| Original research | Peer reviewed |
Cite as: Jacques-Simon R, Millien M, Flanagan JK, et al. A field and laboratory investigation of viral diseases of swine in the Republic of Haiti. J Swine Health Prod. 2013;21(3):130–138.
Also available as a PDF.
SummaryObjective: To confirm the prevalence of teschovirus encephalomyelitis in multiple regions in Haiti and to identify other viral agents present in the swine population. Materials and methods: A field investigation was conducted on 35 swine premises located in 10 regions. Sera from 109 pigs, including 23 sick and 86 apparently healthy pigs, blood samples from 21 sick pigs, and brains, spinal cords, and other tissue samples from eight humanely euthanized pigs were collected and tested. Results: Of the 109 serum samples, 49.5% and 58.7% were positive for antibodies to porcine teschovirus type 1 (PTV-1) and porcine circovirus type 2 (PCV-2), respectively. Additionally, 7.3%, 11.9%, and 22.0% of sera were positive for antibodies to porcine reproductive and respiratory syndrome virus (PRRSV) and swine influenza virus (SIV) H3N2 and H1N1, respectively. Among the 54 sera positive for antibodies to PTV-1, 35 (64.8%) were also positive for antibodies to PCV-2. Classical swine fever virus (CSFV) was isolated from five sera. These results confirm that teschovirus encephalomyelitis is prevalent in multiple regions in Haiti, including areas near the border with the Dominican Republic, and that several other viral disease agents, ie, CSFV, PCV-2, PRRSV, and SIV, are present in the Haitian swine population. Implications: Due to the close proximity of the Hispaniola to Puerto Rico, a territory of the United States, and the large number of direct flights from the Hispaniola to the United States, the risk of introducing the viral diseases mentioned in this paper into the North America swine population is high. | ResumenObjetivo: Confirmar la prevalencia del virus teschovirus causante de encefalomielitis en múltiples regiones en Haití e identificar otros agentes virales presentes en la población porcina. Materiales y métodos: Se realizó una investigación de campo en 35 instalaciones porcinas localizadas en 10 regiones. Se recolectaron y analizaron los sueros de 109 cerdos, incluyendo 23 cerdos enfermos y 86 aparentemente sanos, 21 muestras de sangre de cerdos enfermos, y cerebros, médulas espinales, y otras muestras de tejido de ocho cerdos que fueron eutanasiados. Resultados: De las 109 muestras de suero, 49.5% y 58.7% resultaron positivos a anticuerpos contra el teschovirus porcino tipo 1 (PTV-1 por sus siglas en inglés) y el circovirus porcino tipo 2 (PCV-2 por sus siglas en inglés), respectivamente. Además, 7.3%, 11.9%, y 22.0% de los sueros resultaron positivos a los anticuerpos contra el virus del síndrome reproductivo y respiratorio porcino (PRRSV por sus siglas en inglés) y al virus de la influenza porcina (SIV por sus siglas en inglés) H3N2 y el H1N1, respectivamente. Entre los 54 sueros positivos a los anticuerpos contra el PTV-1, 35 (64.8%) también resultaron positivos a los anticuerpos contra el PCV-2. Se aisló el virus de la fiebre porcina clásica (CSFV por sus siglas en inglés) de cinco sueros. Estos resultados confirman que el teschovirus que produce encefalomielitis es prevalente en múltiples regiones de Haití, incluyendo áreas cerca de la frontera con la República Dominicana, y que algunos otros agentes de enfermedad viral, ie, CSFV, PCV-2, PRRSV, y SIV, están presentes en población porcina Haitiana. Implicaciones: Debido a la cercanía de La Española con Puerto Rico, un territorio de los Estados Unidos, y al gran número de vuelos directos de La Española a los Estados Unidos, el riesgo de introducir las enfermedades virales mencionadas en este escrito a la población porcina de Norte América es alto. | ResuméObjectif: Confirmer la prévalence de l’encéphalomyélite à teschovirus dans de nombreuses régions à Haïti et identifier d’autres agents viraux présents dans la population porcine. Matériels et méthodes: Une enquête sur le terrain a été réalisée sur 35 sites situés dans 10 régions. On a prélevé et testé du sérum provenant de 109 porcs, incluant 23 malades et 86 apparemment en santé; des échantillons de sang provenant de 21 porcs malades; et le cerveau, la moelle épinière, et d’autres échantillons de tissu de huit porcs euthanasiés humainement. Résultats: Des 109 échantillons de sérum, 49,5% et 58,7% étaient positifs, respectivement, pour la présence d’anticorps contre le teschovirus porcin de type 1 (PTV-1) et le circovirus porcin de type 2 (PCV-2). De plus, 7,3%, 11,9%, et 22,0% des sérums étaient positifs pour la présence d’anticorps contre le virus du syndrome reproducteur et respiratoire porcin (PRRSV) et le virus de l’influenza porcin (SIV) H3N2 et H1N1, respectivement. Parmi les 54 sérums positifs pour la présence d’anticorps contre PTV-1, 35 (64,8%) étaient également positifs pour la présence d’anticorps contre PCV-2. Le virus de la peste porcine classique (CSFV) a été isolé de cinq échantillons de sérum. Ces résultats confirment que le teschovirus de l’encéphalomyélite est prévalent dans de nombreuses régions à Haïti, incluant des régions proches de la frontière avec la République Dominicaine, et que plusieurs autres virus responsables de maladie virales, ie, CSFV, PCV-2, PRRSV, et SIV sont présents dans la population porcine haïtienne. Implications: Compte tenu de la proximité rapprochée de l’île d’Hispaniola à celle de Puerto Rico, un territoire appartenant aux États-Unis, et du grand nombre de vols aériens directs en provenance d’Hispaniola vers les États-Unis, le risque d’introduire les maladies virales mentionnées dans cet article dans la population porcine Nord-Américaine est élevé. |
Keywords: swine, viral disease, porcine teschovirus, teschovirus encephalomyelitis, Haiti
Search the AASV web site
for pages with similar keywords.
Received: April 19, 2012
Accepted: August 28, 2012
Teschovirus encephalomyelitis (previously called Teschen disease) is an acute disease that affects pigs, causing central nervous system (CNS) dysfunctions.1 The infectious agent that causes this disease, as well as less pathogenic strains, belong to a single viral species called porcine teschovirus (PTV), in the genus Teschovirus, family Picornaviridae.1,2 There are at least 11 distinct serotypes of PTV: PTV-1 through PTV-11.1,2 Some strains of PTV-1 cause severe teschovirus encephalomyelitis, while other strains, as well as other PTV serotypes, cause milder disease or inapparent infections in pigs.1,2 The first clinical signs of teschovirus encephalomyelitis usually appear after an incubation period of 10 to 20 days and may include fever, anorexia, uncoordinated movements, and locomotive disorders.3 This phase continues with tremors, nystagmus, opisthotonos, general deterioration, and convulsions, and ends with paralysis of hindquarters. The disease affects pigs of all ages, with morbidity and mortality rates of 40% to 60% and 40% to 50%, respectively.3 Porcine teschovirus can enter the body by the oral route and multiply in the gastrointestinal tract and associated lymphoid tissues, including tonsils.2 The virus is excreted in feces and urine for several weeks. The main route of transmission is fecal-oral, directly or indirectly from contaminated food or water.3 Porcine teschovirus infection causes a lasting immunity. As teschovirus encephalomyelitis is now rare in the world, vaccines are no longer available.3
In Haiti, the swine population is currently estimated at 600,000 pigs, distributed mainly in backyard premises (written communication, Drs Max Millien and Rodney Jacques-Simon, 2012). With very little hard currency in the countryside, pigs serve as living savings accounts for farmers and are sold or slaughtered to pay for marriages, medical emergencies, schooling, or seeds for crops.4 Classical swine fever (CSF) was introduced into Haiti for the first time in 1920 and remained endemic until 1984, when the entire swine population of the island of Hispaniola, which is shared by Haiti and the Dominican Republic, was slaughtered because of an outbreak of African swine fever (ASF).5 Classical swine fever re-entered Haiti in 1996 and has since persisted in the swine population.5 For control of CSF, pigs are currently vaccinated once a year with a live attenuated vaccine (Chinese strain).
Outbreaks of severe teschovirus encephalomyelitis in pigs began in the Lower Artibonite Valley and the Lower Plateau of Haiti in February and March 2009. An estimated 1500 backyard pigs became sick, and approximately 700 of them died or were culled in the outbreak.6 Morbidity and mortality were estimated at 60% and 40%, respectively, and PTV-1 was isolated from brain samples of sick pigs at the National Veterinary Services Laboratories (NVSL), United States Department of Agriculture (USDA) in Ames, Iowa, and the Foreign Animal Disease Diagnostic Laboratory (FADDL), Plum Island, New York.6 The entire genome of the Haitian PTV-1 was sequenced, and phylogenetic analyses on the polyprotein of PTV strains indicate that the Haitian isolate is most closely related to other PTV-1 strains, including the strain Konratice which was isolated in Czechoslovakia from pigs with porcine viral encephalomyelitis (Teschen disease).6 One of two pigs inoculated with the Haitian PTV-1 isolate showed typical clinical signs of teschovirus encephalomyelitis, including paralysis of the hindquarters, on day 32 post inoculation at the NVSL (e-mail communication, 2011, John J. Schiltz, DVM, Veterinary Medical Officer of the Diagnostic Virology Laboratory of the NVSL). After the described initial diagnosis of teschovirus encephalomyelitis in Haiti, multiple small outbreaks of the disease were frequently reported from many regions in the country, including areas near the border with the Dominican Republic.
Porcine teschovirus infections often do not produce clinical signs.1 High-virulent strains of PTV were not reported in the Western hemisphere until the outbreak in Haiti. Factors responsible for the expression of the severe teschovirus encephalomyelitis in Haiti are not known. We speculated other disease agents may be present in the Haitian swine population in addition to CSF virus (CSFV), PTV (eg, porcine circovirus type 2 [PCV-2], and porcine reproductive and respiratory syndrome virus [PRRSV]); that the immunosuppressive effect of these agents may have facilitated the expression of PTV; and that adding commercially available vaccines for these agents to the ongoing CSF vaccine program may be beneficial to the control of teschovirus disease. In addition, although other viral agents for swine, including swine influenza virus (SIV), pseudorabies virus (PRV), encephalomyocarditis virus (EMCV), and hemagglutinating encephalomyelitis virus (HEV), did not appear to be an issue in Haiti, information on their presence in the country was not known. We conducted this study to confirm the prevalence of teschovirus encephalomyelitis in multiple regions in Haiti and to determine the presence of other viral disease agents, including PCV-2, PRRSV, SIV, PRV, EMCV, and HEV in the swine population of these regions in the process of developing strategies for the control of the teschovirus disease in the country.
Materials and methods
In this study, sampled animals were treated according to the established standards for the humane handling, care, and use of animals specified in the regulation of the Animal and Plant Health Inspection Service of the USDA regarding animal welfare.7
Field investigation and sample collection
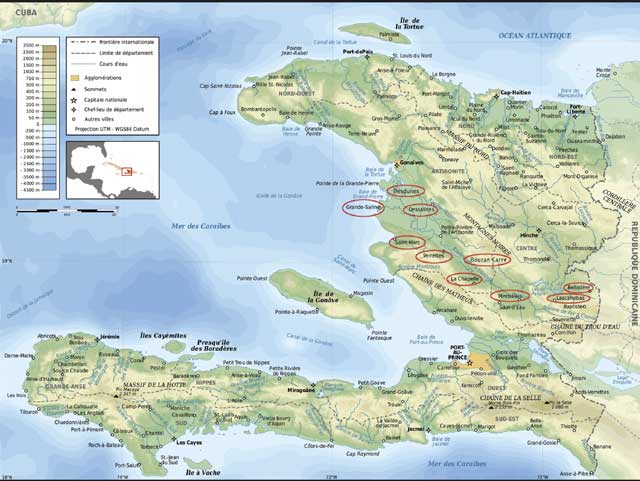
The field investigation was conducted in April 2010 in 10 regions (townships or communes) of Haiti, including regions of Desdunes, Grande-Saline, Dessalines, Saint-Marc, Verrettes, and La Chapelle of the Department of Artibonite, and regions of Boucan Carre, Mirebalais, Belladère, and Lascahobas of the Department of Centre (Figure 1). A total of approximately 60 suspected cases of teschovirus encephalomyelitis were reported from these regions before the investigation. We conducted the study on a convenience sample of 35 swine premises located in these regions. The vast majority of these premises were utilized for raising backyard pigs. During the field investigation, a Haitian Animal Health Authority veterinarian conducted the review with the owner of each premises. A multiple-page questionnaire was used to gather information about the premises and all pigs in the premises, including owner’s name, address of the premises and its latitude and longitude in the global positioning system (GPS), number of pigs, and age, sex, vaccination, movement, and health status of the animals.
Figure 1: A map showing 10 regions of the Republic of Haiti in which a field investigation of viral diseases of swine was conducted in April 2010. The name of each of these regions is circled. The study included 35 premises. Serum samples from 109 pigs, including 23 sick and 86 apparently healthy pigs; ethylenediaminetetraacetic acid- (EDTA-) anticoagulated blood samples from 21 sick pigs; and tissues (brain, spinal cord, tonsil, spleen, and mandibular lymph node) from eight severely ill and euthanized pigs were collected and submitted on ice to the National Veterinary Service Laboratories (NVSL)/Foreign Animal Disease Diagnostic Laboratory on Plum Island, New York, and the NVSL in Ames, Iowa. Serum samples were tested for classical swine fever virus (CSFV) and antibodies to a variety of viral agents including porcine teschovirus type 1 (PTV-1), porcine circovirus type 2 (PCV-2), CSFV, porcine reproductive and respiratory syndrome virus (PRRSV), swine influenza virus, pseudorabies virus, encephalomyocarditis virus, and hemagglutinating encephalomyelitis virus. The blood samples were tested for PRRSV; brains and spinal cords for PTV-1; tonsils for CSFV, PRRSV and PCV-2; and spleens and mandibular lymph nodes for African swine fever virus. This map was adapted with modifications from the Web site of Mindful Generations, Inc, Delano, Minnesota (http://www.mindgens.org/site/our-work/haiti/), with permission. To see more details in this figure, please click image to open a larger version in a new browser window/tab or download the PDF. |
Before sample collection, participating veterinarians of the USDA, the Food and Agriculture Organization of the United Nations, and the Interamerica Institute for Cooperation on Agriculture (IICA) examined all pigs. Pigs showing no apparent clinical signs were categorized as “healthy” and those with apparent clinical signs were categorized as “sick.” Clinical signs observed in sick pigs were CNS disorders, including paresis or paralysis of the hindquarters. Serum samples were collected from a total of 109 pigs from the 35 premises, including 23 sick and 86 apparently healthy pigs. For each animal from which samples were collected, a pig number was assigned as the identifier of the pig and the sample(s) collected from the pig. The numbers of sera collected in each of the 10 regions are shown in Table 1. At the time of the investigation, most of the pigs in the swine population were 1 year old or younger. Of the 109 pigs sampled, 29 (26.6%) were 6 months old or younger, and 55 (50.5%) were over 6 months but not over a year (Table 2); 53 were males and 56 were females. Ethylenediaminetetraacetic acid-(EDTA-) anticoagulated blood samples were also collected from 21 of the 23 sick pigs sampled for sera. Eight pigs with posterior paralysis, from the regions of Saint-Marc, Boucan Carre, and Grande-Saline, were humanely euthanized and brain, spinal cord, tonsils, spleen, and mandibular lymph nodes were collected from each pig. A portion of the brain and spinal cord from each pig was placed in 10% neutral buffered formalin for histopathological analysis. All samples were submitted on ice for laboratory analysis to NVSL/FADDL on Plum Island, New York, and the NVSL in Ames, Iowa.
Table 2: Age distribution of pigs that tested positive in diagnostic assays for viral swine pathogens in several regions of Haiti*
* Details of the field investigation and sample collection are described in Figure 1. Mo = month(s) of age; y = year(s) of age; Ab = antibodies; PTV-1 = porcine teschovirus type 1; PCV-2 = porcine circovirus type 2; CSFV = classical swine fever virus; PRRSV = porcine reproductive and respiratory syndrome virus; SIV = swine influenza virus; RT-PCR = reverse transcription-polymerase chain reaction.
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Detection of antibodies to PTV-1
Porcine kidney cells (PK-15 cells) or primary swine kidney (pSK) grown on coverslips in Leighton tubes (Bellco, Vineland, New Jersey) were inoculated with PTV-1, strain Haiti/2009 (1.5 mL of virus dilution per tube) and incubated at 37°C overnight. The coverslips were fixed in acetone for 5 to 10 minutes and air dried. A volume of 200 µL of each serum sample diluted 1:20 in phosphate buffered saline (PBS), pH 7.2, was added onto a coverslip and incubated at 37°C for 30 minutes in a humid chamber. The coverslips were rinsed in PBS, pH 7.2, and soaked in PBS for 5 minutes. A volume of 200 µL of a 1:75 dilution of goat anti-pig IgG conjugated with fluorescein isothiocyanate was added onto each coverslip and incubated at 37°C for 30 minutes in a humid chamber. The coverslips were rinsed in PBS, pH 7.2, soaked in PBS for 5 minutes, rinsed in reverse osmosis water, and allowed to air dry. After drying, the coverslips were mounted on microscope slides with a mounting medium containing 50% glycerin and 50% PBS and examined under a fluorescence microscope. Porcine teschovirus type-1-infected cells on slides with serum samples positive for antibodies to the virus appeared bright green on a dark background.
Detection of antibodies to CSFV
The HerdChek CSFV Antibody Test Kit (Idexx Laboratories, Inc, Westbrook, Maine) was used for serum samples according to the manufacturer’s instructions. A sample was considered positive for antibodies to a pestivirus if its blocking percentage was 40% or greater. An immunoperoxidase (IP) test was further performed to determine if any borderline positive results were due to antibodies to CSFV.8,9 Briefly, CSFV-infected and uninfected swine kidney cells (SK-6 cells) prepared in microtiter plates were fixed and used in the IP test. After incubation with test serum, bound antibodies were reacted with a protein G-horseradish peroxidase conjugate (Invitrogen Corporation, Carlsbad, California) followed by addition of 3-amino-9-ethylcarbazole, substrate for peroxidase (Vector Laboratories, Inc, Burlingame, California). The plates were washed with PBS, pH 7.2, containing 0.05% Tween 20, and read using an inverted light microscope. A positive reaction was indicated by the presence of a red color in the cytoplasm of the CSFV-infected cells.
Detection of antibodies to PCV-2, PRRSV, and PRV
The SERELISA PCV2 Ab Mono Blocking Detection Kit (Synbiotics Corporation, Kansas City, Missouri), the Idexx PRRS X3 Ab Test (Idexx Laboratories, Inc), and the Idexx PRV/ADV gB Ab Test (Idexx Laboratories, Inc) were used for detection of antibodies to PCV-2, PRRSV, and PRV in serum samples, respectively, according to the manufacturers’ instructions.
Detection of antibodies to SIV, EMCV, and HEV
Three National Veterinary Services Laboratories Standard Operating Protocols were used for detection of antibodies to SIV H1N1 and SIV H3N2, to EMEV, and to HEV, respectively.10-12
Preparation of tissue homogenates and virus isolation
Tissue homogenates of brains, spinal cords, tonsils, spleens, and mandibular lymph nodes collected in this study were prepared in the same manner as for those in the initial diagnosis of teschovirus encephalomyelitis in 2009.6 Cultures of swine kidney cells (SK-6 and IBRS-2 cells) and Vero cells were used for virus isolation from tissue homogenates as described previously.6 Homogenates of tonsils were inoculated onto SK-6 cells, those of brains and spinal cords onto SK-6, Vero, and IBRS-2 cells, and those of spleens and mandibular lymph nodes onto SK-6 and Vero cells. Cultures of brain- and spinal cord-inoculated SK-6 and IBRS-2 cells positive for cytopathic effect (CPE) were subjected to reverse transcription-polymerase chain reaction (RT-PCR) for PTV as described below. Cultures of tonsil-inoculated SK-6 cells negative for CPE and original tonsil samples were analyzed for detection of CSFV antigens with the avidin-biotin complex (ABC) immunohistochemistry assay using VECTASTAIN ABC-AP Kit (Vector Laboratories, Inc) and monoclonal antibody V3 against the glycoprotein 55 of CSFV (Cedi Diagnostics, Lelystad, the Netherlands) according to the manufacturers’ instructions. In addition, a hemadsorption (HAD) test was performed on homogenates of spleens and mandibular lymph nodes for isolation of ASF virus following the procedure of Malmquist and Hay.13
Nucleic acid extraction
Ribonucleic acid (RNA) was extracted from 140 µL of each tissue homogenate of brain, spinal cord, and tonsil, as well as serum, EDTA blood, and CPE-positive cultures of brain- and spinal cord-inoculated SK-6 and IBRS-2 cells using the RNeasy Mini Kit (Qiagen, Inc, Valencia, California) and following the manufacturer’s instructions. Ribonucleic acid from each sample was eluted in 40 µL of RNase-free water and stored at -70°C until RT-PCR was performed. Deoxyribonucleic acid (DNA) was extracted from 200 µL of each homogenate of tonsil samples with the QIAamp DNA Mini Kit (Qiagen, Inc). Deoxyribonucleic acid from each sample was eluted in 100 µL of Buffer AE (10 mM Tris·Cl, 0.5 mM EDTA, pH 9.0) and stored at -70°C until PCR tests were performed.
RT-PCR for PTV and real-time RT-PCR for CSFV
For detection of PTV, two RT-PCR assays were conducted on RNA of brains, spinal cords, and CPE-positive cultures of brain- and spinal cord-inoculated SK-6 and IBRS-2 cells following the procedures of Zell et al.14 The first assay was a nested RT-PCR for detection of PTV-1 through PTV-11. The second assay was specific for PTV-1. For detection of CSFV, a TaqMan real-time RT-PCR was conducted on RNA of serum and tonsil samples according to Risatti et al.15
PCR for PCV-2
Polymerase chain reaction for PCV-2 was conducted on DNA of tonsil samples using the Platinum PCR SuperMix (Invitrogen Corporation) according to the supplier’s protocol. Sequences of primers were as follows: forward primer, 5´-GACAAACGTTACAGGGTGCTGC; reverse primer, 5´-GTTGGCGAGGAGGGTAATGAGG. The 300-bp amplified region is a conserved region within the open-reading frame (ORF) 1 of the genome of PCV-2 encoding the viral “rep protein” (“replicase”). The PCR was performed on a DNA engine Tetrad 2 Thermal Cycler (Bio-Rad Laboratories, Hercules, California) using the following cycling conditions: 95°C for 10 minutes followed by 40 cycles of 94°C for 30 seconds, 58°C for 30 seconds, and 72°C for 30 seconds, with an additional 7-minute extension at 72°C. The products of the PCR were analyzed by agarose gel electrophoresis with ethidium bromide staining. A sample was considered positive for PCV-2 when it generated PCR products of the expected size of 300 bp.
RT-PCR for PRRSV
Ribonucleic acid of tonsil and EDTA blood samples was tested for PRRSV using the Qiagen OneStep RT-PCR Kit (Qiagen, Inc) according to the supplier’s instructions. Sequences of PCR primers were as follows: forward primer, 5´-CAATTCATCTGCGCCGTTCA; reverse primer, 5´-GGTGAGTGCACCATATGAGACAAT. The 894-base amplified region of the PRRSV genome (ORF3-5) is a conserved region encoding glycoproteins of the viral envelope (GP3-GP5). The RT-PCR was performed on a DNA engine Tetrad 2 Thermal Cycler (Bio-Rad Laboratories) using the following cycling conditions: 50°C for 30 minutes, 95°C for 15 minutes followed by 35 cycles of 94°C for 30 seconds, 55°C for 30 seconds, and 72°C for 1 minute, with an additional 10-minute extension at 72°C. The products of the PCR were analyzed by agarose gel electrophoresis with ethidium bromide staining. A sample was considered positive for PRRSV when it generated PCR products of the expected size of 894 bp.
Histopathological analysis
This was performed by a veterinary pathologist in FADDL. Brain tissues that had been fixed in 10% neutral buffered formalin were paraffin embedded, sectioned at 4 µ, mounted on glass slides, stained with hematoxylin and eosin as described by Luna,16 and examined microscopically.
Results
Test results for 109 serum samples collected from different regions are presented in Table 1. Antibodies to PCV-2 and PTV-1 were most prevalent in the study population, and they were distributed in all study regions. More serum samples were collected from pigs older than 6 months but not over 1 year of age than any other age groups, and this age group included the highest percentage of animals that tested positive in all diagnostic assays among all age groups (Table 2). For animals that tested positive, no difference was observed in the proportions of male and female pigs (data not shown). All 109 serum samples tested negative for antibodies to PRV, EMCV, and HEV. As shown in Tables 1 and 2, fifteen of the 109 serum samples were positive by the real-time RT-PCR for CSFV. Five of the 15 RT-PCR-positive samples from four different regions (Desdunes, Dessaline, Saint-Marc, and Lascahobas) were confirmed to contain viable CSFV by virus isolation and the ABC immunohistochemistry assay (Table 1). Among the 15 RT-PCR-positive samples, eight were collected from CSF-vaccinated pigs, six were from nonvaccinated pigs, and one was from a pig of unknown vaccination status. Among the five samples confirmed to contain viable CSFV, four were from CSF-vaccinated pigs and one was from a nonvaccinated pig.
Table 1: Test results of 109 pig sera collected from 10 regions in Haiti *
* Details of the field investigation and sample collection described in Figure 1. † All 109 serum samples tested negative for antibodies to pseudorabies virus, encephalomyocarditis virus, and hemagglutinating encephalomyelitis virus. ‡ Number in parenthesis shows those confirmed to contain viable CSFV by virus isolation and the avidin-biotin complex immunohistochemistry assay. Ab = antibodies; PTV-1 = porcine teschovirus type 1; PCV-2 = porcine circovirus type 2; CSFV = classical swine fever virus; PRRSV = porcine reproductive and respiratory syndrome virus; SIV = swine influenza virus; RT-PCR = reverse transcription-polymerase chain reaction. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Of the 54 samples positive for antibodies to PTV-1, 19 (35.2%) were collected from sick and 35 (64.8%) from clinically healthy pigs. Among these 54 samples, the number also positive for antibodies to other disease agents was 35 (64.8%) for PCV-2, 25 (46.3%) for CSFV, two (3.7%) for PRRSV, four (7.4%) for SIV H3N2, and 10 (18.5%) for SIV H1N1, respectively.
Among the 44 samples positive for antibodies to CSFV (regardless of serological status for PTV), 30 (68.2%) were collected from CSF-vaccinated pigs, 11 (25.0%) from nonvaccinated pigs, and three (6.8%) from animals of unknown vaccination status. Among 65 samples negative for antibodies to CSFV, 29 (44.6%) were collected from CSF-vaccinated pigs and 36 (55.4%) from nonvaccinated pigs.
All of the 21 blood samples collected from pigs with clinical signs (mainly CNS disorders) and tested by RT-PCR for PRRSV were negative.
No gross lesions were observed upon postmortem examination of the eight severely ill pigs with CNS signs from four different regions. Brain samples from these pigs were examined histopathologically. The most significant microscopic finding was multifocal nonsuppurative encephalomyelitis. Lesions were characterized by multifocal perivascular cuffing of lymphocytes and plasma cells, multifocal gliosis, satellitosis, and neuronal necrosis. These lesions were most severe in the cerebellum and the brainstem. All six brain samples and six spinal samples tested were positive by RT-PCR for PTV-1, whereas viable PTV-1 was isolated in cell cultures from only one brain (pig no. 88) and one spinal cord (pig no. 15) (Table 3). Six of eight tonsil samples were positive by PCR for PCV-2, but all eight tonsils were negative by RT-PCR for PRRSV and by both RT-PCR and virus isolation for CSFV. All seven spleen samples and all seven mandibular lymph node samples tested were negative in virus isolation and in the HAD test for ASFV. The six serum samples collected from these pigs and tested by RT-PCR for CSFV were all negative. Among these six sera, all were seropositive for PTV-1, four were seropositive for PCV-2, and two were seropositive for CSFV (Table 3).
Table 3: Laboratory test results on samples from necropsied pigs with neurological signs in a study on viral diseases of swine in 10 regions of Haiti*
* Details of the field investigation and sample collection are described in Figure 1. † Pigs 15 and 16 originated in Saint-Marc, pigs 57 and 61 in Boucan Carre, and pigs 28, 31, 88, and 92 in Grande-Saline. Pig 31 had been vaccinated against CSF; pigs 15, 28, 57, 61, and 88 were not vaccinated; and vaccination status was unknown for pigs 16 and 92. ‡ Multifocal nonsuppurative encephalomyelitis. RT-PCR = reverse transcription-polymerase chain reaction; PTV = porcine teschovirus; VI = virus isolation; CSFV = classical swine fever virus; PRRSV = porcine reproductive and respiratory syndrome virus; PCV-2 = porcine circovirus type 2; HAD = hemadsorption; ASFV = African swine fever virus; SIV = swine influenza virus; Ab = antibodies; ND = not done; NS = no sample was available for the test; Pos = positive (for histopathological analysis on brains, “Pos” refers to multifocal nonsuppurative encephalomyelitis); Neg = negative.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Discussion
Results of this study confirm that teschovirus encephalomyelitis is prevalent in multiple regions in Haiti, including areas near the border with the Dominican Republic, and that several other viral disease agents, ie, CSFV, PCV-2, PRRSV, and SIV, are present in the Haitian swine population. The fact that 15 of the 109 serum samples were positive in the real-time RT-PCR for CSFV and five of these 15 samples from four different regions were confirmed to contain viable CSFV by virus isolation and the ABC immunohistochemistry assay demonstrates CSFV circulation.
Subsequent to this study, an investigation was conducted in the Dominican Republic in August 2010.17 The investigation revealed that teschovirus encephalomyelitis has spread to regions of the Dominican Republic close to the border with Haiti, and that other viral disease agents, ie, CSFV, PCV-2, PRRSV, and SIV, are also present in the swine population of these regions.17
The viral disease agents in the swine population of the Hispaniola, such as PTV-1 and CSFV, are highly contagious and can be introduced to other Caribbean countries, as well as to Central and North American countries by the movement of swine and contaminated products.
In Haiti, no established regulations for movement of commercial swine between different regions exist.3 Pigs are usually grown at home. Individual farmers buy pigs in local live-animal markets in their towns and carry them back home to fatten them. We speculate that this uncontrolled movement of animals has facilitated the spread of PTV-1 and other disease agents in the country.
In this study, PTV-1 was detected in all six brain samples and all six spinal cord samples tested by RT-PCR, whereas viable PTV-1 was isolated by cell cultures from only one brain and one spinal cord among these RT-PCR-positive samples. The negative results in isolation of PTV-1 from the balance of brain and spinal cord samples positive by RT-PCR were likely related to virus degradation or inactivation. The fact that 64.8% of sera positive for antibodies to PTV-1 came from healthy pigs indicates that many pigs were immune to PTV-1 prior to sampling.
It was surprising that 44.6% of serum samples negative for antibodies to CSFV were from pigs vaccinated with a live attenuated CSF vaccine (Chinese strain). Vaccination for CSF is conducted once a year for the entire swine population in Haiti. The Chinese strain is widely considered to be one of the most effective vaccines against CSF, and, in general, it provides lifelong immunity.18 The negative CSF serological results from pigs vaccinated with the Chinese strain are likely due to improper handling or administration of the vaccine. During the field investigation, we learned that vaccines were not always stored and transported in cold conditions even in the summer. Improvement of the cold chain, together with vaccination traceability, have been identified as priorities by several CSF control projects in Haiti, including the European Union-funded project Caribbean Agriculture and Fisheries Programme.
Approximately half of the 109 pigs sampled were positive for antibodies to PTV-1 and PCV-2, and the majority of the PTV-1 antibody-positive pigs were also positive for antibody to PCV-2. In addition, both PTV-1 and PCV-2 were detected simultaneously in specimens from five necropsied pigs. These results seem to support the assumption that there is a relationship between PCV-2 infection and the teschovirus disease. However, it is difficult to ascertain this relationship on the basis of the data from this study.
Humoral mechanisms are important in preventing PTV infection.19,20 Several commercial PCV-2 vaccines are available, and research has shown increases in growth rates and final weights of finishing pigs vaccinated with PCV-2 vaccines.21 Currently, a major strategy for control of teschovirus encephalomyelitis in pigs in the Hispaniola is to develop and apply an autogenous teschovirus vaccine using the Haitian PTV-1 isolate as the vaccine virus. While development and production of the autogenous vaccine are in process, a field trial on a PCV-2 vaccine is being conducted in PTV-affected regions in Haiti with the hope that the PCV-2 vaccine may improve the immunity of the swine population and thus reduce the occurrence of teschovirus disease. This field trial is based on the assumed relationship between PCV-2 infection and teschovirus disease.
This study was an international collaboration of multiple organizations. There were limitations pertaining to the design and execution of the study due to various factors. For example, more serum samples should have been taken. We collected 109 sera from 35 swine premises located in 10 regions, based on a plan to collect sera from 100 to 110 pigs with three pigs sampled per premises. In addition, there was a big variation in the number of samples from different regions. In order to collect a sample from a pig, it was necessary to obtain permission from the owner of the pig. Sometimes, it was difficult to obtain such permission due to concerns of the owner about a potential negative impact of sampling on the health of the animal. Only two and four pigs were sampled in La Chapelle and Mirebalais, respectively, but as many as 31 serum samples were collected in Grande-Saline. We were able to euthanize sick pigs and collected tissue samples from these pigs in four regions but not in all 10 regions. In spite of these limitations, the authors believe that the findings from this study are of importance.
Implication
Due to the close proximity of the Hispaniola to Puerto Rico, a territory of the United States, and the large number of direct flights from the Hispaniola to Miami, Newark, New York, and other cities, there is a high risk of introducing these viral pathogens into the North American swine population.
Acknowledgments
The field and laboratory investigation on swine diseases in Haiti was a result of collaboration of several organizations, including animal-health authorities of the Republic of Haiti and Dominican Republic, Food and Agriculture Organization of the United Nations, the Institute of International Cooperation in Agriculture, APHIS/International Services, and APHIS/VS/NVSL. The study was performed within a multipartner project (USDA/APHIS, IICA, CIRAD, CaribVET) called Veterinary Epidemiologist Para-epidemiologist (VEP). This VEP project is under the strategy of the Caribbean animal-health network CaribVET. The authors thank Maria Teresa Frias Lepoureau, Lester J. Pérez, and Carmen Laura Perera of the CENSA (Cuba) and Marion Petit Sinturel of CIRAD for their technical advice for the project. The authors also thank Elizabeth Lautner, Paul Hauer, and Beverly Schmitt of the NVSL for their instrumental advice and support to the project and Ethan Macnow of Plum Island Animal Disease Center for his assistance in preparing the map of Haiti used in the manuscript.
Disclaimer: The views expressed in this publication are those of the authors and do not necessarily reflect the views of the Food and Agriculture Organization of the United Nations.
Conflict of interest
None reported.
References
1. World Organization for Animal Health. Teschovirus encephalomyelitis. In: Manual of Diagnostic Tests and Vaccines for Terrestrial Animals. Paris, France: OIE; 2008:1146-1151.
2. The Center for Food Security and Public Health of Iowa State University. Teschovirus encephalomyelitis and porcine teschovirus infection. 2009. Available at: www.cfsph.iastate.edu/Factsheets/pdfs/enterovirus_ encephalomyelitis.pdf. Accessed 6 February 2013.
3. Food and Agriculture Organization. Teschovirus encephalomyelitis in the Republic of Haiti. 2009. Available at ftp://ftp.fao.org/docrep/fao/012/ak137e/ak137e00.pdf. Accessed 6 February 2013.
4. Anonymous. Haiti – The economy. Available at: http://www.mongabay.com/reference/country_studies/haiti/ECONOMY.html. Accessed 11 February 2013.
5. FAO, OIRSA, OIPORC and OIE. Continental plan for the eradication of classical swine fever (CSF) from Americas. 2009. Available at: www.rlc.fao.org/es/prioridades/transfron/ppc/pdf/mpingl.pdf. Accessed 6 February 2013.
6. Deng MY, Millien M, Jacques-Simon R, Flanagan JK, Bracht AJ, Carillo C, Barrett RW, Fabian A, Mohamed F, Moran K, Rowland J, Swanson SL, Jenkins-Moore M, Koster L, Thomsen BV, Mayr G, Pyburn D, Morales P, Shaw J, Burrage TY, White W, McIntosh MT, Metwally S. Diagnosis of teschovirus encephalomyelitis in the Republic of Haiti. J Vet Diagn Invest. 2012;24:671-678.
7. Animal and Plant Health Inspection Service, United States Department of Agriculture, Code of Federal Regulations, Title 9, Part 3, Subpart F – Specifications for the humane handling, care, treatment, and transportation of warm-blooded animals other than dogs, cats, rabbits, hamsters, guinea pigs, nonhuman primates, and marine mammals. 2012. Available at: http://www.law.cornell.edu/cfr/text/9/3/subpart-F. Accessed 11 February 2013.
8. Saunders CS, Wilder ME. Disease screening with enzyme-labeled antibodies. J Infect Dis. 1974;129:362–364.
9. Afshar A, Dulac GG, Bouffard A. Application of peroxidase labeled antibody assays for the detection of porcine IgG antibodies to hog cholera and bovine viral diarrhea viruses. J Virol Methods. 1989;23:252–262.
10. Beach T. National Veterinary Services Laboratories Standard Operating Protocol SOP-BPA-0801.05. Hemagglutination-inhibition test for detection of antibodies to swine influenza virus, H1N1 or H3N2. Ames, Iowa: National Veterinary Services Laboratories; 2010.
11. Beach T. National Veterinary Services Laboratories Standard Operating Protocol SOP-BPA-2109.03. Virus neutralization test for detection of antibodies against encephalomyocarditis virus. Ames, Iowa: National Veterinary Services Laboratories; 2010.
12. Beach T. National Veterinary Services Laboratories Standard Operating Protocol SOP-BPA-0803.02. Hemagglutination-inhibition test for detection of antibodies to hemagglutinating encephalomyelitis virus. Ames, Iowa: National Veterinary Services Laboratories; 2010.
13. Malmquist WA, Hay D. Haemadsorption and cytopathic effect produced by African swine fever virus in swine bone marrow and buffy coat cultures. Amer J Vet Res. 1960;21:104-108.
14. Zell R, Krumbholz A, Henke A, Birch-Hirschfeld E, Stelzner A, Doherty M, Hoey E, Dauber M, Prager D, Wurm R. Detection of porcine enteroviruses by nRT-PCR: differentiation of CPE groups I-III with specific primer sets. J Virol Methods. 2000;88:205-218.
15. Risatti GR, Callahan JD, Nelson WM, Borca MV. Rapid detection of classical swine fever virus by a portable real-time reverse transcriptase PCR assay. J Clin Microbiol. 2003;41:500-505.
16. Luna LG. Routine staining procedures, hematoxylin and eosin stains. In: Luna LG, ed. Manual of Histologic Staining Methods of the Armed Forces Institute of Pathology. 3rd ed. New York, New York: McGraw-Hill Book Company; 1968:32-46.
17. Ventura A, Gonzalez W, Barrette R, Swenson S, Bracht A, Rowland J, Fabian A, Moran K, Mohamed F, O’Hearn E, Jenkins-Moore M, Toms D, Shaw J, Morales P, Pyburn D, Carrillo C, Mayr G, McIntosh M, Deng M. Virus and antibody diagnostics for swine samples of the Dominican Republic collected in regions near the border to Haiti. International Scholarly Research Network Virology. Volume 2013, Article ID 425831. Available at: http://dx.doi.org/10.5402/2013/425831. Accessed 6 February 2013.
18. Greser-Wilke I, Moennig V. Vaccination against classical swine fever virus: limitations and new strategies. Anim Health Res Rev. 2004;5:223-226.
19. Bangari DS, Pogranichniy RM, Gillespie T, Stevenson GW. Genotyping of porcine teschovirus from nervous tissue of pigs with and without polioencephalomyelitis in Indiana. J Vet Diagn Invest. 2010;22:594-597.
20. Knowles N. Porcine enteric picornaviruses. In: Straw BE, Zimmerman JJ, D’Allaire S, Taylor DJ, eds. Diseases of Swine. 9th ed. Ames, Iowa: Blackwell Publishing; 2006:337-345.
21. Shelton NW, Tokach MD, Dritz SS, Goodband RD, Nelssen JL, DeRouchey JM, Usry JL. Effects of porcine circovirus type 2 vaccination on nursery and finishing pig performance under a PRRS challenge. Swine Day 2009:28-32. Kansas State University. Agricultural Experiment Station and Cooperative Extension Service, Manhattan, KS. Available at: http://krex.k-state.edu/dspace/handle/2097/2148?show=full. Accessed 22 January 2013.