IRT) twice daily for 4 days (N = 90 values per box plot). Some data were excluded because of illness in sows or inability to analyze the IRC images. Infrared measurements were made at the eight anatomical sites described in Figure 1. Total time for measurements was approximately 10 minutes per sow. 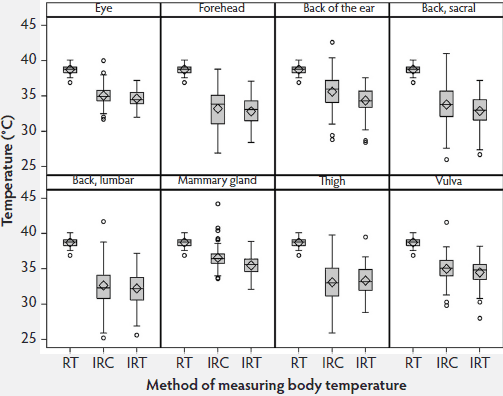 |
Phase 2
Data distribution in Phase 2 was similar to that in Phase 1 (Figure 3). Table 1 shows the median differences and interquartile ranges between the Phase 2 RT and infrared temperatures, sorted by anatomical site and method. The median differences between the RT and IRC temperatures were lower than those between the RT and IRT temperatures. At all sites, temperatures measured with the IRC were higher (average 36.93°C) than those measured with the IRT (average 35.00°C).
Table 1: Minimum, median, maximum, and range values for temperatures of sows measured by infrared camera (IRC), infrared thermometer (IRT), and digital rectal thermometer ( rectal temperature; RT) (Phase 2 of the study)
* Infrared thermography was performed at four of the anatomical sites described in Figure 1. Temperature measurements were made twice at an approximately 48-hour interval. † Range = maximum value – minimum value. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Figure 3: Phase 2 of study: body temperatures of 30 sows measured by digital rectal thermometer (rectal temperature; RT), infrared camera (IRC), and infrared thermometer (IRT) once every second day at four of the anatomical sites used in Phase 1 (the eye, the back of the ear, the mammary gland, and the vulva, described in Figure 1 (N = 68 values per box plot). Some data were excluded because of illness in sows or inability to analyze the IRC images. Total time for measurements was approximately 6 minutes per sow. 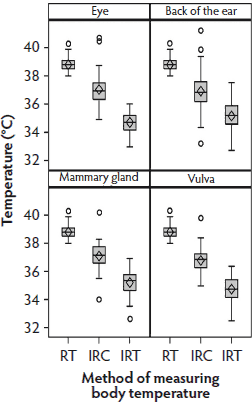 |
The Bland-Altman plot for RT-IRC differences and arithmetic means demonstrated that most values were within the 95% limit of agreement, but the range of individual temperature differences was considerably larger than those in the RT-IRT Bland-Altman plot (Figure 4).
Figure 4: Phase 2 of study: Bland-Altman plots of body temperatures of 30 sows measured by digital rectal thermometer (rectal temperature; RT) and infrared camera (IRC) at anatomical sites listed in Figure 3 and described in Figure 1 (N = 58 values per plot). Some data were excluded because of illness in sows or inability to analyze the IRC images. 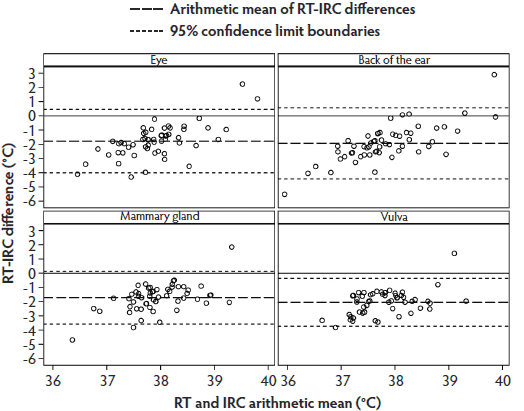 |
The mean range of the difference between RT and IRT across all locations (4.21°C) was significantly higher (P = .02) than the mean range of the difference between RT and IRC (6.67°C).
The Bland-Altman plot for RT and IRT temperatures (Figure 5) showed a similar data distribution as that for RT and IRC, although the individual temperature differences tended to be close to the arithmetic mean. Most values were within the 95% limit of agreement.
Figure 5: Phase 2 of study: Bland-Altman plots of body temperatures of 30 sows measured by digital rectal thermometer (rectal temperature; RT) and by infrared thermometer (IRT) at anatomical sites listed in Figure 3 and described in Figure 1 (N = 58 values per plot). Some data were excluded because of illness in sows or inability to analyze the IRT images. 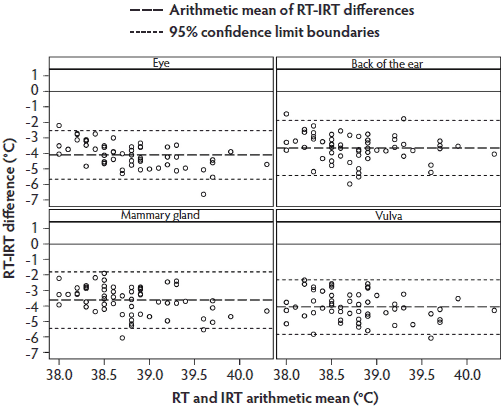 |
All data concerning the study sows, including those showing no clinical signs, were analyzed to determine if it is possible to detect fever in sows by one of the infrared methods. Rectal temperatures above the 90% quantile (≥ 39.8°C) were found in 10 individuals. When body temperature was measured at the back of the ear with the IRC, seven of these 10 sows were classified as febrile, with a 90% quantile of ≥ 38.1°C. When temperature was measured at the eye with the IRC, six of the 10 sows were classified as febrile, with a 90% quantile of ≥ 36.9°C.
Overall, the median difference between the IRC temperatures and the RT (1.8°C) was lower than that between the IRT temperatures and the RT (3.8°C).
Discussion
As observed in Phase 1 of the study, body surface temperatures measured with the infrared techniques at the lumbar and sacral areas of the back and at the thigh were not in close agreement with the RT. Temperature ranges at these locations were also highly variable compared with the RT ranges, possibly because the skin at locations such as the eye and back of the ear is not as thick as it is in the dorsal regions, as described by Sumena et al.21 Furthermore, the dorsal regions have a thick layer of fat which acts as insulation. In swine, the thickest layer of subcutaneous fat can be found in the dorsal neck region.21
Temperatures measured with the IRC and the IRT were lower than those measured with the rectal thermometer. The body location yielding the IRC and IRT measurements most similar to the RT was the mammary gland, followed by the vulva. However, for various reasons, including the suckling of the piglets and the recumbent positions of the sows, the mammary gland is not suitable for IRC and IRT measurements in practice, as the temperature of a piglet rather than the mammary gland might be recorded. The vulva is also not appropriate, as urine and excrement can negatively influence the measurements, as described by Knizkova et al.7 Therefore, the back of the ear and the area around the eye are possible locations for further investigation of infrared body-temperature measurements. The results of this study support those of Johnson et al4 and Traulsen et al.2 In both phases of this study, variation between the IRC temperatures and the RT was greater than that between the IRT temperatures and the RT in all locations except the mammary gland and vulva. However, IRC temperatures were higher than IRT temperatures, so the mean difference between the infrared temperature and the reference temperature was smaller for the IRC than for the IRT. One explanation for this finding is that it was possible to identify the highest temperature in every pixel of the video recordings obtained using the camera, while the IRT measured only the average temperature of the target area. Another explanation could be that evaporation or the nature and color of the skin surface influenced one method more than the other. The smaller variation between single measurements is more important than the difference between the infrared and the rectal temperature measurements when the goal is to monitor animal temperature over a period of days. Smaller variation is necessary to detect changes as early as possible, since variations as large as, or even larger than, the magnitude of expected changes require more measurements and hence more time to show significant differences.
The Bland-Altman plot showed that the lack of agreement between the RT and the infrared temperatures precludes replacement of the RT in clinically normal sows. This conclusion supports those of other authors.8-10 The 95% confidence interval for the IRC was closer to zero than that for the IRT, but bias was worse for the IRC than for the IRT. Variability of the IRT was lower than that of the IRC, as shown by the smaller 95% limit of agreement in the Bland-Altman plot, and IRT would therefore be preferable to IRC to detect changes in body-surface temperature.
Seven of 10 sows were identified as febrile using the IRC method at the back of the ear, while six of the 10 sows were identified as febrile using the IRC method at the eye location. This result supports those of Johnson et al,4 Schaefer et al,5 and Loughmiller et al.22 Loughmiller et al22 used infrared techniques to measure temperature at the scapula of pigs, concluding that it is possible to detect a febrile response using body-surface temperature. The greater degree of agreement between measurements made at the back of the ear and the eye locations seems to be due to the anatomy and the unusual method of thermoregulation in pigs. Because swine have only a few sweat glands and are forced to cool down by increasing the rate of blood flow through the skin,14,15 infrared technology may be a good method to detect this increase. Steward et al11 proposed that changes in peripheral blood flow can be detected with infrared technology, and our results agree with this. Vianna and Carrive23 conducted a study to detect changes in the temperature at the tail in connection with a fear reaction and demonstrated that it was possible to detect a decrease in temperature via infrared images. Our results also show that it is possible to detect changes in skin temperature.
The distance between the thermal camera and the animal strongly influences measured temperature, as described by Johnson et al.4 Infrared methods could be improved by further standardizing the locations of the measurements. During our measurements, some sows were moving considerably. Minimizing the size of the target area may also improve infrared techniques, especially for the IRT method. Measuring body temperature at the eye location may be difficult because of the eyelid opening and closing, and movement of the ears can also influence measurements.
In conclusion, the results of these studies showed that single-time measurements of body-surface temperature with either IRC or IRT did not deliver adequately reproducible results under field conditions. Further investigations and development (ie, computer programming) is needed before this method can be used routinely by practitioners as a monitoring tool. However, monitoring body temperatures of sows using infrared technology individually 1 week before farrowing could provide an average temperature for each sow. A rise in temperature during parturition and post partum might be a warning signal. The temperatures obtained using the infrared methods did not have a high level of agreement with the RT in clinically normal sows, but the RT and infrared measurements were in agreement in some febrile sows. Further studies including more febrile sows are necessary to confirm that fever can be detected using infrared techniques with a sensitivity of approximately 90%. This method provides the opportunity to measure body temperature continuously without contacting or stressing the animals and with a minimal risk of injuries.
Implications
• Under the conditions of this study, the eye and back of the ear are the most promising locations for practical application of infrared methods to measure body temperature in sows.
• Variability should be small to detect changes in body-surface temperature for long-term monitoring; therefore, the IRT is more useful than the IRC because, under the conditions of this study, variability was smaller for the IRT.
• Using the infrared techniques investigated, single body-temperature measurements are not appropriate to detect fever in swine.
Acknowledgements
The authors thank Diplom-Informatiker Christian Manteuffel (Leibniz-Institute for Farm Animal Biology Dummerstorf, Department of Behavioral Physiology) and Big Dutchman Pig Equipment GmbH for their assistance. Subsidies were received from funds provided by the Federal Ministry of Nutrition, Agriculture and Consumer Protection via the German Federal Agency for Agriculture and Food in the framework of the program to support innovation.
Conflict of interest
None reported.
References
1. Plonait H. Geburt, Puerperium und perinatale Verluste [Birth, puerperium and perinatal mortality]. In: Waldmann KH, Wendt M, Plonait H, Bickhardt K, eds. Lehrbuch der Schweinekrankheiten [Textbook of Pig Diseases]. 4th ed. Hannover, Germany: Parey Verlag; 2004:493–502.
2. Traulsen I, Naunin K, Müller K, Krieter J. Application of infrared thermography to measure body temperature of sows. Züchtungskunde. 2010;82:437–446.
3. Röhlinger P, Grunow C, Reichmann A, Zimmerhackel M. Voruntersuchungen zur Ermittlung der Anwendungsgebiete der Infrarotmeßtechnik in der Veterinärmedizin [Preliminary studies to determine the use of infrared technique in veterinary medicine]. Monatshefte für die Veterinärmedizin. 1979;287–291.
4. Johnson SR, Rao S, Hussey SB, Morley PS, Traub-Dargatz JL. Eye thermographic temperature as an index to body temperature in ponies. J Equine Vet Sci. 2011;31:63–66.
5. Schaefer AL, Cook N, Tessaro SV, Deregt D, Desroches G, Dubeski PL, Tong AKW, Godson DL. Early detection and prediction of infection using infrared thermography. Can J Anim Sci. 2004;84:73–80.
6. Scolari SC, Clark SG, Knox RV, Tamassia MA. Vulvar skin temperature changes significantly during estrus in swine as determined by digital infrared thermography. J Swine Health Prod. 2011;19:151–155.
7. Knizkova I, Kunic P, Gürdil G, Pinar Y, Selvi K. Applications of infrared thermography in animal production. Anadolu J Agricult Sci. 2007;22:329–336.
8. Chen PH, White CE. Comparison of rectal, microchip transponder, and infrared thermometry techniques for obtaining body temperature in the laboratory rabbit (Oryctolagus cuniculus). J Amer Assoc Anim Sci. 2006;45:57–63.
9. Dewulf J, Koenen F, Laevens H, de Kruif A. Infrared thermometry is not suitable for the detection of fever in pigs. Vlaams Diergeneeskundig Tijdschrift. 2003;72:373–379.
10. Wendt M, Eickhoff K, Koch R. Die Messung der Hauttemperatur als Methode zur Erkennung fieberhaft erkrankter Schweine [Measuring the skin temperature as a method to detect pigs with elevated body temperature]. Deutsche Tierärztliche Wochenschrift. 1997;104:29–33.
11. Stewart M, Webster JR, Schaefer AL, Cook NJ, Scott SL. Infrared thermography as a non-invasive tool to study animal welfare. Anim Welf. 2005;14:319–325.
12. Oka T, Oka K, Hori T. Mechanisms and mediators of psychological stress-induced rise in core temperature. Psychosom Med. 2001;63:476–486.
13. Nakamura K. Central circuitries for body temperature regulation and fever. Amer J Physiol Regul-Integr Comp Physiol. 2011;301:R1207-R1228.
14. Montagna W, Yun JS. The skin of the domestic pig. J Invest Dermatol. 1964;43:11–21.
15. Moritz AR, Henriques FC. Studies of thermal injury. Part 2: The relative importance of time and surface temperature in the causation of cutaneous burns. Amer J Path. 1947;23:695–720.
16. Godynicki VS, El-Bab MRF, Schwarz R. The vascular pattern in the skin of the pig at the time of birth. Anatomia Histologia Embryologia. 1985;14:304–315. doi:10.1111/j.1439-0264.1985.tb00826.x.
17. Ingram DL, Weaver ME. A quantitative study of blood vessels of the pig’s skin and the influence of environmental temperature. Anat Rec. 1969;163:517–524.
18. European Council. Council Directive 98/58/EC of 20 July 1998 concerning the protection of animals kept for farming. Official Journal of the European Communities 8.8.98. Available at: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:1998:221:0023:0027:EN:PDF. Accessed 13 April 2013.
19. Bland JM, Altman DG. Measuring agreement in method comparison studies. Stat Methods Med Res. 1999;8:135–160. doi:10.1177/096228029900 800204.
20. Grouven U, Bender R, Ziegler A, Lange S. Vergleich von Messmethoden [Comparison of measuring methods]. Deutsche Medizinische Wochenschrift. 2007;132:e69–e73.
21. Sumena KB, Lucy KM, Chungath JJ, Ashok N, Harshan KR. Morphology of the skin in Large White Yorkshire pigs. Ind J Anim Res. 2010;44:55–57.
22. Loughmiller JA, Spire MF, Dritz SS, Fenwick BW, Hosni MH, Hogge SB. Relationship between mean body surface temperature measured by use of infrared thermography and ambient temperature in clinically normal pigs and pigs inoculated with Actinobacillus pleuropneumoniae. Amer J Vet Res. 2001;62:676–681.
23. Vianna DML, Carrive P. Changes in cutaneous and body temperature during and after conditioned fear to context in the rat. Euro J Neurosci. 2005;21:2505–2512.