Gomes Neto JC, Gauger PC, Strait EL, et al. Mycoplasma-associated arthritis: Critical points for diagnosis. J Swine Health Prod. 2012;20(2):82–86
| Diagnostic notes | Peer reviewed |
SummaryMycoplasma hyosynoviae and Mycoplasma hyorhinis are known causative agents of arthritis in postweaned swine. Data from the Iowa State University Veterinary Diagnostic Laboratory shows that diagnosis of M hyosynoviae- and M hyorhinis-associated arthritis has increased in swine in the Midwest United States. This diagnostic note summarizes disease characteristics of both pathogens and describes appropriate procedures to diagnose Mycoplasma-associated arthritis. An accurate diagnosis is critical to establishing effective treatment and prevention measures in affected herds. | ResumenLos Mycoplasma hyosynoviae y Mycoplasma hyorhinis son agentes causales conocidos de la artritis porcina postdestete. Información del laboratorio de diagnóstico veterinario de la universidad estatal de Iowa indica que el diagnóstico del M hyosynoviae y del M hyorhinis asociados a artritis en cerdos ha aumentado en el medio-oeste de los Estados Unidos. Esta nota diagnóstica resume las características de enfermedad de ambos patógenos y describe los procedimientos apropiados para diagnosticar la artritis asociada a Mycoplasma. Un diagnóstico adecuado es crítico para establecer un tratamiento y las medidas preventivas adecuadas en piaras afectadas. | ResuméMycoplasma hyosynoviae et Mycoplasma hyorhinis sont reconnus comme des agents étiologiques d’arthrite chez les porcs en période post-sevrage. Les résultats provenant du Iowa State Veterinary Diagnostic Laboratory montrent que les diagnostics d’arthrite associée à M hyosynoviae et M hyorhinis ont augmenté chez les porcs du midwest américain. Ce bref rapport résume les caractéristiques des conditions associées à ces deux agents pathogènes et décrit les procédures appropriées pour diagnostiquer les arthrites causées par les mycoplasmes. Un diagnostic précis est essentiel afin d’établir un traitement efficace et des mesures préventives dans les troupeaux affectés. |
Keywords: swine, arthritis, Mycoplasma hyorhinis, Mycoplasma hyosynoviae
Search the AASV web site
for pages with similar keywords.
Received: August 23, 2011
Accepted: November 30, 2011
Arthritis is defined as inflammation of the intra-articular tissue of one or more joints. It is characterized by an increased volume of intra-articular fluid with specific features that vary depending on the cause. Features that may have diagnostic significance include color, turbidity, hemorrhage, or exudate. In addition, arthritis may be classified according to the cause, the duration (ie, acute, chronic), or the components of the exudate, ie, serous, fibrinous, purulent, macrophagic, lymphoplasmacytic.1
Infectious arthritis in swine is commonly associated with bacteria. Pathogens isolated from arthritic joints may include Erysipelothrix rhusiopathiae, Streptococcus suis, Haemophilus parasuis, Actinobacillus suis, Arcanobacterium pyogenes, Staphylococcus species, Salmonella choleraesuis, Mycoplasma hyorhinis, and Mycoplasma hyosynoviae.2-5 Infectious arthritis is an increasing concern to swine producers as it may contribute to compromised animal welfare and decrease profitability of the operation. Despite concerns and a long history of recognition, specific information is often lacking regarding the pathogenesis, epidemiology, and control of infectious arthritis in modern swine-production systems.
The Iowa State University Veterinary Diagnostic Laboratory (ISU-VDL) diagnosed 431 cases of lameness between 2003 and 2010. Overall, 69% (298 of 431) of the clinical cases had evidence of infectious arthritis. Mycoplasma species accounted for an average of 17% of arthritis cases over all 8 years, with an increasing frequency in recent years, up to 37% (41 of 111) in 2010 (Figure 1). Mycoplasma hyorhinis was diagnosed more frequently in animals ≤ 10 weeks of age. Conversely, M hyosynoviae was diagnosed more often in animals > 10 weeks of age (Figure 2). However, due to the inherent bias of diagnostic data and lack of random sampling, it is difficult to correlate these numbers to the actual disease prevalence in the field.
| Figure 1: Frequency of diagnosis of Mycoplasma hyosynoviae and Mycoplasma hyorhinis in cases of swine lameness submitted to the Iowa State University Veterinary Diagnostic Laboratory (Ames, Iowa) between 2003 and 2010. 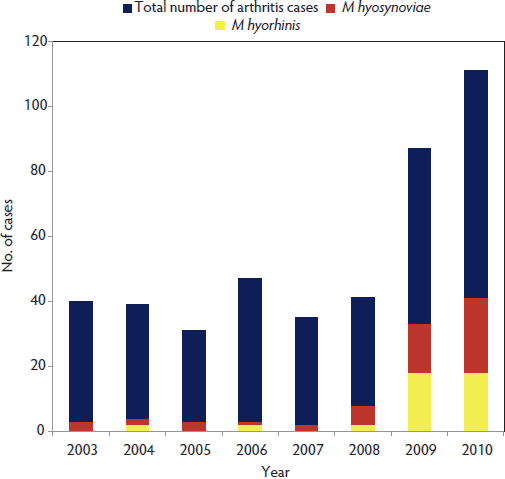 |
Figure 2: Frequency of Mycoplasma hyosynoviae and Mycoplasma hyorhinis cases diagnosed by age from the total number of cases of swine lameness submitted to the Iowa State University Veterinary Diagnostic Laboratory (Ames, Iowa) between 2003 and 2010.
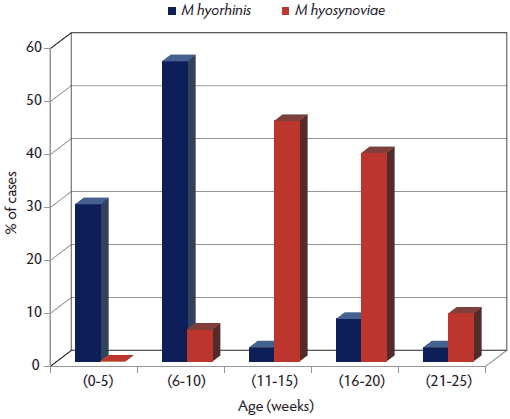 |
In response to the increasing number of Mycoplasma-associated arthritis cases diagnosed at the ISU-VDL, this article will describe disease characteristics as well as proper sampling and diagnostic tests necessary to establish a diagnosis of M hyorhinis- and M hyosynoviae-associated arthritis in swine.
Pathogenesis
Mycoplasma hyorhinis colonizes the upper respiratory tract of pigs, and may be isolated from swine lungs with or without pneumonia.6,7 However, systemic disease is more commonly diagnosed after weaning.8 The process of systemic dissemination remains unknown, but affinity for serosal surfaces may lead to acute inflammation of the serosa of body cavities and synovium (membrane lining the joint cavity). If not effectively mitigated by the immune response or medication, infections at those sites may become chronic, resulting in compromised growth and performance.9 Serofibrinous pleuritis, pericarditis, and peritonitis are typical lesions that may generate fibrous adhesions in chronic stages.4,6,8
Mycoplasma hyosynoviae may cause arthritis in pigs approximately 2 to 3 weeks post exposure.10 Tonsils are the primary site of infection, which may persist to the adult age and occasionally cause systemic disease at that time.6,8,10,11 There is an incubation period of approximately 3 to 10 days that may often follow stress, such as movement or vaccination.8,10,11 The acute phase of infection lasts approximately 2 weeks, during which time the pathogen may spread to the joints and various tissues (eg, spleen, lung) throughout the body by a hematogenous route.10,11
Many factors may influence Mycoplasma-associated arthritis disease expression, including maternal or herd immunity, strain virulence, management practices or biosecurity, breed, and environment.8 However, it is currently unknown how each of these factors may contribute to disease pathogenesis.
Clinical signs
Mycoplasma hyorhinis may be associated with polyserositis and polyarthritis, generally occurring in 3- to 10-week-old pigs,4 although occasionally it may occur in older animals.4,8 Clinical signs typically occur 3 to 10 days after exposure and continue for approximately 10 to 14 days, depending on the severity of disease and presence of co-infections. Pigs appear unthrifty and lethargic and may demonstrate reluctance to move, lameness, and swollen joints. In addition, conjunctivitis and otitis have been associated with M hyorhinis infection.6,8,12,13
Mycoplasma hyosynoviae lameness generally occurs in 3- to 5-month-old pigs, appearing acutely in one or more legs.6,14 Clinical signs that may occur in a portion of or all affected animals include lameness, stiffness, swollen joints, pain or discomfort, and reluctance to stand.14,15 Clinical signs may persist for 3 to 10 days, after which lameness gradually decreases in severity. Many animals recover without treatment; however, clinical signs may persist, especially when co-infections or joints lesions such as osteochondritis dissecans are present. Swollen joints may or may not be observed in lame pigs with infectious arthritis. However, discomfort during ambulation and reluctance to stand can be frequently observed in the majority of affected pigs and suggests further evaluation.
Animal and sample selection and procedures
Nursery, grower, or finisher pigs demonstrating lameness, swollen joints, reluctance to stand, shifting hind-limb lameness, or a painful, uncomfortable gait may be selected for sampling. Acutely affected pigs without prior antibiotic treatment are preferred for diagnostic testing. Chronically affected pigs that have demonstrated prolonged lameness, joint abscesses, recumbency, and emaciation often have other co-infections that compromise the accuracy of diagnosis, and these animals should be avoided. Selected animals should be humanely euthanized immediately prior to sampling. Pigs that have already died should be avoided due to potential chronicity, death due to a noninfectious process, lack of a proper antemortem examination, or combinations of these.
Determining the correct sampling procedures to use will depend on the objectives of the investigation. Basically, two different outcomes may be pursued. The first is that of a definitive diagnosis for the clinical disease present in a particular situation. The second may be more epidemiological, that is, detection of the pathogen within a specific population to determine prevalence, incidence, or age of colonization.
Although M hyosynoviae and M hyorhinis may be present as commensal organisms in the upper respiratory tract of unaffected animals,8,11 confirming Mycoplasma-associated arthritis requires detection of the pathogen in affected joints and characteristic microscopic lesions in synovial tissue.
Following clinical evaluation, necropsy should include an evaluation of all joints and body systems. Gross observation of serofibrinous to fibrinopurulent polyserositis and arthritis are suggestive of M hyorhinis infection; however, similar lesions may be caused by infection with Haemophilus parasuis and Streptococcus suis.6 Mycoplasma hyosynoviae-affected joints tend to have large amounts of yellow or brown viscous fluid of a serofibrinous or serosanguineous nature. The synovial membrane may appear dark in color, edematous, proliferative, or hyperemic.6,14
Synovial fluid from elbow, stifle, hock, and shoulder must be collected using a sterile syringe and appropriately sized needle (eg, selection based on age) after removing the skin and sterilizing the region with a small propane flame to decrease bacterial contamination (Figure 3A). Affected whole legs may be submitted intact to the diagnostic laboratory as an alternative to tissue samples and joint fluid.
Figure 3: A. Collecting joint fluid from a 6-week-old pig demonstrating lameness prior to diagnostic testing. B. Swabbing the intra-articular cavity for diagnostic testing. Photos courtesy of PCG, Iowa State University Veterinary Diagnostic Laboratory, Ames, Iowa.
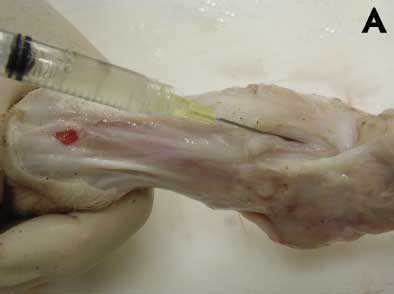 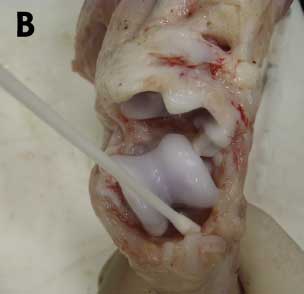 |
Lung tissues should be submitted when fibrinous pleuritis is present and M hyorhinis infection is suspected. Pericardial, thoracic, and peritoneal fluid may also be submitted if excess effusions or polyserositis are present. Fresh and fixed heart, as well as other abdominal tissues such as liver and spleen, should be submitted in cases where polyserositis is prominent.
An alternative sampling option when insufficient synovial fluid is available may include multiple swabs aseptically collected after carefully opening the joint (Figure 3B). Multiple pieces of synovium should be collected in 10% formalin and included for microscopic evaluation. The presence of characteristic microscopic lesions in synovium, in addition to pathogen detection, is required for a definitive diagnosis.
Synovial fluid from live animals can be collected from swollen joints using a sterile syringe and needle after surgical preparation of the affected area. An advantage of this procedure is that euthanasia can be avoided; however, this technique causes discomfort to the animal and usually requires sedation for proper sampling.
Cross-sectional samples derived from weaning- to finishing-age pigs may be used to determine the prevalence of Mycoplasma infection within a population, or prevalence may be determined longitudinally by following a specific group of animals over time. Cross-sectional studies can be used to determine the number of positive animals over time in a specific population. A disadvantage of this method is that one group of animals may not represent the prevalence of Mycoplasma infection in the entire herd. Therefore, horizontal or serial studies may be more appropriate to determine the approximate time pigs become colonized with M hyosynoviae or M hyorhinis or the prevalence of positive animals over time. However, either method may require evaluation of multiple groups depending on the diagnostic question and the herd situation (eg, herds that have a stable parity flow may require fewer groups to establish a pattern).
Nasal swabs and tonsil scrapings should be collected individually and not pooled for sample analysis. Mycoplasma hyorhinis may be detected in nasal swabs; however, tonsil scrapings are more appropriate for detecting M hyosynoviae.
Submission of samples
Sample submission to a diagnostic laboratory should include a complete and thorough history and clinical evaluation. A complete history should include a description and the duration of clinical signs, age and total number of affected animals, mortality, and differential diagnosis where applicable.
Synovial fluid or effusions should be transferred to polystyrene tubes prior to submission, which helps avoid leakage, cross-contamination, and damage during shipping. Synovial swabs and fluids, tissue samples, effusions, and intact joints or legs should be submitted on ice and shipped within 24 hours of collection to a diagnostic laboratory. Swabs should be in transport medium (eg, Amies medium) to prevent dehydration. Tissues submitted in 10% formalin should be properly identified and packaged appropriately to minimize the possibility of leakage. If appropriate conditions and instruments for sampling are not available, submission of affected whole legs or a live, acutely affected animal is preferred. All samples submitted to a diagnostic laboratory should be fresh and kept cool prior to and during shipment.
Diagnostic tests
Real-time polymerase chain reaction (PCR) is the most sensitive assay available to detect Mycoplama species. Real-time PCR assays have been developed for both M hyorhinis and M hyosynoviae and may be conducted directly on clinical specimens (such as joint fluid), joint swabs, nasal swabs, or tonsil scrapings when applicable. Bacterial culture is available upon request and is necessary if further evaluation of an isolate is warranted, such as antibiotic-resistance profiles. However, bacterial cultures are time-consuming and can be overgrown by other bacterial species. Mycoplasma species require specialized medium for growth; therefore, a specific request for Mycoplasma culture is required.
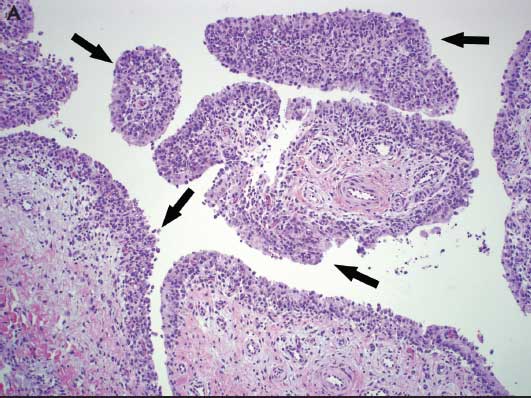
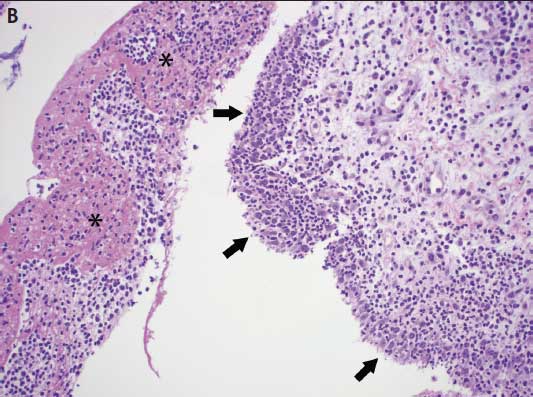
Microscopic lesions characteristic of Mycoplasma infection are necessary to confirm a diagnosis of Mycoplasma-associated arthritis. Mycoplasma hyosynoviae microscopic lesions may include hyperplasia of the synovium, moderate synovial villous hypertrophy, edema, and mononuclear cell infiltration with mild fibrosis of the capsule.6,14 Mycoplasma hyorhinis typically induces a mixture of mononuclear and polymorphonuclear cells in serosal and synovial membranes, which may lead to erosion of cartilage and pannus formation in chronically affected joints (Figure 4).6,8
Figure 4: A. Photomicrograph from a 12-week-old pig demonstrating lameness in which Mycoplasma hyosynoviae was detected by real-time polymerase chain reaction (PCR) in joint fluids. Chronic proliferative synovitis with mononuclear inflammation of the synovial membrane and hyperplasia and hypertrophy of synoviocytes (arrows) (H&E stain, original magnification 100×). B. Photomicrograph from a 6-week-old pig exhibiting swollen joints in which Mycoplasma hyorhinis was detected by real-time PCR in joint fluids. Asterisks (*) indicate fibrinosuppurative inflammation of the synovial membrane with a mixture of mononuclear and polymorphonuclear cells in the subsynovial connective tissues. Arrows indicate hyperplasia and hypertrophy of synoviocytes (H&E stain, original magnification 100×). Photos courtesy of DMM, Iowa State University Veterinary Diagnostic Laboratory, Ames, Iowa.
  |
Maternal antibodies against Mycoplasma species may be present for some time after weaning in positive herds, although the duration of maternal immunity has not been widely studied. Antibody detection in postweaned animals may help determine the exposure status of that particular group when followed over time. Conversely, it is not uncommon to find PCR-positive animals with no detectable antibody titers (JCGN, personal observation). In pigs experimentally infected with M hyosynoviae, antibody titers persisted for as long as 6 months post inoculation.16 Mycoplasma infection demonstrated in tonsils was correlated with a significantly higher antibody response in carrier pigs. In addition, slaughter pigs with higher antibody titers were more likely to demonstrate Mycoplasma in tonsils.17
Acknowledgement
The authors appreciate the cooperation of the ISU-VDL in making the database available for assessment.
References
1. Weisbrode SE, Doige DE. Bone and joints. In: Macgavin MD, Carlton WW, Zachary JF. Thomson’s Special Veterinary Pathology. 3rd ed. Saint Louis, Missouri: Mosby Inc. 2001:528–529.
2. Friis NF, Hansen KK, Schirmer AL, Aabo S. Mycoplasma hyosynoviae in joints with arthritis in abattoir baconers. Acta Vet Scand. 1992;33:205–210.
3. Hariharan H, MacDonald J, Carnat B, Bryenton J, Heaney S. An investigation of bacterial causes of arthritis in slaughter hogs. J Vet Diagn Invest. 1992;4:28–30.
4. Magnusson U, Wilkie B, Mallard B, Rosendal S, Kennedy B. Mycoplasma hyorhinis infection of pigs selectively bred for high and low immune response. Vet Immunol Immunopathol. 1998;61:83–96.
5. Turner GV. A microbiological study of polyarthritis in slaughter pigs. J S Afr Vet Assoc. 1982;53:99–101.
6. Kobisch M, Friis NF. Swine mycoplasmoses. Rev Sci Tech Off Int Epiz. 1996;15:1569–1605.
7. Lin JH, Chen SP, Yeh KS, Weng CN. Mycoplasma hyorhinis in Taiwan: Diagnosis and isolation of swine pneumonia pathogen. Vet Microbiol. 2006;115:111–116.
8. Thacker EL. Mycoplasmal diseases. In: Straw BE, Zimmerman JJ, D’Allaire S, Taylor DJ, eds. Diseases of Swine. 9th ed. Ames, Iowa: Blackwell Publishing Professional. 2006:707–710.
9. Browning GF, Marenda MS, Markham PF, Noormohammadi AH, Whitear KG. Mycoplasma. In: Gyles CL, Prescott JF, Songer JG, Thoen CO, eds. Pathogenesis of Bacterial Infections in Animals. 4th ed. Ames, Iowa: Blackwell Publishing Professional. 2010:549–573.
10. Hagedorn-Olsen T, Nielsen NC, Friis NF. Induction of arthritis with Mycoplasma hyosynoviae in pigs: Clinical response and re-isolation of the organism from body fluids and organs. J Vet Med A. 1999;46:317–325.
11. Hagedorn-Olsen T, Nielsen NC, Nielsen J. Progression of Mycoplasma hyosynoviae infection in three pigs herds. Development of tonsillar carrier state, arthritis, and antibodies in serum and synovial fluid in pigs from birth to slaughter. J Vet Med A. 1999;46:555–564.
12. Friis NF, Kokotovic B, Svensmank B. Mycoplasma hyorhinis isolation from cases of otitis media in piglets. Acta Vet Scand. 2002;43:191–193.
13. Morita T, Fukuda H, Awakura T, Shimiada A, Umemura T, Kazama S, Yagihashi T. Demonstration of Mycoplasma hyorhinis as a possible primary pathogen of porcine otitis media. Vet Pathol. 1995;32:107–111.
14. Nielsen EO, Nielsen NC, Friis NF. Mycoplasma hyosynoviae in grower-finisher pigs. J Vet Med A. 2001;48:475–486. doi:10.1046/j.1439–0442.2001.00378.x.
15. Ross RF, Switzer WP, Dunmay JR. Experimental production of Mycoplasma hyosynoviae arthritis in swine. Am J Vet Res. 1971;32:1743–1749.
16. Zimmerman BJ, Ross RF. Antibody response of swine experimentally infected with Mycoplasma hyosynoviae. Vet Microbiol. 1982;7:135–146.
17. Nielsen EO, Lauritsen KT, Friis NF, Enoe C, Hagedorn-Olsen T, Jungersen G. Use of a novel serum ELISA method and the tonsil-carrier state for evaluation of Mycoplasma hyosynoviae distributions in pig herds with or without clinical arthritis. Vet Microbiol. 2005;111:41–50.