| Brief communication | Peer reviewed |
Cite as: Collins AM, Fell SA, Barchia IM. Cleaning and disinfection with Virkon S significantly reduces Lawsonia intracellularis survival and transmission to naive pigs. J Swine Health Prod. 2013;21(3):144–147.
Also available as a PDF.
SummaryLawsonia intracellularis infection was transmitted to naive pigs introduced into pens contaminated by pigs with proliferative enteropathy. However, cleaning pens with a pressure hose then disinfecting with potassium peroxymonosulfate was able to prevent transmission of infection in a second group of naive pigs. | ResumenLa infección por Lawsonia intracellularis se transmitió a cerdos libres introducidos en corrales contaminados por cerdos con enteropatía proliferativa. Sin embargo, la limpieza de corrales con una manguera de presión seguida de la desinfección con peroxymonosulfato de potasio logró prevenir la transmisión de la infección en un segundo grupo de cerdos libres. | ResuméL’infection par Lawsonia intracellularis a été transmise à des porcs naïfs introduits dans des enclos contaminés par des porcs atteints d’entéropathie proliférative. Par contre, un nettoyage des enclos avec de l’eau sous pression puis désinfection avec du peroxymonosulfate de potassium a permis d’empêcher la transmission de l’infection à un second groupe de porcs naïfs. |
Keywords: swine, Lawsonia intracellularis, transmission, disinfection
Search the AASV web site
for pages with similar keywords.
Received: February 21, 2012
Accepted: August 17, 2012
Lawsonia intracellularis is an important pathogen of pigs, causing acute intestinal hemorrhage (proliferative hemorrhagic enteropathy; PHE) in naive adult pigs and the more chronic form of proliferative enteropathy (PE) in growing pigs.1 Lawsonia intracellularis is predominantly transmitted between pigs via ingestion of contaminated feces.2,3 Pigs clinically affected with PE can shed at least 3 × 108 L intracellularis per gram of feces,4 so less than 1 g of contaminated feces contains sufficient L intracellularis to infect a naive pig.5 Outbreaks of PE are associated with frequent commingling of pigs, increasing herd size, and introduction of new pigs.6,7 In addition, the risk of L intracellularis infection was approximately six times greater with continuous-flow management than with all-in, all-out (batch) management,8 suggesting that management practices that improve biosecurity and hygiene may aid in the control of disease.
Cleaning pens with a pressure hose will remove most feces, but bacteriostatic or bactericidal disinfectants may also be needed to reduce bacterial survival. In vitro studies have indicated that the iodophor povidone iodine and quaternary ammonium compounds are both effective against L intracellularis in the absence of feces.3,9 However, the efficacy of many disinfectants may be reduced in swine housing environments in the presence of fecal organic matter.
Survival of L intracellularis in conventional swine facilities can be investigated only by monitoring transmission of infection to naive pigs, as L intracellularis viability cannot be determined by fecal culture. This study investigated survival and transmission of L intracellularis after pressure cleaning and disinfecting contaminated pens with the oxidizing agent potassium peroxymonosulfate (Virkon S; Antec International, Sudbury, Suffolk, United Kingdom).
Materials and methods
Animal experiments were performed according to the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes and approved by the institute’s Animal Ethics Committee.
Study design
Inoculated pigs. Thirteen nursery pigs (Large White × Landrace) were selected from a closed herd with no clinical signs of PE. Piglets and sows were tested 8 days post farrowing for fecal shedding of L intracellularis by quantitative real-time polymerase chain reaction (qPCR), described previously.4 At weaning (3 weeks of age), the pigs were moved to a research facility that had been cleaned and disinfected with Virkon S. Pigs were housed in two adjacent pens with wire mesh dividers and 50% concrete slatted floors, with ad libitum access to water. A commercial pelleted diet containing oxytetracycline (300 g per tonne) was fed from 3 to 17 weeks of age. Minimum and maximum temperatures within the barn were recorded daily.
Blood and feces were collected weekly from each pig between 4 and 17 weeks of age and tested for serum IgG antibodies to L intracellularis by an indirect fluorescent antibody test (IFAT) and by the qPCR for L intracellularis in feces. Oxytetracycline was removed from the diet when the pigs were 17 weeks of age, and the pigs were divided into two groups (n = 6 and 7), moved into a separate room within the same building, and housed in two cleaned and empty pens 10 m apart. No other pigs were housed in the facility. Five days later, all 13 pigs were orally inoculated via gastric tube with approximately 5 × 109 L intracellularis extracted from the mucosa of a naturally infected finisher pig with gross and histological lesions of PHE.5 Between 7 and 21 days post inoculation (dpi), each group of pigs was moved between the two pens three times per week to ensure similar numbers of L intracellularis were shed in each pen. Blood samples were collected at 0 and 21 dpi and tested by IFAT for serum IgG to L intracellularis, and feces were collected from individual pigs at 0, 4, 7, 10, 14, 18, and 21 dpi for quantification of L intracellularis shed in feces by qPCR. Nucleic acids were extracted from 0.2 g of individual fecal samples using a MagMax Viral RNA isolation kit (Ambion Life Technologies, Carlsbad, California) on a Kingfisher magnetic particle processor (Thermo Fisher, Vantaa, Finland) according to the manufacturer’s instructions. Amplified L intracellularis DNA was quantified with seeded fecal standards in a real-time PCR assay.
At 21 dpi, the pigs were euthanized and intestinal sections were preserved for histopathology and immunohistochemistry (IHC). Sections of formalin-fixed tissue were stained for the presence of L intracellularis antigen using a monoclonal antibody (IgG4) directed against an outer membrane protein of L intracellularis10 (which does not react by Western blot with Campylobacter species commonly found in pig intestine11), using positive and negative control sections. The percentage area affected with adenomatosis (hematoxylin and eosin stain) and containing intracellular L intracellularis (IHC) was averaged from 20 individual scores (0% to 100% of crypt area) for each ileal section.
Once emptied, one pen was cleaned with cold water using a high-pressure hose to remove feces from the floors, walls, drinkers, and feeders. A 1% w/v solution of Virkon S was sprayed over all surfaces in the pen later the same day and the pen was left to dry naturally over 14 days. The other pen was left empty and not cleaned over the same 2-week period.
Naive pigs exposed to clean and dirty pens. Two groups of naive weaner pigs (3 weeks old) from the same closed herd were introduced into either the dirty pen (n = 10) or the cleaned and disinfected pen (n = 12) two weeks after the original pens were emptied and cleaned. Pigs in the dirty pen were allowed to voluntarily ingest feces contaminated with L intracellularis. Strict quarantine was maintained between the pens, with dedicated boots and clothing for each pen. Blood and feces were collected weekly from each pig over a 56-day period to monitor for L intracellularis infection by IFAT and fecal qPCR.
Serological and PCR testing, histological examination, and calculations
A previously described IFAT assay was used to determine serum IgG antibody titres to L intracellularis.12 One-twentieth volume of DNA extracted with a MagMax kit was amplified using a published quantitative real-time PCR method (qPCR).13 A standard curve was plotted from the five concentrations of seeded feces (108 to 104 L intracellularis per gram feces), and the number of L intracellularis organisms in each sample was determined using the regression equation from the standard curve (R2 = 0.988). The specificity, sensitivity, and reproducibility of this qPCR have been described previously.4 The number of L intracellularis organisms shed per gram of feces was determined for each inoculated pig and averaged for the period between 14 and 21 dpi. The numbers of L intracellularis shed per gram of feces were log10 transformed for the inoculated pigs and then correlated with antibody titer and the percentage area of adenomatosis. The number of L intracellularis organisms shed per gram of feces was determined for each naive weaner pig housed in either a cleaned or dirty pen. The mean number of Lawsonia intracellularis organisms shed by naive pigs housed in the dirty pen was determined by log10 transforming the qPCR result with 95% confidence intervals (GenStat, 13th ed; VSN International, Oxford, United Kingdom). As L intracellularis organisms were not detected in the feces of any naive pig housed in the cleaned pen, we needed to use a statistical test to compare the outcome for pigs in dirty pens to the observed zero results for pigs in cleaned pens. In addition, due to the high variability in the number of L intracellularis shed and the small sample size, we approached the statistical analysis using proportions of positive pigs. We used a one-sided Agresti Coull test to analyze the effect of cleaning and disinfecting pens. Although we did not detect L intracellularis shedding in any of the 12 pigs housed in cleaned pigs, we cannot be certain that all pigs would be negative for shedding if we had a sample size of 1000 pigs. Therefore, we assumed a measurement error of 5% for the qPCR to detect L intracellularis. The critical value to test the treatment was determined using the following formula: critical proportion = p + z1-α √(p(1-p)) ÷ n, where p = measurement error of 5%, α = .05, n = sample number (10), and z1-α = 1.645. Therefore, for the cleaning and disinfection treatment to significantly reduce L intracellularis shedding, the critical proportion of positive pigs in dirty pens was > 0.16.
Results
Monitoring infection in finisher pigs orally inoculated with L intracellularis
Daily temperatures in the pens varied from an overnight minimum of 9°C to a daytime maximum of 18°C from the day of infection to 11 weeks later. Neither L intracellularis DNA nor serum IgG antibodies were detected by qPCR or IFAT, respectively, in any pig prior to oral inoculation at 17 weeks of age. All inoculated pigs seroconverted by 21 dpi and shed between 1.2 × 105 and 5 × 107 L intracellularis per gram of feces between 14 and 21 dpi. Diarrhea was not observed in inoculated pigs. The area affected with histological lesions of PE varied substantially between pigs (5% to 98% of ileal sections). The number of L intracellularis shed at 18 and 21 dpi correlated well with the histopathology score (r = 0.77 and r = 0.68, respectively) and antibody titer at 21 dpi in individual pigs (r = 0.77 and r = 0.78 respectively).
Monitoring L intracellularis infection in naive pigs introduced into cleaned and dirty pens
Diarrhea was not observed in the naive pigs following their introduction into the cleaned or dirty pens. Lawsonia intracellularis DNA was detected in the feces of eight of the 10 pigs in the dirty pen, beginning as early as 21 days post entry and persisting in one pig until 49 days post entry (Figure 1). Lawsonia intracellularis DNA was not detected in the 12 pigs introduced into the cleaned pen over the same period. The mean number of L intracellularis shed was low throughout the trial, with only three pigs shedding > 2 × 105 L intracellularis at a single time point. The proportions of pigs housed in dirty pens shedding L intracellularis at 21, 28, and 35 days post entry (0.4, 0.5, and 0.4, respectively) were significantly higher than the critical theoretical proportion of positive pigs housed in the cleaned pens (0.16). However, by days 42 and 49 post entry, the proportions of pigs housed in dirty pens shedding L intracellularis (0.1 and 0.1, respectively) were not significantly different from the theoretical proportion of positive pigs housed in the cleaned pens (0.16). Antibodies to L intracellularis were detected in the same eight PCR-positive pigs introduced into the dirty pen, beginning at 28 days post entry, with a peak in titer at 42 days post entry. No L intracellularis antibodies were detected in pigs introduced into the cleaned pen for 56 days post entry.
Figure 1: The estimated population mean number of Lawsonia intracellularis (LI) organisms (log10 transformed) shed per gram of feces, with 95% confidence intervals, in 10 previously naive nursery pigs housed in a pen that had housed finisher pigs inoculated with LI. The pen had been left empty for 2 weeks and was not cleaned or disinfected before introduction of nursery pigs at 3 weeks of age. Number of LI organisms was determined by quantitative polymerase chain reaction. 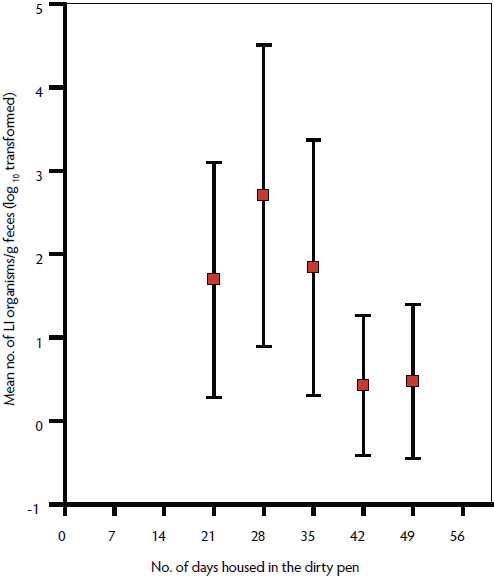 |
Discussion
To the authors’ knowledge, this is the first study to identify cleaning practices that can significantly reduce the survival and transmission of L intracellularis in a conventional swine-production environment. This study highlights the combined effectiveness of cleaning and treatment with Virkon S to reduce numbers of L intracellularis in conventional concrete slatted pens and prevent transmission to naive pigs. However, this study did not investigate whether pressure-hosing alone can prevent L intracellularis transmission.
The bactericidal activity of similar concentrations of potassium peroxymonosulfate (Virkon S) against L intracellularis was demonstrated previously in vitro in the presence of organic matter and over a wide range of L intracellularis concentrations (105 to 108 per g or mL of feces).3,9 Potassium peroxymonosulfate acts by forming hydroxyl radicals that oxidize proteins, carbohydrates, and lipids in the membranes of both bacterial and mammalian cells. Membrane disruption is followed by oxidation of intracellular molecules, including enzymes and nucleic acids.14,15 Disruption of cultured L intracellularis membranes by 1% Virkon S has been demonstrated by electron microscopy.9
Of equal interest is the finding that L intracellularis bacteria were able to survive in a contaminated pen for 2 weeks at temperatures between 9°C and 18°C and infect naive pigs in dirty pens. Lawsonia intracellularis survival for > 2 weeks was demonstrated in an earlier study where naive pigs were orally dosed with feces containing similar numbers of L intracellularis.3 However, in the current study, we relied on voluntary ingestion of contaminated feces by naive pigs in conventional pig housing conditions. We have no way of quantifying the number of viable L intracellularis ingested by the naive pigs in earlier studies3 or the current study. However, pigs dosed with as few as 105 L intracellularis develop infection, although the onsets of fecal shedding and seroconversion are delayed relative to pigs dosed with 107 or 109 L intracellularis,5,16 as was demonstrated in the current study. The inability to detect L intracellularis infection in two pigs in the dirty pens may be due to the low dose of viable L intracellularis or the limited sensitivity of our assays.
This study demonstrates the importance of cleaning and good biosecurity in swine housing facilities to aid in the control of PE. Cleaning concrete-floored pens and disinfecting with Virkon S between batches of pigs can reduce survival and transmission of L intracellularis to naive pigs. This is supported by the significantly lower risk of L intracellularis infection in batch-reared pigs (all-in, all-out) than in pigs raised in continuous-flow production systems.9 The ease of cleaning pen floors will have an impact on the efficacy of improved hygiene practices to reduce disease. Partially slatted floors were identified as a risk factor for diarrhea in grower-finisher pigs in an English postal survey,17 which may be explained by the build-up of manure and the difficulty in cleaning slatted floors.
Further studies are needed to test the effects of washing without disinfection and the efficacy of quaternary ammonium compounds, iodophors, and other disinfectants in the presence of organic matter in conventional and straw-based housing.
Implications
• L intracellularis can survive in feces in contaminated pig pens for at least 2 weeks at temperatures between 9° and 18°C.
• Naive pigs introduced into dirty pens can ingest sufficient viable L intracellularis in this environment to become infected.
• Power hosing and disinfecting pens with Virkon S can significantly reduce the survival and transmission of L intracellularis to naive pigs.
Acknowledgments
The authors wish to thank Dr Graeme Eamens and Jocelyn Gonsalves for their veterinary and laboratory support. This research was funded by the Australian government through the Pork Cooperative Research Centre.
Conflict of interest
None reported.
References
1. Lawson GHK, Gebhart CJ. Proliferative enteropathy. J Comp Path. 2000;122:77–100.
2. Jordan DM, Knittel JP, Schwartz KJ, Roof MB, Hoffman LJ. A Lawsonia intracellularis transmission study using a pure culture inoculated seeder-pig sentinel model. Vet Microbiol. 2004;104:83–90.
3. Collins AM, Love RJ, Pozo J, Smith SH, McOrist S. Studies on the ex-vivo survival of Lawsonia intracellularis. Swine Health Prod. 2000;8:211–215.
4. Collins AM, Fell S, Pearson H, Toribio JA. Colonisation and shedding of Lawsonia intracellularis in experimentally inoculated rodents and in wild rodents on pig farms. Vet Microbiol. 2011;150:384–388.
5. Collins AM, Love RJ. Re-challenge of pigs following recovery from proliferative enteropathy. Vet Microbiol. 2007;120:381–386.
6. Smith SH, McOrist S, Green LE. Questionnaire survey of proliferative enteropathy on British pig farms. Vet Rec. 1998;142:690–693.
7. Love RJ, Love DN, Edwards MJ. Proliferative haemorrhagic enteropathy in pigs. Vet Rec. 1977;100:65–68.
*8. Collins AM, Love RJ. Risk factors associated with Lawsonia intracellularis infection. Proc Australasian Pig Science Assoc. Fremantle, Australia. 2003;28.
9. Wattanaphansak S, Singer RS, Gebhart CJ. Evaluation of in vitro bactericidal activity of commercial disinfectants against Lawsonia intracellularis. J Swine Health Prod. 2010;18:11–17.
10. McOrist S, Boid R, Lawson GHK, McConnell I. Monoclonal antibodies to intracellular campylobacter-like organisms of porcine proliferative enteropathies. Vet Rec. 1987;121:421–422.
11. McOrist S, Boid R, Lawson GHK. Antigenic analysis of Campylobacter species and an intracellular Campylobacter-like organism associated with porcine proliferative enteropathies. Infect Immun. 1989;57:957–962.
12. Knittel JP, Jordan DM, Schwartz KJ, Janke BH, Roof MB, McOrist S, Harris DL. Evaluation of antemortem polymerase chain reaction and serologic methods for detection of Lawsonia intracellularis-exposed pigs. Am J Vet Res. 1998;59:722–726.
13. Nathues H, Holthaus K, Gross Beilage E. Quantification of Lawsonia intracellularis in porcine feces by real-time PCR. J Appl Microbiol. 2009;107:2009–2016.
14. McDonnell G. Peroxygens and other forms of oxygen: Their use for effective cleaning, disinfection and sterilization. In: Zhu, PC, ed. New Biocides Development: The Combined Approach of Chemistry and Microbiology. ACS Symposium Series; 2007;967: 292–308. doi:10.1021/bk-2007–0967.ch013.
15. Russell AD. Similarities and differences in the responses of microorganisms to biocides. J Antimicrobiol Chemotherapy. 2003;52:750–763.
16. Paradis MA, Gebhart CJ, Toole D, Vessie G, Winkelman NL, Bauer SA, Wilson JB, McClure CA. Subclinical ileitis: Diagnostic and performance parameters in a multi-dose mucosal homogenate challenge model. J Swine Health Prod. 2012;20:137–141.
17. Pearce GP. Epidemiology of enteric disease in grower-finisher pigs: a postal survey of pig producers in England. Vet Rec. 1997;144:338–342.
*Non-refereed reference.