| Commentary | Peer reviewed |
Cite as: Martineau GP, Le Treut Y, Guillou D, et al. Postpartum dysgalactia syndrome: A simple change in homeorhesis? J Swine Health Prod. 2013;21(2):85–93.
Also available as a PDF.
SummaryMastitis, metritis, and agalactia syndrome (MMA) is a clear entity often reported as postpartum dysgalactia syndrome (PDS). However, MMA may represent only a small emerging part of an iceberg represented by PDS. Until now, investigators have compiled a list of risk factors for PDS related to nutrition, housing, and management practices and suggested that endotoxins and cytokines may play a central role in development of PDS. However, the pathophysiology of PDS has never been defined. The goal of this paper is to fill this gap, basing our proposal on the most recent published scientific literature and on the concept of homeorhesis developed by Bauman and Currie in the 1980s. Homeorhesis, a term that encompasses dynamic systems that return to a trajectory, refers to orchestrated changes in metabolism of body tissues to prioritize a physiological state (such as gestation or lactation) and brings a new perspective to this multifactorial disease that we will try to clarify using a transdisciplinary approach. Indeed, it appears that the clinical approach to PDS must simultaneously take into account physiology, endocrinology, innate immunology, and ethology. | ResumenEl síndrome de mastitis, metritis, y agalactia (MMA por sus siglas en inglés) es una entidad clara a menudo reportada como síndrome de disgalactia postparto (PDS por sus siglas en inglés). Sin embargo, la MMA puede constituir solamente la punta del iceberg representado por PDS. Hasta ahora, los investigadores han recopilado una lista de factores de riesgo para el PDS relacionados con la nutrición, alojamiento, y prácticas de manejo y sugieren que las endotoxinas y las citokinas pueden jugar un papel central en el desarrollo del PDS. Sin embargo, la patofisiología del PDS nunca ha sido definida. El propósito de este estudio es llenar este espacio, basando nuestra propuesta en literatura científica recientemente publicada y en el concepto de homeorhesis desarrollado por Bauman y Currie en la década de 1980. La homeorhesis, un término que abarca sistemas dinámicos que regresan a una trayectoria, se refiere a cambios orquestados en el metabolismo de tejidos corporales para dar prioridad al estado fisiológico (tal como la gestación ó la lactancia) y ofrece una nueva perspectiva a esta enfermedad multifactorial que trataremos de clarificar utilizando un acercamiento multidisciplinario. De hecho, parece que el abordar el PDS clínicamente debe tomarse en cuenta simultáneamente la fisiología, endocrinología, inmunología innata, y la etología. | ResuméLe syndrome de mammite, agalactie, et métrite (MMA) est une entité clinique reconnue qui est souvent rapportée sous l’appellation syndrome de dysgalactie postpartum (PDS). Par contre, le MMA pourrait représenter seulement une petite partie émergente d’un iceberg représenté par le PDS. Jusqu’à récemment, les chercheurs ont compilé une liste des facteurs de risque pour le PDS qui sont reliés à la nutrition, l’hébergement, et les pratiques de gestion et ont suggéré que les endotoxines et les cytokines pourraient jouer un rôle central dans le développement du PDS. Par contre, la pathophysiologie du PDS n’a jamais été définie. L’objectif de cet article est de combler cette lacune, en basant notre proposition sur la littérature scientifique la plus récemment publié et sur le concept d’homéorhésie développé par Bauman et Currie durant les années 1980. L’homéorhésie est un terme qui englobe les systèmes dynamiques qui reviennent à leur trajectoire, réfère à une cascade de changement dans le métabolisme des tissus visant à prioriser un état physiologique (tel que la gestation ou la lactation) et à amener une nouvelle perspective à cette maladie multifactorielle que nous voulons clarifier en utilisant une approche trans-disciplinaire. En effet, il semble que l’approche clinique envers le PDS peut simultanément prendre en considération la physiologie, l’endocrinologie, l’immunologie naïve, et l’éthologie. |
Keywords: swine, mastitis, metritis, and agalactia syndrome; postpartum dysgalactia syndrome; homeorhesis
Search the AASV web site
for pages with similar keywords.
Received: October 27, 2011
Accepted: March 29, 2012
Mastitis, metritis, and agalactia syndrome (MMA) is a clear entity1 that has been described for at least 50 years.2 More recently it has been called postpartum dysgalactia syndrome (PDS).3 However, MMA syndrome may be considered the emerging part of an iceberg represented by PDS, which is the more important and underestimated part and therefore the least obvious and most dangerous. In 1982, the name “periparturient hypogalactia syndrome” was proposed4 to characterize the disease, and in 1998, it was named “postpartum dysgalactia syndrome” (PDS),5 which is now well recognized and accepted in the scientific community.3
Homeorhesis, derived from Greek and standing for “similar flow,” is a concept encompassing dynamic systems which return to a trajectory, as opposed to systems that return to a particular state, defined as homeostasis.6 The word “homeorhesis” was proposed by Conrad Waddington (1905-1975), biologist, paleontologist, and embryologist. It refers to “coordinated changes in metabolism of body tissues necessary to support a (dominant) physiological state.”6 While well reported in scientific papers,7,8 homeorhesis, in contrast to homeostasis, is not very common in the daily swine veterinary language. In the animal science area, homeorhesis is primarily used in connection with cattle production.6,7,9,10
Taking into account recent scientific information,3 we propose that PDS is a consequence of an unsuccessful transition from the homeorhesis of gestation to the homeorhesis of lactation, that we will call a “dys-homeorhesis change.” The transition is very complex and depends on an extensive series of biological adaptations that include many, perhaps most, body tissues and all nutrient classes. Therefore, the pathophysiology of PDS is similar to that of postweaning diarrhea, in which pathology results because of a difficult transition between two completely different diets.11
Postpartum dysgalactia syndrome
Postpartum dysgalactia syndrome is usually investigated using either an infectious or an epidemiological approach. The infectious approach, which starts most commonly with coliform mastitis as the primary event, has been recently well reviewed.12 The hypothesis that an infectious agent is at the root of PDS has existed for years and is still current, as shown by the prominence of research trying to isolate and type associated Escherichia coli strains, although the hypothesized link between E coli and PDS is not always easy to demonstrate.13-15 Epidemiological studies on PDS report findings of two types of observational studies, longitudinal16,17 and cross-sectional.18 Very recent work conducted in Belgium19 identified four major risk factors for PDS: moving the sow to the farrowing stable 4 days before farrowing, rather than > 7 days before, as is common in the commercial swine industry; ad libitum feeding shortly after farrowing; farrowing induction; and supervision of farrowing, as summarized in a table by Maes et al.3 Discovery that farrowing induction and farrowing supervision were risk factors for PDS was surprising and unexpected by these authors.
There have been many studies and reports on the infectious component of E coli mastitis. Mastitis is at the heart of the model of PDS that focuses on the acute mastitis paradigm, generating full chapters in books such as Diseases of Swine.20 In a recent literature review by Gerjets and Kemper,12 entitled “Coliform mastitis in sows: a review,” the authors report that clinical mastitis is not as prevalent in sows in Germany as it was in the past. As any temporary decrease in colostrum ingestion causes pathological consequences in the litter, the possible connection between PDS and neonatal diarrhea becomes clear. In 2009 and 2010, we, and Svensmark21 in Denmark, reported a new neonatal porcine diarrhea syndrome (NNPD)22 that has been frequently reported since then.23-26 Neonatal piglet diarrhea has emerged in many European countries (eg, Denmark, Sweden, and France) despite carefully implemented vaccination against enterotoxigenic E coli and good management procedures, taking into account biosecurity, hygiene, colostrum intake, nursing, and a high standard of care.27 The etiology of NNPD has been unknown until recently, and in most cases microbiological testing is insufficient to explain the cause.
In herds with NNPD, a preliminary study28 showed that duration of farrowing is longer in affected herds, and longer in affected than in non-affected litters. Therefore, the pathophysiology of disturbances in farrowing, in litters affected with neonatal diarrhea, has been investigated.29-31 The outcome of this work was a surprising correlation: piglets affected with neonatal diarrhea had ingested a high volume of colostrum.29 However, Le Dividich et al32 observed no diarrhea in neonatal piglets bottle-fed colostrum in amounts up to 561 g per kg body weight per 24 hours. The reason for the observation by Sialleli et al,29,30 that diarrheic piglets had consumed a large volume of colostrum, remains to be elucidated.
Homeorhesis
A dys-homeorhesis change is at the heart of PDS (Figure 1). In 1980, Bauman and Currie6 reviewed the regulation of nutrient partitioning during pregnancy and lactation. They defined homeorhesis as “the orchestrated or coordinated changes in metabolism of body tissues necessary to support a physiological state.”6 The central nervous system coordinates homeorhetic adaptations through the endocrine system. Bauman and Currie6 characterized the “key features” of homeorhetic control: its path, ie, hours or days versus seconds or minutes for homeostatic regulation; its simultaneous influence on various and apparently functionally unrelated tissues and systems; and its mediation through altered responses to homeostatic signals.
At farrowing (which may be considered an acute process), the homeorhetic drivers must shift from a pregnancy pattern to a lactation pattern, synchronizing among multiple organs and organ systems the metabolic and hormonal milieu of the sow.33
The well-coordinated developmental change in adipose tissue metabolism in late pregnancy is a good example of homeorhesis. Overall, the homeorhetic controls operating during lactogenesis alter adipose tissue metabolism, decreasing lipid synthesis and increasing lipid mobilization, to support a new physiological state. However, homeostatic controls also are operating throughout this period to balance environmental challenges, eg, stress or the daily rhythms in circulating metabolites associated with feeding. The study of Metz and van den Bergh34 focused on these changes in dairy cows at parturition.
During farrowing, huge individual variations in hormonal and biochemical changes are well described.35 However, little data indicates how these changes are coordinated. Recent data from studies in rats indicate that changes in mammary, liver, and adipose transcriptomes at the onset of lactation are coordinated through molecular clocks, ie, small molecules acting as metabolic messengers.8 These authors suggest principles to explain how molecular clocks might affect metabolism, on the basis of the review by Michele.36 Genes expressed in adipose tissue are believed to regulate both feed intake and whole-body energy metabolism.37
In pregnant sows, a huge acceleration of fetal growth occurs after day 70, but some major changes in fetal composition also occur.38 Very accurate data have been obtained in normoprolific gilts. However, one has to take into account that we now have to manage hyperprolific sows, therefore the changes in fetal composition are probably bigger.
Pathophysiology of PDS
Risk factors for PDS were identified as a result of a large questionnaire survey in commercial pig farms in Belgium.19 The aim of the study was to investigate management practices and strategy-related risk factors. On the basis of these results, the same team then proposed a pathophysiological explanation of PDS involving nutrition, housing, and management practices.3 The authors do not mention homeorhesis, taking into account the changes occurring physiologically in gestation and lactation metabolisms, related to energy, protein, water, minerals, and anion gap. We believe the unsuccessful change in homeorhesis (ie, the “dys-homeorhesis change”) when shifting from gestation to lactation is at the heart of PDS and propose a novel pathophysiological explanation summarized in Figure 1.
Figure 1: Schematic pathophysiology of postpartum dysgalactia syndrome (PDS) adapted from Martineau et al.1 Red arrows link risk factors within a box, blue arrows illustrate a connection between boxes, and white arrows illustrate the link between a box and a change of homeorhesis. This material is reproduced with permission of John Wiley and Sons, Inc. 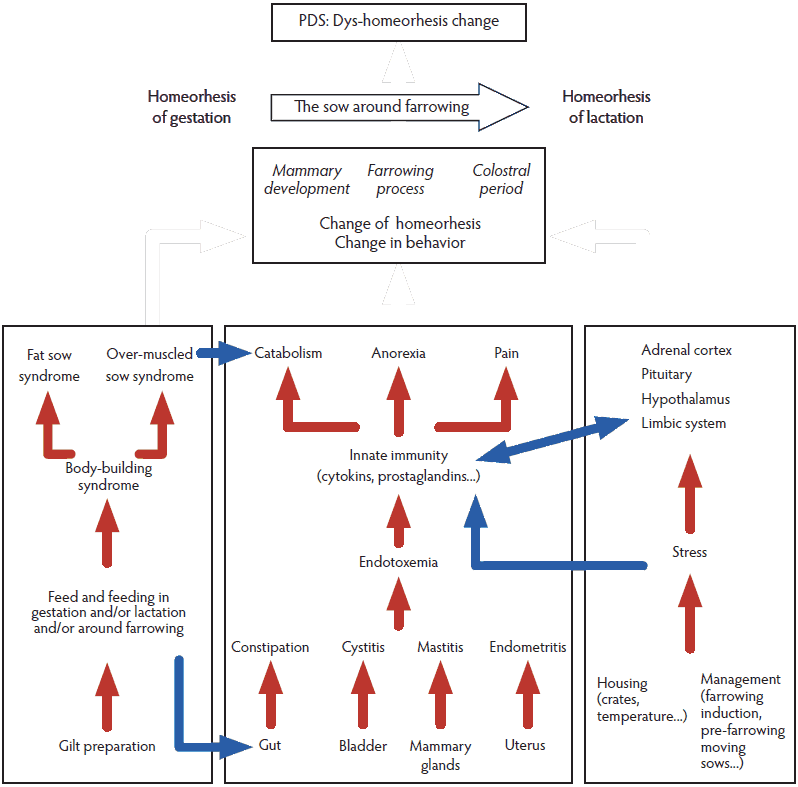 |
Hormones and their regulation are an essential component of the homeorhesis transition. An example of the complexity of the interactions and feedback loops is shown in Figure 2.39 Question marks show the several remaining issues regarding the interactions between reproductive and stress hormones, but it seems appropriate to emphasize that we need “a favorable hormonal milieu” for gestation and for lactation.40
Figure 2: Major hormonal interactions (positive [+] and negative [-]) on lactogenesis (Foisnet et al41). Question marks show the several remaining issues regarding the interaction between reproductive and stress hormones. IGF-1 = insulin-like growth factor-1. 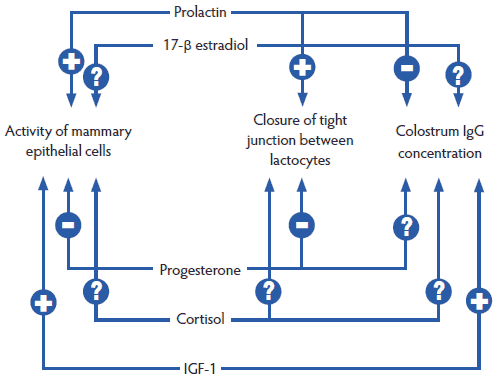 |
Foisnet et al41 demonstrated the consequences of asynchrony between progesterone and prolactin on colostrum production in sows showing no clinical signs. They concluded that sows producing a low yield of colostrum were characterized by a leaky mammary epithelium and reduced synthesis of lactose, related to delayed hormonal change before farrowing. These sows had greater plasma progesterone the day before farrowing, and tended to have lower plasma prolactin levels 1 to 2 days before farrowing, than did sows with a high yield of colostrum. Consequences of poor colostrum production on production parameters (such as average daily weight gain of the piglets) have been reported, as have decreased concentrations of prolactin42 and relaxin43 in late gestation. These hormones are absolutely necessary for mammary development. Both sow-related processes (mammary development and colostrum production) are important at the time of farrowing.41 Thus, a “slight” asynchronism may lead to a major problem (PDS), similar to the butterfly effect.44 As hormonal changes start several days before farrowing, PDS also often starts a few days before farrowing.
Our understanding of the physiopathology of PDS implies three components (Figure 1): the body-building syndromes,45 the endotoxemia component linked to the innate immunity system, and stress-related mechanisms. Although they are closely related, we will now separate them for a didactical approach and highlight for each the new research findings available in the literature.
First, feed and feeding in gestation have straightforward consequences on body composition. One can note that, although there should be a natural peak of water intake just before parturition,46 this is rarely possible where a liquid feeding system is used (eg, as in France, Denmark, the Netherlands, and Spain). Surprisingly, while physiological and behavioral changes are well described in sows,35,41,47-50 a few exceptions remain, for example, drinking behavior around the time of parturition is not often discussed, although it may consequently interfere with homeorhesis.1
When studying homeorhesis in gestation, it is necessary to take into account the fact that major changes have occurred in our sows since 2000. The two major changes are related to hyperprolificacy and carcass leanness. In 1998, Eggert et al37 had already observed that pig genotypes differed not only in the total amount of backfat, but also in the relative amounts of the three individual backfat layers. Backfat is a critical energy source for sows during periods of negative energy balance, and the relative contributions of each layer as a source of energy appear to differ. More broadly, fat has been shown important for endocrine regulation, including that of reproduction.51 From a chronological point of view, we had the “thin sow syndrome” during the late 1970s, followed a few years later by the “fat sow syndrome,” and then what we called the “accordion sow syndrome,”52 describing sows that are too fat at farrowing, lose too much weight during lactation, are reconditioned because they are too thin, then gain too much weight and enter the same loop again. Recently, we have seen the emergence of a new body-building syndrome associated with new sow genetic lines, the “over-muscled sow syndrome.”53-55 Even if fat sows are classically predisposed to PDS,56 recent observations by Vanderhaeghe et al57 provide new information regarding the consequences of sows having too little backfat at farrowing. In her study, sows with low backfat levels (< 16 mm) at parturition have a higher risk for stillbirths compared to sows with medium backfat levels (16 to 23 mm). Contrary to the general opinion,58 fat sows (in the Vanderhaeghe et al study, > 23 mm backfat)57 did not have a similarly increased risk.
These observations, in association with those of Bories et al,28 underline the importance of prepartum catabolism on farrowing and post partum. Prepartum catabolism seems a common feature in modern sows. Oliviero et al59 have shown that parameters related to catabolism (circulating non-esterified fatty acids [NEFAs] and creatinine) increased significantly a few days before farrowing, with a peak occurring a few hours before or after parturition. The observation concerning NEFAs is in agreement with the results of Le Cozler et al.47 In contrast, the metabolic markers of circulating energy-containing feed in the diet (urea, insulin, and glucose) decreased significantly when farrowing was approached, to a minimal value on the day of parturition.59 At the time of farrowing, internal body reserve (fat and muscles) becomes the main source of energy.7
Mammary glands are often and classically reported as a source of endotoxemia.12,60 Development of coliform mastitis is associated with the varying levels of local expression of regulatory cytokines in response to intramammary E coli inoculation and infection. Sows that develop clinical signs of mastitis have significantly lower pre-inoculation levels of interleukin-1b (IL-1b) mRNA than sows that remain clinically healthy.61 According to Zhu et al,61 an increase in expression of IL-6 mRNA is observed in the inoculated mammary glands of sows that developed clinical mastitis. This finding suggests that development of clinical signs due to coliform mastitis (eg, high fever) is associated with the degree of local expression of regulatory cytokines in response to intramammary E coli inoculation.
Initial recognition of microbes by cells of the immune system is largely based on pattern-recognition receptors, including Toll-like receptors.62,63 Of these, Toll-like receptor 4 is the main pattern-recognition receptor for lipopolysaccharides from gram-negative bacteria. No single organ is responsible for the origin of endotoxins, although the gut microflora is now recognized as probably the most important source. Indeed, in addition to its classical metabolic functions, the gut microflora has a major effect on innate immunity.63 A change in composition of the gut microbial community occurs at farrowing in sows,64 probably reflecting a peripartal digestive transit slow-down, which in turn is probably associated with concurrent hormonal changes. As sows approach farrowing, mild constipation is thus a common state.65 Even doubling the crude fiber content of the diet (from 3.8% to 7.0%) as the sow approaches farrowing reduces but does not prevent prolonged constipation.56 Besides the direct effect of fiber on digestive transit, an indirect effect has been described (but not explained) wherein sows fed high-fiber diets had a higher postpartum level of plasma prolactin than did control sows.49 This participates in making the “gut axis” so important in regard to the pathophysiology of PDS. In agreement with Reiner et al,66 we believe that a major pathophysiological component of PDS is triggered by bacterial endotoxins. As a consequence, these authors put emphasis on distinguishing fever and hyperthermia and the controversial cut-off between the two. Klopfenstein et al,67 for example, thoroughly discussed the value of 39.5°C that is often reported for diagnosis of fever and PDS. Rectal temperature is easy to measure, and it remains the parameter chosen to differentiate control sows from PDS sows. According to Reiner et al,66 the increase in rectal temperature is probably associated with the release of cytokines via macrophage activation. This would explain why an efficient treatment of PDS with non-steroidal anti-inflammatory drugs (NSAIDs) is often reported.68,69
Innate immunity, which has been clearly related to the mammary gland in cattle,70 is less recognized by practitioners than is adaptive immunity. Recently, a team from Poland71 showed that sows that developed PDS had higher levels of two proinflammatory cytokines, tumor necrosis factor α (TNFα) and IL-6, 12 to 24 hours and 2 to 3 days before farrowing, respectively. These cytokines also increased after experimental infection with E coli or administration of endotoxins,72,73 which may be explained by the fact TNFα is mainly produced by endotoxin-activated macrophages and is part of the activation processes of other cytokines, such as IL-6.74 Interestingly, recent results also support the hypothesis that polymorphisms of IL-6 and TNFα are associated with variations in amount of body fat within pig breeds.75 For example, the TNF gene is in a region where several quantitative trait loci for backfat thickness were also mapped.76
Finally, stress is a classical pathway involved in PDS.3 Papadopoulos et al19 reported that many risk factors for PDS are related to stress, such as late introduction of sows into farrowing crates. Bäckström et al77 reported that incidence of MMA is greater in sows subjected to major changes in their environment, such as being moved from pasture to crates a few days before farrowing. However, allowing the sows to adapt to the new environment (farrowing crates) for 25 days before parturition, rather than 3 to 5 days, was not associated with a lower incidence of problem litters.46 It has been demonstrated that confinement at the time of strong nest-building motivation, immediately prior to parturition, resulted in physiological stress in the sow.78 Thus, a period of acclimatization to the new farrowing-lactation environment appears to be necessary to reduce stress.
Three recent studies showed a relationship between stress and farrowing duration59 and between control of stress and colostrum production.79,80 This indirect evidence attributes a major role in PDS to stress.
An example of the linkage between the three components (body building, endotoxemia, and stress) of our proposed pathophysiology of PDS is the close relationship between the stress pathway and that of innate immunology.81 Thus, a current hypothesis of the effects of stress on immunity, which is based largely on data from rodents and humans, suggests that stress disrupts the balance between cellular immunity (T helper 1; Th1) and humoral immunity (T helper 2; Th2) in an attempt to achieve homeostasis (but not homeorhesis).82 This hypothesis holds that stress hormones influence the production of T helper cell (Th1 and Th2) cytokines, which determine the type of immune response that prevails.83 Cytokines provide the link between the innate and adaptive immune systems and help maintain T-cell homeostasis during infection.84 Stress can suppress, enhance, or have no effect on the immune status of an animal. Many of the conflicting findings reported may be partially explained by the animal (eg, genotype, age, social status), types of stressors, and management timing.
Evolution from MMA to PDS
We propose to consider that MMA syndrome is the emerging part of an iceberg represented by PDS, with MMA now less common than it was 20 years ago. The following hypotheses may explain this shift. During the past 2 decades, radical changes in feeding management of sows have occurred in Western Europe. These are not fully implemented worldwide, while genetic lines have been harmonized across countries much faster. It is now unusual to feed a single sow diet throughout the reproductive cycle. The common practice is to feed different gestating and lactating sow diets, or to use a sophisticated multiple-phase diet – a practice that is increasingly observed. “Single” diets were formulated to provide 160 to 165 g per kg crude protein (CP) with ingestion of 480 to 495 g CP per sow per day (3 kg feed allowance per day). Current practice has reduced the protein by 33% during the gestation period (130 g CP per kg, 2.5 kg feed allowance per day). Concurrent with these changes in protein levels is a shift in energy sources: diets previously based on grains (high starch) now include a larger share of by-products rich in plant cell walls, with positive effects on MMA prevention as speculated by Göransson85 and recently verified.86,87 Development of a specific net-energy system for sows88 allowed cost-effective, early implementation of such feeding strategies. These changes are depicted in Figure 3, showing the evolution of crude fiber (CF) and neutral detergent fiber (NDF) in gestating sow diets, based on trials reported at annual swine research meetings in France from 1985 to 2012 (Journées de la Recherche Porcine, nutrition sessions).89 The list of papers is available at www.aasv.org/. [The references are included at the end of this web page.] All trials related to sow feeding and showing the full ingredient composition of the gestation or lactation diets or both were selected to build the database. Nutrient contents were calculated from 75 recipes designed for gestating sows using the same database to ease comparison (EvaPig version 1.3.0.4; Ajinomoto-Eurolysine and INRA; 2012; available at http://www.evapig.com/x-home-en). A steady and progressive trend of increasing fiber content was found for both descriptors (an increase of 0.93 g per kg CF or 1.74 g per kg NDF per year). Diets used in these research studies did not necessarily reflect the field situation. However, they were close enough to “real-life diets” for the research results to be accepted and implemented.
Figure 3: Steady rise of crude fiber (CF) and neutral detergent fiber (NDF) content in gestating sow diets reported at the nutrition sessions of the Journées de la Recherche Porcine (France) from 1985 to 2012.89 The list of papers is available at www.aasv.org/. The references are included at the end of this web page. 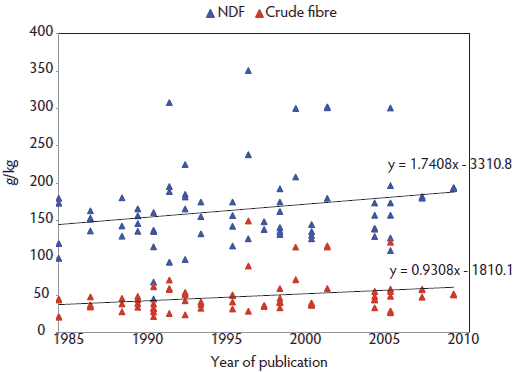 |
The last nutrient to be mentioned here is calcium (Ca). In the early 1990s, recommendations from many extension services were 1.10% Ca, but this then dropped to 0.70% to 0.80%, thus drastically reducing the amounts of calcium carbonate included in sow diets.90 Today, more fermentable fiber, as well as less protein and carbonate (pH buffer), are available to the digestive microbes, which should promote a healthier microbial balance and metabolism. Nevertheless, the modern sow faces more acute challenges, for example, due to its higher milk production. In the first 3 days postpartum, a “good” sow should produce enough milk to sustain growth of 14 piglets at 200 g per day each, ie, 2800 g per day, corresponding to 11.2 kg milk.91 Feed intake in early lactation is not sufficient to cover such nutritional requirements, and the deficit has increased accordingly. Moreover, body reserves did not improve to compensate for this: despite greater mean body size, mean backfat thickness has decreased.92,93 These consequences of genetic selection challenge the lifetime of feeding strategies that were based on management of body condition. Altogether, the situation gathers elements questioning the ability of the sow to cope with the change in status from pregnant to high-milk-producing.
Conclusions
As reported by Pijoan et al94 in 2004, there is an evolution among diseases. Acute mastitis is much less common today. However, early postpartum disorders are more frequent, for example, neonatal diarrhea22,30 or low weaning weight (common consulting request to one author [GPM] by practitioners; unpublished data). These “new” problems occur in very good herds where productivity is very high and management skills are developed. Thus, numerous different but interacting mechanisms that are reflected by PDS affect the optimum expression of the sows’ productive capacity in a way that is much less clinically visible than was the MMA syndrome.
The central concept for an understanding of how a slight change related to the animals or to their management can lead to major (although not spectacular) problems is homeorhesis and its critical shift occurring around the time of farrowing. Therefore, at parturition, “the orchestrated or coordinated changes in metabolism of body tissues necessary to support a (dominant) physiological state”6 have to shift from a priority of gestation to a priority of lactation. Many of these modifications occur before farrowing, but remain asymptomatic until revealing animals such as piglets are present. The shift from homeorhesis of gestation to homeorhesis of lactation has not yet been taken into account in proposals for the pathophysiology of PDS, probably because physiology, endocrinology, and clinical innate and adaptive immunology all must be simultaneously considered.
Conflict of interest
None reported.
References
1. Martineau GP, Farmer C, Peltoniemi O. Mammary system. In: Zimmerman JJ, Karriker LA, Ramirez A, Schwartz KJ, Stevenson GW, eds. Diseases of Swine. 10th ed. United Kingdom: John Wiley and Sons, Inc. 2012;18:270–293.
2. Tharp VL, Amstutz HE. Mastitis, metritis and agalactia. In: Dunne HW, ed. Diseases of Swine. Ames, Iowa: Iowa State University Press. 1958;36:501–507.
3. Maes D, Papadopoulos G, Cools A, Janssens GPJ. Postpartum dysgalactia in sows: pathophysiology and risk factors. Tierarztliche Praxis. 2010;38:515–520.
*4. Martineau GP, Smith BB, Doizé B. Pathogenesis, prevention and treatment of lactational insufficiency in sows. Vet Clinics N Amer Food Anim Pract. 1992;8:661–683.
5. Martineau GP. Postpartum dysgalactia syndrome and mastitis in sows. In: Aiello E, ed. Merck Veterinary Manual. 8th ed. Whitehouse Station, New Jersey: Merck and Co, Inc. 1998:1020–1024.
6. Bauman DE, Currie WB. Partitioning of nutrients during pregnancy and lactation: a review of mechanisms involving homeostasis and homeorhesis. J Dairy Sci. 1980;63:1514–1529.
7. Bauman DE. Regulation of nutrient partitioning during lactation: homeostasis and homeorhesis revisited. In: Cronje PB, ed. Ruminant Physiology: Digestion, Metabolism, Growth and Reproduction. United Kingdom: CAB International. 2000;18:311–328.
8. Casey T, Patel O, Dykema K, Dover H, Furge K, Plaut K. Molecular signatures reveal circadian clocks may orchestrate the homeorhetic response to lactation. PLoS ONE 4(10):e7395. doi:10.1371/journal.pone.0007395.
*9. Bauman DE. Homeorhesis: past, present and future. Elanco Swine Symposium. Indianapolis, Indiana: Eli Lilly and Co. 2010:1–20.
10. Bell AW, Bauman DE. Adaptations of glucose metabolism during pregnancy and lactation. J Mammary Gland Biol Neoplasia. 1997;2:265–278.
11. Martineau GP, Morvan H. Les facteurs prédisposants de la pathologie digestive au sevrage [Predisposing risk factors for postweaning digestive disorders]. In: Martineau GP. Maladies d’élevage des porcs. 2nd ed. Paris, France: Editions France Agricole. 2010;4:138–141.
12. Gerjets I, Kemper N. Coliform mastitis in sows: a review. J Swine Health Prod. 2009;17:97–105.
13. Gerjets I, Traulsen I, Reiners K, Kemper N. Comparison of virulence gene profiles of Escherichia coli isolates from sows with coliform mastitis and healthy sows. Vet Microbiol. 2011. doi:10.1016/j.vetmic.2011.05.002.
14. Kemper N, Preissler R. Bacterial flora on the mammary gland skin of sows and in their colostrum. J Swine Health Prod. 2011;19:112–115.
15. Kemper N, Gerjets I. Bacteria in milk from anterior and posterior mammary glands in sows affected and unaffected by post-partum dysgalactia syndrome (PPDS). Acta Vet Scand. 2009;51:26–32.
16. Leon E, Madec F. Etude de la phase périnatale chez le porc dans trois élevages. 1. La pathologie de la truie à la mise bas [Epidemiological observations about paripartum disorders in the pig in three farms. 1. The farrowing house]. Journées de la Recherche Porcine en France. 1992;24:89–98.
17. Leon E, Madec F. Etude de la phase périnatale chez le porc dans trois élevages. 2. Santé et performances du porcelet en phase d’allaitement [Epidemiological observations about peripartum disorders in the pig in three farms. 2. The suckling piglet]. Journées de la Recherche Porcine en France. 1992;24:99–108.
18. Papadopoulos GA. Lactation physiology in sows: impact of feeding strategies and risk factors for postpartum dysgalactia syndrome. PhD thesis. Ghent, Belgium: Ghent University; 2008:229.
19. Papadopoulos GA, Vanderhaeghe C, Janssens GPJ, Dewulf J, Maes DGD. Risk factors associated with postpartum dysgalactia syndrome in sows. Vet J. 2010;184:167–171.
20. Fairbrother JJ. Coliform mastitis. Diseases of the mammary glands. In: Straw BE, Zimmerman JJ, D’Allaire S, Taylor DJ, eds. Diseases of Swine. 9th ed. Ames, Iowa: Blackwell Publishing. 2006:665–671.
*21. Svensmark B. New neonatal diarrhoea syndrome in Denmark. Proc 1st European Symposium of Porcine Health Management. Copenhagen, Denmark. 2009:27.
*22. Gin T, Le Guennec J, Morvan H, Martineau GP. Clinical and laboratory investigations in 10 French pig herds dealing with enzootic neonatal diarrhea. Proc IPVS. Vancouver, Canada. 2010:758. Poster 452.
*23. Kongsted H, Angen O, Kokotovic B, Jorsal SE, Nielsen JP. New neonatal porcine diarrhea in Denmark – a clinical description. Proc IPVS. Jeju, Korea. 2012:72.
*24. Jonach B, Boye M, Jensen TK. Histopathological, morphological and fluorescence in situ hybridization investigation of new neonatal porcine diarrhea in Denmark. Proc IPVS. Jeju, Korea. 2012:1076.
*25. Wallgren P, Merza M. New neonatal porcine diarrhoea. I. Clinical outcome in an affected herd. Proc IPVS. Jeju, Korea. 2012:75.
*26. Wallgren P, Mattsson S, Merza M. New neonatal porcine diarrhoea. II. aspects on etiology. Proc IPVS. Jeju, Korea. 2012:76.
*27. Melin L, Wallgren P, Mattsson S, Stampe M, Löfstedt M. Neonatal diarrhoea in piglets from E. coli vaccinated sows in Sweden. Proc IPVS. Vancouver, Canada. 2010:290.
28. Bories P, Vautrin F, Boulot S, Père MC, Sialelli JN, Martineau GP. Analysis of physio-metabolic parameters in relation with long or complicated farrowings in the sow. Journées de la Recherche Porcine en France. 2010;42:233–239.
29. Sialelli JN, Lautrou Y, Oswald I, Quiniou N. Is there a relationship between sows’ characteristics and neonatal diarrhoea occurrence? Proposed answers from measurements performed in commercial units. Journées de la Recherche Porcine en France. 2009;41:167–172.
*30. Sialelli JN, Vautrin F, Lautrou Y, Oswald I, Quiniou N, Martineau GP. Farrowing progress (chronopart) and enzootic neonatal diarrhoea: observational study in nine commercial herds. Proc IPVS. Vancouver, Canada. 2010:752. Poster 446.
*31. Sialelli JN, Vautrin F, Bories P, Boulot S, Pere MC, Martineau GP. Chronopart in catheterized commercial sows in conventional herds: Physiological, biochemical and hormonal follow-up in “easy” farrowing and “difficult” farrowing sows. Proc IPVS. Vancouver, Canada. 2010:125.
32. Le Dividich J, Herpin P, Rosario-Ludovino RM. Utilization of colostral energy by the newborn pig. J Anim Sci. 1994;72:2082–2089.
33. Collier RJ, McNamara JP, Wallace CR, Dehoff MH. A review of endocrine regulation of metabolism during lactation. J Anim Sci. 1984;59;498–510.
34. Metz SHM, van den Bergh SG. Regulation of fat mobilization in adipose tissue of dairy cows in the period around parturition. Netherlands J Agric Sci. 1977;55:198.
35. Devillers N, Le Dividich J, Prunier A. Physiologie de la production de colostrum chez la truie [Physiology of colostrum production in sows]. INRA Productions Animales. 2006;19:29–38.
36. Michele MB. Collective behavior in gene regulation: Metabolic clocks and cross-talking. 2008;275:2356–2363.
*37. Eggert JM, Belstra BA, Richert BT, Schinckel AP. Total backfat and individual backfat layer changes of primiparous sows during late gestation and lactation. Proc Swine Day. Purdue University. 1998:65–74. Available at: http://www.ansc.purdue.edu/swine/swineday/sday98/10.pdf. Accessed 16 October 2012.
38. McPherson RL, Ji F, Wu G, Blanton JR, Kim SW. Growth and composition changes of fetal tissues in pigs. J Anim Sci. 2004;82:2534–2540.
39. Foisnet A. Variabilité de la production de colostrum par la truie: implication des changements endocriniens et métaboliques en période péripartum [Variability of colostrum production by sows: the involvement of sow physiology around parturition]. PhD thesis. Institut Supérieur des Sciences Agronomiques, agro-alimentaires, horticoles et du Paysage. Université de Rennes, France. 2010:250.
40. Neville MC, Berga AC. Cellular and molecular aspects of the hormonal control of mammary function. In: Neville MC, Neifert MR, eds. Lactation Physiology Nutrition and Breast-Feeding. New York, New York: Plenum Press. 1983:141–177.
41. Foisnet A, Farmer C, David C, Quesnel H. Relationships between colostrum production by primiparous sows and sow physiology around parturition. J Anim Sci. 2010;88:1672–1683.
42. Farmer C. The role of prolactin for mammogenesis and galactopoiesis in swine. Livest Prod Sci. 2001;70:105–113.
43. Hurley WL, Doane RM, O’Day-Bowman MB, Winn RJ, Mojonnier LE, Sherwood OD. Effect of relaxin on mammary development in ovariectomized pregnant gilts. Endocrinology. 1991;128:1285–1290.
*44. Smith P. The butterfly effect. Proc Aristotelian Society. 1990;91:247–267.
*45. Martineau GP. Body building syndromes in sows. Proc AASP. Denver, Colorado. 1990; 345–348.
46. Klopfenstein C, D’Allaire S, Martineau GP. Effect of adaptation to the farrowing crate on water intake of sows. Livest Prod Sci. 1995;43:243–252.
47. Le Cozler Y, David C, Beaumal V, Johansen S, Dourmad JY. Effect of the feeding level during rearing on performance of Large White gilts. Part 2: effect on metabolite profiles during gestation and lactation, and on glucose tolerance. Reprod Nutr Dev. 1998;38:377–390.
48. Meunier-Salaün MC, Gort F, Prunier A, Schouten WGP. Behavioural patterns and progesterone, cortisol and prolactin levels around parturition in European (Large-White) and Chinese (Meishan) sows. Appl Anim Behav Sci. 1991;31:43–59.
49. Quesnel H, Meunier-Salaün MC, Hamard A, Guillemet R, Etienne M, Farmer C, Dourmad JY, Père MC. Dietary fiber for pregnant sows: Influence on sow physiology and performance during lactation. J Anim Sci. 2009;87:532–543.
50. Farmer C, Devillers N, Rooks JA, Le Dividich J. Colostrum production in swine: from the mammary glands to the piglets. CAB Reviews: Perspectives in Agriculture, Veterinary Science, Nutrition and Natural Resources. 2006;3:1–16.
51. Frisch RE. The right weight: body fat, menarch and fertility. Proc Nutrition Society. 1994;53:113–129.
52. Martineau GP, Morvan H. Le syndrome de la truie accordéon [The accordion sow syndrome]. In: Martineau GP. Les maladies d’élevage des porcs. Paris, France: Editions France Agricole. 2010:424–427.
*53. Solignac T, Martineau GP. The over-muscled sow syndrome: a new emerging syndrome in a hyperprolific sow herds: clinical consequences, preliminary observations. Proc IPVS. Vancouver, Canada. 2010:1174. Poster 868.
*54. Solignac T, Keita A, Pagot E, Martineau GP. The over-muscled sow syndrome: a new emerging syndrome in a hyperprolific sow herds. Preliminary observations on farrowing duration. Proc IPVS. Vancouver, Canada. 2010:124.
*55. Solignac T, Martineau GP. The over-muscled sow syndrome: a new emerging syndrome in a hyperprolific sow herds. Effect of back fat and back lean on prolificacy. Proc IPVS. Vancouver, Canada. 2010:1175. Poster 869.
56. Oliviero C, Kokkonen T, Heinonen M, Sankari S, Peltoniemi O. Feeding sows with different amount of fibre during late pregnancy, farrowing and early lactation: Impact on the intestinal function, the energy balance and the litter. Res Vet Sci. 2009;86:314–319.
57. Vanderhaeghe C, Dewulf J, De Vliegher S, Papadopoulos GA, de Kruif A, Maes D. Longitudinal field study to assess sow level risk factors associated with stillborn piglets. Anim Repro Sci. 2010;120:78–83.
58. Muirhead MR, Alexander TJL. Managing and treating disease in the farrowing and suckling period. In: Managing Pig Health and the Treatment of Disease. Sheffield, United Kingdom: 5M Enterprises Ltd. 1997;8:231.
59. Oliviero C, Heinonen M, Valros A, Peltoniemi O. Environmental and sow related factors affecting the duration of farrowing. Anim Reprod Sci. 2010;119:85–91.
*60. Gerjets I, Kruse S, Krieter J, Kemper N. Diagnosis of MMA affected sows: bacteriological differentiation, temperature measurement and water intake. Proc IPVS. Durban, South Africa. 2008:236.
61. Zhu Y, Magnusson U, Fossum C, Berg M. Escherichia coli inoculation of porcine mammary glands affects local mRNA expression of Toll-like receptors and regulatory cytokines. Vet Immunol Immunopathol. 2008;125:182–189.
62. Aderem A, Ulevitch RJ. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511.
63. Mulder I, Schmidt B, Stokes CR, Lewis M, Bailey M, Aminov RI, Prosser JI, Gill BP, Pluske JR, Mayer CD, Musk CC, Kelly D. Environmentally acquired bacteria influence microbial diversity and natural innate immune responses at gut surfaces. BMC Biol. 2009;7:79–99.
*64. Walker N, Cintora M, Durand H, Le Treut Y. Influence of a live yeast on the faecal microflora of gestating and lactating sows. J Anim Sci. 2008;86(E-suppl 2):293. Abstract 294.
65. Kamphues J, Tabeling R, Schwier S. Die Kotqualität von Sauen unter dem Einfluss verschiedener Fütterungs-und Haltungsbedingungen [Feces quality of sows under the influence of different feeding and housing conditions]. Deutsche Tierarztliche Wochenschrifte (Hannover). 2000;107:380.
66. Reiner G, Hertampf B, Richard HR. Dysgalactia in the sow post partum – a review with special emphasis on pathogenesis. Tierarztliche Praxis. 2009;37:305–318.
67. Klopfenstein C, Farmer C, Martineau GP. Diseases of the mammary glands. In: Straw BE, Zimmerman JJ, D’Allaire S, Taylor DJ, eds. Diseases of Swine. 9th ed. Ames, Iowa: Blackwell Publishing; 2006:57–74.
*68. Hoy S, Friton G. Preliminary findings of the pharmaco-economic benefit evaluation of meloxicam (Metacam) treatment on litter performance of sows with MMA syndrome. Proc IPVS. Hannover, Germany. 2004;597.
*69. Lamana J, Ubiergo A, Rubio S, Salleras JM. Post-farrowing treatment of sows with meloxicam or flunixin meglumine on the preweaning weight gain of the low birth weight piglets in subclinical MMA. Proc IPVS. Ames, Iowa. 2006. Poster 32–05:476.
70. Rainard P, Riollet C. Innate immunity of the bovine mammary gland. Vet Res. 2006;37:369–400.
71. Szczubial M, Urban-Chmiel R. Tumour necrosis factor-α and interleukin-6 concentration in the serum of sows with MMA syndrome. Bulletin of the Veterinary Institute in Pulawy. 2008;52:267–270.
72. Wang JF, Wang M, Ma JL, Jiao LG, Zhou XY, Lindberg JE. The influence of intramammary lipopolysaccharide infusion on serum Ca, P, vitamin D, cytokines and cortisol concentrations in lactating sows. J Vet Med A. 2006;53:113–118.
73. Zhu Y, Berg M, Fossum C, Magnusson U. Proinflammatory cytokine mRNA expression in mammary tissue of sows following intramammary inoculation with Escherichia coli. Vet Immunol Immunopath. 2007;116:98–103.
74. Riollet C, Rainard P, Poutrel B. Differential induction of complement fragment C5a and inflammatory cytokines during intramammary infections with Escherichia coli and Staphylococcus aureus. Clin Diag Lab Immunol. 2000;7:161–167.
75. Szydlowski MA, Buszka A, Mackowski M, Lechniak D, Switonski M. Polymorphism of genes encoding cytokines IL6 and TNF is associated with pig fatness. Livest Sci. 2010;136:150–156.
76. Hu ZL, Fritz ER, Reecy JM. Animal QTLdb: a livestock QTL database tool set for positional QTL information mining and beyond. Nucleic Acids Res. 2007;35(suppl 1):D604-D609. doi 10.1093/nar/gkl946. Available at: http://www.animalgenome.org/cgi-bin/QTLdb/SS/index. Accessed 16 October 2012.
77. Bäckström L, Morkoc AC, Connor J, Larson R, Price W. Clinical study of mastitis-metritis-agalactia in sows in Illinois. JAVMA. 1984;185:70–73.
78. Lawrence AB, Petherick JC, McLean KA, Deans LA, Chirnside J, Vaughan A. The effect of environment on behaviour, plasma cortisol and prolactin in parturient sows. Appl Anim Behav Sci. 1994;39:313–330.
*79. Biermann J, Jourquin J, van Gelderen R, Goossens L. Facilitating nursing behavior of sows at farrowing has a positive effect on colostrum distribution. Proc IPVS. Vancouver, Canada. 2010:1187. Poster 991.
*80. Tseng S, Jourquin J, Goossens L. Facilitation of nursing behavior in sows during colostrum phase improves piglet condition. Proc IPVS. Vancouver, Canada. 2010:1188. Poster 882.
81. Salak-Johnson JL, McGlone JJ. Making sense of apparently conflicting data: stress and immunity in swine and cattle. J Anim Sci. 2007;85(E suppl):E81-E88.
82. Moynihan JA, Cohen N. Stress and immunity. In: Schneiderman N, McCabe P, Baum A, eds. Perspectives in Behavioral Medicine. Stress and Disease Processes. Hillsdale, New Jersey: Lawrence Erlbaum Associates Inc. 1992;2:27–54.
83. Elenkov IJ, Webster EL, Torpy DJ, Chrousos GP. Stress, corticotrophin-releasing hormone, glucocorticoids, and the immune/inflammatory response: acute and chronic effects. Ann NY Acad Sci. 1999;876:1–11.
84. Bot A, Smith KA, Von Herrath M. Molecular and cellular control of T1/T2 immunity at the interface between antimicrobial defense and immune pathology. DNA Cell Biol. 2004;23:341–350.
85. Göransson L. The effect of feed allowance in late pregnancy on the occurrence of agalactia post partum in the sow. J Vet Med A. 1989;36:505–513.
86. Tabeling R, Schwier S, Kamphues J. Effects of different feeding and housing conditions on dry matter content and consistency of faeces in sows. J Anim Phys Anim Nutr. 2003;87:116–121.
87. Sarandan H, Neufeld N, Neufeld K. Effects of a new lignocellulose product for fibre supplementation on MMA symptoms, reproductive performance and faecal quality in a pig farm with evident MMA problems in Romania. Lucrari Stiintifice Medicina Veterinara Timisoara. 2008;16:851–858.
88. Noblet J, Shi XS, Dubois S. Metabolic utilization of dietary energy and nutrients for maintenance energy requirements in sows: basis for a net energy system. Br J Nutr. 1993;70:407–419.
89. Journées de la Recherche Porcine [Swine Research Days]. Paris, France: INRA IFIP. 2012. Available at: http://www.journees-recherche-porcine.com/texte/index.htm. Accessed 16 October 2012.
90. Mahan DC. Mineral nutrition of the sow: a review. J Anim Sci. 1990;68:573–582.
91. Noblet J, Etienne M. Estimation of sow milk nutrient output. J Anim Sci. 1989;67:3352–3359.
92. Mejia-Guadarrama CA, Pasquier A, Dourmad JY, Prunier A, Quesnel H. Protein (lysine) restriction in primiparous lactating sows: effects on metabolic state, somatotropic axis, and reproductive performance after weaning. J Anim Sci. 2002;80:3286–3300.
93. Hughes PE, Smits RJ, Xie Y, Kirkwood RN. Relationships among gilt and sow live weight, P2 backfat depth, and culling rates. J Swine Health Prod. 2010;18:301–305.
*94. Pijoan C, Torremorell M, Dee S. Pig health and production. Past, present, future. Proc IPVS. Hannover, Germany. 2004;1:1–3.
*Non-refereed references.
Supplemental references for Figure 3
Boone C, Cadoret A, Père MC, Etienne M, Mourot J, Effets du taux et de la nature des lipides du régime de gestation et de lactation des truies sur le développement des tissus adipeux des porcelets. Journées de la Recherche Porcine. 2001;33:157-163.
Buron G, Gatel F. Utilisation de la féverole (Vicia faba) par la truie en reproduction. Journées de la Recherche Porcine. 1992 ;24:187-194.
Cambeilh D, Meymerit C, Cazaux JG, Castaing J, Skiba F. Incidence de la réduction de l’apport de phosphore dans les aliments pour les truies en gestation-lactation. Journées de la Recherche Porcine. 2005;37:7-16.
Castaing J, Cambeilh D. Inertage du maïs grain humide entier comparé au maïs sec ou au blé pour la truie en gestation-lactation. Journées de la Recherche Porcine. 1996;28:355-364.
Castaing J, Cambeilh D. Influence de la granulométrie des aliments sur les performances et le comportement des truies en gestation-lactation. Journées de la Recherche Porcine, 2001;33:165-172.
Castaing J, Cambeilh D, Coudure R. L’ensilage de maïs grain humide avec ou sans rafle pour la truie en gestation-lactation. Journées de la Recherche Porcine, 1993;25:181-192.
Castaing J, Cambeilh D, Etienne M, Courboulay V. Comparaison, chez la truie reproductrice, d’un régime simple à base de céréales à deux régimes complexes isoénergétiques par l’association de fibres et de matières grasses. Journées de la Recherche Porcine. 1999;31:207-214.
Christon R, Lionet H, Racon B, Saminadin G, Gaudru L, Schaeffer B, Cerneau P. Effet d’une incorporation de lipides dans le régime de la truie en lactation, sur son niveau d’ingestion, ses performances et celles de sa portée. Journées de la Recherche Porcine., 1998;30:265-273.
Courboulay V, Gaudré D. Faut-il distribuer des aliments enrichis en fibres aux truies en groupe ? Journées de la Recherche Porcine. 2002;34:225-232.
De Quelen F, Boudry G, Fillaut M, Mourot J. Variation de la composition en acides gras du lait de truie au cours de la lactation en fonction de la teneur en acide alpha-linolénique du régime. Journées de la Recherche Porcine. 2010;42:139-140.
Dourmad JY, Influence du niveau alimentaire de gestation sur les performances de reproduction et le comportement alimentaire de la truie en lactation. Journées de la Recherche Porcine. 1989;21:109-114.
Dourmad JY, Etienne M. Etude des besoins en lysine et en thréonine de la truie en gestation. Journées de la Recherche Porcine. 1998;30:201-207.
Dourmad JY, Etienne M, Noblet J. La reconstitution des réserves corporelles chez la truie multipare en gestation. Influence du niveau de mobilisation au cours de la lactation précédente. Journées de la Recherche Porcine. 1994;26:277-284.
Etienne M. Utilisation comparée du blé, du maïs et de l’orge par la truie en gestation. Journées de la Recherche Porcine. 1985;17:433-440.
Etienne M. Apports énergétiques de gestation et accrétion de protéines chez la truie nullipare. Journées de la Recherche Porcine. 1991;23:69-74.
Etienne M, Dourmad JY. Effets de la consommation de tourteau de colza normal ou à faible teneur en glucosinolates sur la reproduction chez la truie. Journées de la Recherche Porcine. 1987;19:231-238.
Etienne M, Dourmad JY, Barrios A, Noblet J. La reconstitution des réserves corporelles chez la truie multipare en gestation: influence des apports d’énergie. Journées de la Recherche Porcine. 1991;23:75-84.
Etienne M, Dourmad JY, Evrard J. Effets de la consommation de tourteau de colza à très basse teneur en glucosinolates pendant la croissance et la gestation chez la truie. Journées de la Recherche Porcine. 1993;25:193-202.
Etienne M, Dourmad JY, Noblet J, Castaing J. Association de parois végétales et de graisses dans le régime de lactation des truies. Effets sur l’utilisation digestive, la composition du lait et des porcelets. Journées de la Recherche Porcine. 1999;31:199-205.
Etienne M, Dourmad JY, Noblet J, Le Bellego L, Van Cauwenberghe S. Contribution à la détermination du besoin en valine des truies. Journées de la Recherche Porcine. 2005;37:195-202.
Etienne M, Noblet J, Dourmad JY, Fortune H. Etude du besoin en lysine des truies en lactation. Journées de la Recherche Porcine. 1989;21:101-108.
Etienne M, Dourmad JY, Obindzinski W. Effets du tourteau de colza à basse teneur en glucosinolates pendant la gestation chez la truie. Journées de la Recherche Porcine. 1990;22:243-250.
Etienne M, Oswald I, Bony S, Lallès JP, Pinton P, Trépier B, Lessard M. Effets de la contamination par le déoxynivalénol (DON) de l’aliment des truies reproductrices. Journées de la Recherche Porcine. 200;38:233-240.
Gatel M, Buron G, Leuillet M. Utilisation du pois protéagineux de printemps par la truie en gestation-lactation. Journées de la Recherche Porcine. 1987;19:223-230.
Guillemet R, Hamard A, Quesnel H, Père MC, Etienne M, Dourmad JY, Meunier-Salaün MC. Comportement alimentaire et performances de reproduction chez la truie en lactation : impact d’un aliment fibreux pendant la gestation. Journées de la Recherche Porcine. 2006;38:453-460.
Gatel F, Buron G, Leuillet M. Utilisation comparée de régimes de gestation à base de blé ou d’orge par la truie au cours de plusieurs cycles de reproduction. Journées de la Recherche Porcine. 1989;21:93-100.
Gerfault V, Mourot J, Etienne M, Mounier A. Influence de la nature des lipides dans le régime de gestation de la truie sur ses performances et la composition corporelle des porcelets à la naissance. Journées de la Recherche Porcine. 1999;31:191-197.
Giguère A, Lambert R, Girard CL, Laforest JP, Matte JJ. L’acide folique, le conditionnement utérin et le contrôle des performances de reproduction chez les truies nullipares. Journées de la Recherche Porcine. 1999;31:33-38.
Girard C, Matte JJ, Robert S, Farmer C, Martineau GP. Evolution des concentrations sériques de nutriments mineurs chez des truies reproductrices soumises à des régimes à haute teneur en fibres. Journées de la Recherche Porcine. 1992;24:195-200.
Martel-Kennes Y, Cormier I, Green D, Guillou D, Hall RE, James B, Moore J, Nash M, Niver J, Roy M. Effet de la granulométrie des céréales dans l’aliment de lactation sur les performances des truies et des porcelets. Journées de la Recherche Porcine., 2006;38:171-176.
Matte JJ, Girard CL. Le besoin en acide folique chez la truie gravide. Utilisation métabolique des folates comme critère de mesure. Journées de la Recherche Porcine. 1996;28:365-370.
Matte JJ, Laforest JP, Farmer C, Girard CL. Le contrôle de la survie embryonnaire chez le porc: l’effet de l’acide folique sur certaines caractéristiques du milieu utérin et sur le développement embryonnaire. Journées de la Recherche Porcine. 1994;26:293-298.
Mosnier E, Ramaekers P, Meunier-Salaün MC, Etienne M. Consommation d’aliment de la truie après la mise-bas: relation avec le tryptophane plasmatique et la réactivité émotionnelle. Journées de la Recherche Porcine. 2008;40:159-166.
Paboeuf F, Dourmad JY, Quentric O, Calvar C, Landrain B, Roy H. Impact de la concentration en nutriments de l’aliment sur les performances de lactation des truies et de leur portée. Journées de la Recherche Porcine. 2002;34:81-87.
Paboeuf F, Ramonet Y, Corlouër A, Dourmad JY, Cariolet R, Meunier-Salaün MC. Impact de l’incorporation de fibres dans un régime de gestation sur les performances zootechniques et le comportement des truies. Journées de la Recherche Porcine. 2000;32:105-113.
Quiniou N, Etienne M, Mourot J, Noblet J. Apport supplémentaire d’aliment ou de lipides pendant les 10 derniers jours de gestation et conséquences sur les performances de mise-bas et de lactation. Journées de la Recherche Porcine. 2008;40:151-158.
Quiniou N, Gaudré D, Rapp S, Guillou D. Influence de la température ambiante et de la concentration en nutriments de l’aliment sur les performances de lactation de truies primipares. Journées de la Recherche Porcine. 2000;32:275-282.
Quiniou N, Mourot J, Etienne M, Richard S. Quel est l’impact d’un apport d’énergie sous forme de lipides pendant la gestation et/ou la lactation sur les performances des truies allaitantes et celles des porcelets jusqu’à l’abattage? Journées de la Recherche Porcine. 2006;38:177-184.
Quiniou N, Mourot J, Richard S, Etienne M, Coudray L. Influence d la nature de l’énergie allouée à la truie pendant la gestation et la lactation sur ses performances de lactation et celles de sa portée et sur la composition corporelle des porcs au sevrage et à l’abattage. Journées de la Recherche Porcine. 2005;37:203-210.
Ramonet Y, Meunier-Salaün MC, Dourmad JY. Effets d’une incorporation de parois végétales dans la ration alimentaire sur l’activité comportementale des truies gestantes. Journées de la Recherche Porcine. 1997;29:167-174.