Shen HG, Loiacono CM, Halbur PG, et al. Age-dependent susceptibility to porcine circovirus type 2 infections is likely associated with declining levels of maternal antibodies. J Swine Health Prod. 2012;20(1):17–24
| Original research | Peer reviewed |
SummaryObjectives: To investigate the relationships between age, porcine circovirus type 2 (PCV2) viremia, and pathologic lesions by evaluating the age of porcine circovirus associated disease- (PCVAD-) affected pigs submitted to Iowa State University Veterinary Diagnostic Laboratory between 2003 and 2010 and by experimentally infecting pigs of different ages. Materials and methods: A total of 3565 PCVAD cases were selected and ages of PCVAD-affected pigs summarized. Sixty-two pigs were randomly assigned to three groups and inoculated with PCV2 at 2 weeks (AGE-2; n = 21), 7 weeks (AGE-7; n = 20), or 12 weeks of age (AGE-12; n = 21). A portion of the pigs in each group arbitrarily selected by the farm manager were vaccinated with an adjuvanted vaccine (Mycoplasma hyopneumoniae) at 1 week of age. All pigs were euthanized 14 days post inoculation (DPI). Lesions and viremia were compared among groups. Results: Among PCVAD cases received from 2003 to 2010, 47.6% were 12 to 16 weeks of age. AGE-12 pigs had significantly lower levels of PCV2 antibody compared with AGE-2 and AGE-7 pigs at the time of inoculation, and AGE-12 pigs had significantly higher levels of PCV2 viremia at 7 and 14 DPI. Mycoplasma hyopneumoniae-vaccinated pigs in the AGE-12 group had higher levels of PCV2 viremia compared to nonvaccinated pigs; however, this effect was not seen in younger pigs. Implications: Under field conditions, 12- to 16-week-old pigs are most commonly affected by PCVAD. Under experimental conditions, 12-week-old pigs are more susceptible to PCV2 infection than are 2- and 7-week-old pigs. | ResumenObjetivos: Investigar las relaciones entre edad, viremia del circovirus porcino tipo 2 (PCV2 por sus siglas en inglés), y lesiones patológicas mediante la evaluación de la edad de los cerdos afectados con la enfermedad asociada a circovirus porcino (PCVAD por sus siglas en inglés) enviados al Laboratorio de Diagnóstico Veterinario de la Universidad del Estado de Iowa entre 2003 y 2010 y de cerdos experimentalmente infectados de diferentes edades. Materiales y métodos: Se seleccionaron un total de 3565 casos de PCVAD y se resumieron las edades de los cerdos afectados por PCVAD. Se asignaron al azar sesenta y dos cerdos a tres grupos y se inocularon con PCV2 a las 2 semanas (EDAD-2; n = 21), 7 semanas (EDAD-7; n = 20), ó 12 semanas de edad (EDAD-12; n = 21). Se vacunó una porción de los cerdos de cada grupo, seleccionado de manera arbitraria, por el administrador de la granja con una vacuna con adyuvante (Mycoplasma hyopneumoniae) a la semana de edad. Se practicó la eutanasia a todos los cerdos a los 14 días post inoculación (DPI por sus siglas en inglés). Se compararon las lesiones y la viremia entre los grupos. Resultados: Entre los casos de PCVAD recibidos de 2003 a 2010, 47.6% fueron de 12 a 16 semanas de edad. Los cerdos de EDAD-12 tuvieron niveles significativamente más bajos de anticuerpos de PCV2 comparados con los cerdos de EDAD-2 y EDAD 7 al momento de la inoculación, y los cerdos de EDAD-12 tuvieron niveles significativamente más altos de viremia de PCV2 a los 7 y 14 DPI. Los cerdos vacunados con M hyopneumoniae en el grupo de EDAD-12 tuvieron niveles más altos de viremia de PCV2 comparado con los cerdos no vacunados; sin embargo, este efecto no se observó en los cerdos más jóvenes. Implicaciones: Bajo condiciones de campo, los cerdos de 12 a 16 semanas de edad son afectados más comúnmente por el PCVAD. Bajos condiciones experimentales, los cerdos de 12 semanas de edad son más susceptibles a una infección de PCV2 que los cerdos de 2 y 7 semanas de edad. | ResuméObjectifs: Étudier les relations entre l’âge, la virémie au circovirus porcin de type 2 (PCV2), et les lésions pathologiques en évaluant l’âge des porcs affectés par la maladie associée au circovirus porcin (PCVAD) soumis au Iowa State University Veterinary Diagnostic Laboratory entre 2003 et 2010 et en infectant de manière expérimentale des porcs d’âges variés. Matériels et méthodes: Un total de 3565 cas de PCVAD ont été sélectionnés et les âges des animaux affectés de PCVAD résumés. On assigna de manière aléatoire 62 porcs dans trois groupes qui furent inoculés avec du PCV2 à l’âge de 2 semaines (AGE-2; n = 21), 7 semaines (AGE-7; n = 20), ou 12 semaines (AGE-12; n = 21). Une portion des porcs dans chaque groupe sélectionnés de manière arbitraire par le gérant de la ferme ont été vaccinés avec un vaccin avec adjuvant (Mycoplasma hyopneumoniae) à 1 semaine d’âge. Tous les porcs ont été euthanasiés 14 jours post inoculation (DPI). Une comparaison des lésions et de la virémie a été faite entre les groupes. Résultats: Parmi les cas de PCVAD reçus entre 2003 et 2010, 47.6% avaient entre 12 et 16 semaines d’âge. Les porcs du groupe AGE-12 avaient des niveaux d’anticorps contre le PCV2 significativement plus bas que ceux des groupes AGE-2 et AGE-7 au moment de l’inoculation, et les porcs AGE-12 avaient une virémie significativement plus élevée aux jours 7 et 14 DPI. Comparativement aux animaux non-vaccinés, les porcs vaccinés contre M hyopneumoniae dans le groupe AGE-12 avaient une virémie à PCV2 plus élevée; toutefois, cet effet n’était pas observé chez les animaux plus jeunes. Implications: Dans des conditions de terrain, les porcs âgés de 12 à 16 semaines sont les plus fréquemment affectés par PCVAD. Sous des conditions expérimentales, les porcs âgés de 12 semaines sont plus susceptibles aux infections par PCV2 que ceux âgés de 2 et 7 semaines.
|
Keywords: swine, porcine circovirus type 2, porcine circovirus associated disease, age susceptibility, PCV2, PCVAD
Search the AASV web site
for pages with similar keywords.
Received: April 20, 2011
Accepted: August 2, 2011
Porcine circovirus type 2 (PCV2) was first identified as the primary cause of postweaning multisystematic wasting syndrome (PMWS) in pigs in Canada.1 In addition, PCV2 is also a component of various other clinical syndromes, including the porcine respiratory disease complex (PRDC),2,3 porcine dermatitis and nephropathy syndrome (PDNS),4 chronic enteritis,5 and reproductive failure.6 The spectrum of clinical manifestations associated with PCV2 infections is referred to as porcine circovirus associated disease (PCVAD). The most frequent gross lesions associated with PCVAD are enlargement of lymph nodes and non-collapsed, tan-mottled lungs.7 The most characteristic microscopic lesions of PCVAD are lymphoid depletion or histiocytic replacement of follicles in lymphoid tissues or both.7-9 Serological surveys have shown that a high percentage of pig herds are infected with PCV2.10,11 Subclinical PCV2 infections are very common, and only a small percentage of infected pigs develop PCVAD.12 Several effective commercial PCV2 vaccines became available in 2006 and are now widely used in the US pig population.13 Vaccines for PCV2 have been shown to reduce PCV2 viremia and lesions under experimental conditions14-16 and improve the average daily weight gain and the number of pigs marketed under field conditions.17,18
Age at infection can impact the severity of many diseases of animals. The PMWS form of PCVAD is most commonly seen between 5 and 12 weeks of age,19 PDNS between 6 and 17 weeks of age,20,21 and PRDC between 8 and 26 weeks of age.3 Age-dependent resistance to another member of the Circoviridae family, psittacine feather and beak disease virus, has been observed. Juvenile birds between 0 and 3 years of age are more susceptible to disease.22 Few studies on age-dependent susceptibility are available in the peer-reviewed literature for pigs. However, it is well known that younger pigs are more susceptible to certain enteric pathogens, such as rotavirus, which typically affects 1- to 5-day-old pigs23 and causes little to no disease in older pigs.24 Interestingly, it has also been found that susceptibility of pigs to some Escherichia coli isolates increases with age, which was found to be related to changes in pilus-mediated adhesion.25 In an in vitro study, pulmonary alveolar macrophages from 4-week-old pigs were found to be more permissive to porcine reproductive and respiratory syndrome virus (PRRSV) infection compared to those from 4-month-old pigs.26 In addition, PRRSV viremia was longer and excretion rates were higher in younger pigs (4 to 8 weeks of age) compared to older pigs (16 to 24 weeks of age).27
In the present study, our objectives were to investigate the relationship between age and levels of PCV2 viremia and PCV2-associated lesions and expression of PCVAD by evaluating the age of PCVAD-affected pigs submitted to the Iowa State University Veterinary Diagnostic Laboratory (ISU-VDL) between 2003 and 2010 and by experimentally infecting pigs with PCV2 at different ages representing different production stages (suckling, nursery, grow-finish).
Materials and methods
Retrospective analysis of laboratory submissions (2003-2010)
Included data. Cases diagnosed as PCVAD at the ISU-VDL, Ames, Iowa, between January 1, 2003, and December 31, 2010, were obtained from the ISU-VDL Laboratory Information Management System (LIMS) database. In the ISU-VDL LIMS, all cases are classified using diagnostic codes assigned by case-coordinators responsible for the cases. The following ISU-VDL-specific diagnostic codes are associated with PCV2: PCV (systemic infection), PMWS (postweaning multisystemic wasting syndrome), PNEU PCV (lung-pneumonia, PCV), ENTE PCV (intestines-enteritis, PCV), PDNS (porcine dermatitis and nephropathy syndrome), and ABOR PCV (abortion, PCV). In this investigation, we selected for all diagnostic codes listed above with the exception of ABOR PCV. The cases identified were verified individually to confirm that the diagnostic code matched criteria used for diagnosis of PCVAD (presence of characteristic microscopic lesions, presence of intra-lesional PCV2 antigen). A case was defined by submission and not by individual pigs. The age of the pigs was recorded in rounded weeks (eg, week 0 would be < 3.5 days old, week 1 would be 3.5 to < 10.5 days old), with the exception of pigs ≥ 27 weeks, which were considered to be 27 weeks old due to the low numbers in this age group.
Experimental inoculation
The experimental protocol was approved by the Iowa State University Institutional Animal Care and Use Committee (IACUC) and the Institutional Biosafety Committee (IBC).
Animal source. Sixty-two segregated-early-weaned (SEW) pigs were obtained from a high-health status commercial herd, negative by monthly serological monitoring for PRRSV and swine influenza virus (SIV). The sows in this herd were seropositive for PCV2 as determined by an enzyme-linked immunosorbent assay (ELISA)28 based on the open reading frame 2 (ORF2) protein of PCV2; however, PCVAD had not been reported in the breeding herd or in their offspring. Porcine circovirus type 2 vaccination was not used in the breeding herd at the time the pigs were obtained for the study, and the dams of the pigs were never vaccinated against PCV2. Approximately 100 pigs were individually ear tagged, and blood samples were collected from the pigs at 1 week of age on the farm and tested for anti-PCV2 serum antibodies using a PCV2 ELISA.28 Samples with a sample-to-positive (S:P) ratio ≥ 0.2 were considered positive for PCV2-specific antibodies. Pigs were sorted by rising S:P ratio, and the 62 pigs with the lowest S:P ratios were selected for the study. The pig with the highest S:P ratio at the time of selection that was included in the trial at 1 week of age had an S:P ratio of 0.8. At 2 weeks of age, the pigs were brought to the Livestock Infectious Disease Isolation Facility at Iowa State University, Ames, Iowa.
Experimental design. The experimental design is summarized in Table 1. Approximately half of the 62 selected pigs were arbitrarily selected by the farm manager at 1 week of age and were vaccinated using an inactivated Mycoplasma hyopneumoniae bacterin (RespiSure-One; Pfizer Animal Health, Exton, Pennsylvania). Each vaccinated pig received 1 mL of the bacterin intramuscularly in the right side of the neck. The number of vaccinated pigs in each group is indicated in Table 1. Blood samples were collected upon entry into the research facility at 2 weeks of age and weekly thereafter, and the serum was tested by PCV2 ELISA. The pigs were blocked by vaccination status and arbitrarily assigned to three groups: PCV2 inoculation at 2 weeks of age (AGE-2; n = 21), at 7 weeks of age (AGE-7;
n = 20), and at 12 weeks of age (AGE-12; n = 21). At 14 days post inoculation (DPI), all pigs from each treatment group were necropsied. A negative control group was not included in the experimental study as the main study outcome was comparison of PCV2 viremia and lesions in different age groups. All three groups came to the research facility at the same time, and since AGE-12 pigs remained PCV2 DNA-negative until the time of inoculation, unintended PCV2 infection by natural exposure to a PCV2-viremic pig from the source herd had not occurred.
Table 1: Numbers of segregated-early-weaned pigs that received treatment of the total number in the group for pigs vaccinated with an inactivated Mycoplasma hyopneumoniae bacterin* and challenged at 2, 7, or 12 weeks of age with PCV2†
* RespiSure-One; Pfizer Animal Health, Exon, Pennsylvania. † Intranasal challenge with PCV2 isolate 40895 at a dose of 104.5 TCID50. Pigs were challenged at 2 weeks of age (AGE-2), 7 weeks of age (AGE-7), or 12 weeks of age (AGE-12). PCV2 = porcine circovirus type 2; TCID50 = median tissue culture infectious dose.
|
Clinical evaluations. Pigs were monitored every day for presence of clinical signs such as inappetence, lethargy, and visible weight loss. In addition, rectal temperatures were measured and respiratory disease was evaluated at the same time every other day using the following scoring system: 0 = normal, 1 = sporadic sneezing, 2 = rapid respiratory rate with shallow respirations of short duration, and 3 = prolonged and labored respiration.
PCV2 inoculation. To ensure that all pigs received the same inoculum at the same titer, the PCV2 inoculum for all pigs was prepared ahead of time, divided into three aliquots, and stored at -80ºC. Pigs in all groups received PCV2 isolate 40895 (from a case of naturally occurring PCVAD in Iowa29) at a dose of 104.5 median tissue culture infective doses intranasally. For each inoculation, one aliquot was thawed under cold tap water and used within 1 hour.
Serological testing. A PCV2 ELISA based on a recombinant ORF2 capsid protein of PCV2 was performed on serum samples as previously described.28
Evaluation of gross and microscopic lesions. At necropsy, the severity of macroscopic lung lesions (0% to 100% of lung involvement) was estimated and the size of lymph nodes was scored from 0 (normal) to 3 (four times normal size)30 by a pathologist not blinded to age of the pigs at necropsy. Tissue samples were collected from the superficial inguinal, external iliac, mediastinal, tracheobronchial, and mesenteric lymph nodes, lung, liver, spleen, tonsil, kidney, ileum, and colon, placed in 10% neutral buffered formalin, and routinely processed for microscopic examination. Lungs were infused with formalin at the time of necropsy. Microscopic lesions were evaluated by a pathologist blinded to the status of the treatment groups. Lymphoid depletion and histiocytic replacement of follicles in lymph nodes, tonsil, and spleen were scored from 0 (none) to 3 (severe).30 Lesions in the liver, kidney, and heart were scored for severity of lymphohistiocytic inflammation from 0 (none) to 3 (severe).
Immunohistochemistry. Sections of lymph nodes (superficial inguinal, external iliac, mediastinal, tracheobronchial, and mesenteric), tonsil, and spleen were examined by immunohistochemistry (IHC) as described31 to detect PCV2 antigen. The amount of PCV2 antigen was scored from 0 (none) to 3 (strongly positive) by a pathologist blinded to the treatment status of the animals.30
DNA extraction and quantitative PCR. Deoxyribonucleic acid was extracted from all serum samples using a commercial DNA isolation kit (QIAamp DNA Blood Mini Kit; Qiagen, Valencia, California). Deoxyribonucleic acid extracts were then used to quantify the amount of PCV2 DNA by real-time PCR as previously described.32
Statistical analysis
The data analysis for this project was performed using SAS software, version 9.2 (SAS Institute, Cary, North Carolina). For parametric data, differences among groups were determined by analysis of variance (ANOVA). If significant differences were identified (P < .05), pairwise testing using Tukey-Kramer adjustment was then performed. In addition, PCV2 viremia data obtained on 7 and 14 DPI (number of genomic copies per mL serum) were log10 transformed and analyzed using the three-way ANOVA model. Explanatory variables included group (age), vaccination status, antibody status, and their interactions. Binary responses (PCV2 antigen prevalence as determined by IHC stains) were analyzed using a logistic regression model. In addition, for nonparametric data (including microscopic and IHC scores), a Kruskal-Wallis ANOVA test was performed to test for differences among groups. If significant differences were identified (P < .05), a pairwise Wilcoxon test was performed.
Results
Retrospective analysis of laboratory submissions (2003-2010)
Age prevalence of PCVAD in field cases collected 2003-2010. From 2003 to the 2010, 3688 pigs were recorded as PCVAD cases in ISU-VDL LIMS, which included 92 subclinical cases (without lesions or with mild microscopic lesions limited to individual lymphoid tissues and low amounts of intralesional PCV2 antigen) and 3596 clinical cases. Of note, the 92 subclinical cases were identified among cases originally classified as PCVAD and do not represent all subclinical PCV2 cases submitted to the ISU-VDL. Among the 3596 clinical PCVAD cases, 31 were removed due to missing age information, resulting in 3565 cases which were included in the analysis in this study. As shown in Figure 1, the peak of PCVAD diagnoses at the ISU-VDL occurred in 2006 (1050 cases) and has since been decreasing. The average age of pigs with PCVAD was 13.1 ± 0.1 weeks, with 47.5% of the PCVAD pigs between 12 and 16 weeks of age (Figure 2). Interestingly, the mean age was highest in 2008 (14.1 ± 0.3 weeks) and has declined through 2009 (12.8 ± 0.3 weeks) and 2010 (11.7 ± 0.4 weeks) (Figure 1).
Figure 1. Mean age in weeks (± SE) of pigs diagnosed with porcine circovirus associated disease in cases submitted to the Iowa State University Veterinary Diagnostic Laboratory (ISU-VDL), Ames, Iowa, from 2003 to 2010. The age of the pigs was expressed in rounded weeks. The red dotted line indicates the total number of cases submitted to the ISU-VDL during this time period. 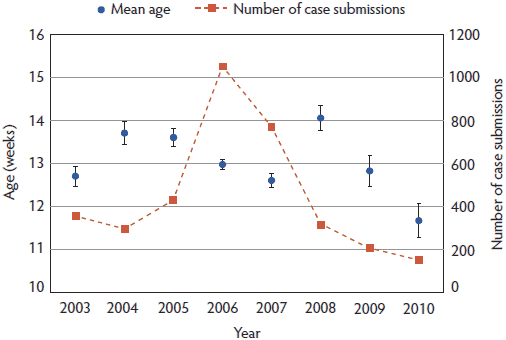 |
Figure 2. Numbers of porcine circovirus associated disease cases at different pig ages submitted to the Iowa State University Veterinary Diagnostic Laboratory, Ames, Iowa, between 2003 and 2010. The black line represents a trend line and was added to account for the apparent bias in recording even weeks rather than odd weeks on the submission form. The age of the pigs was expressed in rounded weeks with the exception of pigs ≥ 27 weeks of age, which were classified as 27 weeks old. The percentages represent the numbers of cases 0-11 weeks of age (1209 cases; 33.3%), 12-16 weeks of age (1694 cases; 47.5%, red frame), and 17-27 weeks of age (662 cases; 18.6%). 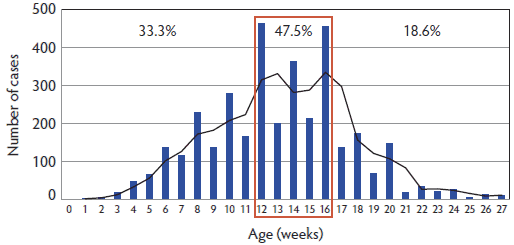 |
Experimental inoculation
Clinical signs. None of the pigs developed fever (rectal temperatures > 40ºC) or had visible weight loss at any time during the experiment. Respiratory scores were 0 or 1 in all pigs throughout the study.
Anti-PCV2 IgG antibody levels. Numbers of seropositive pigs in the three treatment groups are shown in Table 2. At the time of PCV2 inoculation and at 7 DPI, AGE-12 pigs had significantly (P < .05) lower PCV2 S:P ratios than AGE-2 and AGE-7 pigs (Table 2). By 14 DPI, no significant differences in antibody levels were observed among the different ages. When M hyopneumoniae-vaccinated and nonvaccinated pigs were compared within each age group, only AGE-7 vaccinated pigs had significantly higher PCV2 S:P ratios (P < .05), compared with AGE-7 nonvaccinated pigs, at DPI 14 (Table 3).
Table 2: Prevalence and mean group PCV2 antibody presented as ELISA* sample-to-positive (S:P) ratios and viremia levels† presented as log10 PCV2 DNA copies/mL in the three treatment groups at 0, 7, and 14 DPI‡.
* ELISA based on a recombinant ORF2 capsid protein of PCV2.28 † PCV2 DNA quantified by real-time polymerase chain reaction.32 ‡ Treatment groups described in Table 1. abc Values with different superscripts within a column and within a parameter represent significant differences between treatment groups (P < .05; pairwise testing using the Tukey-Kramer adjustment). PCV2 = porcine circovirus type 2; ELISA = enzyme-linked immunosorbent assay; ORF2 = open reading frame 2; DPI = days post inoculation. |
||||||||||||||||||||||||||||||||||
Table 3: Prevalence and mean group PCV2 antibody (ELISA* S:P ratios) and viremia levels† in pigs in the same treatment group, either vaccinated or not vaccinated against Mycoplasma hyopneumoniae, at 0, 7, and 14 DPI‡.
* ELISA based on a recombinant ORF2 capsid protein of PCV2.28 † PCV2 DNA quantified by real-time polymerase chain reaction32 and presented as log10 PCV2 DNA copies/mL. ‡ Treatment groups described in Table 1. ab Values with different superscripts within a column and within a parameter represent significant differences between pigs in the same treatment group either vaccinated or not vaccinated against M hyopneumoniae (P < .05; pairwise testing using the Tukey-Kramer adjustment). PCV2 = porcine circovirus type 2; ELISA = enzyme-linked immunosorbent assay; S:P = sample-to-positive ratio; DPI = days post inoculation; ORF2 = open reading frame 2. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PCV2 DNA in serum. The detailed PCR results are summarized in Tables 2, 3, and 4. Main effects “age,” “antibody status,” and the interactions “age by vaccination status” and “age by antibody status” were significant for DPI 7. Main effects “age” and “antibody status” were significant for DPI 14. Specifically, among AGE-2 pigs, 71.4% (DPI 7) and 66.7% (DPI 14) of pigs were positive for PCV2 DNA after challenge (five of 21 were negative on both DPI 7 and 14), while 100% of pigs in the AGE-7 and AGE-12 groups were positive for PCV2 DNA (Table 2). The log10 PCV2 DNA loads in AGE-12 pigs were significantly higher than those in AGE-2 and AGE-7 pigs (Table 2). When the effect of PCV2 antibody at the time of inoculation was evaluated, seronegative AGE-2 pigs had significantly higher levels of PCV2 DNA than did seropositive AGE-2 pigs at 7 and 14 DPI, in contrast to AGE-7 and AGE-12 groups (Table 4). When vaccinated and nonvaccinated pigs were compared within each age group, nonvaccinated AGE-12 pigs had lower amounts of PCV2 in serum, which was significant on DPI 7 (Table 3).
Table 4: Prevalence and group mean PCV2 viremia levels* of seronegative and seropositive pigs† at the time of inoculation with PCV2 at 7 and 14 DPI‡.
* PCV2 DNA quantified by real-time polymerase chain reaction32 and presented as log10 PCV2 DNA copies/mL. † Treatment groups described in Table 1. ‡ Sera tested by ELISA based on a recombinant ORF2 protein of PCV2, with sample-to-positive ratio ≥ 0.2 the cutoff for a positive test. ab Values with different superscripts within a column and within a parameter represent significant differences between PCV2 seronegative and PCV2 seropositive pigs in the same group (P < .05; pairwise testing using the Tukey-Kramer adjustment). PCV2 = porcine circovirus type 2; DPI = days post inoculation; ELISA = enzyme-linked immunosorbent assay; ORF2 = open reading frame 2. |
|||||||||||||||||||||||||||
Microscopic lesions and PCV2 antigen in tissues. Lymphoid depletion and histiocytic replacement were observed in lymphoid tissues in 10 of 21 AGE-2 pigs, four of 20 AGE-7 pigs, and five of 21 AGE-12 pigs, with overall lymphoid scores of 1.7 ± 0.6, 0.5 ± 0.3, and 0.9 ± 0.5, respectively. There were no significant differences in overall lymphoid scores among challenged groups of different ages. Porcine circovirus type 2 antigen was detected in lymphoid tissues of 13 of 21 AGE-2 pigs, 11 of 20 AGE-7 pigs, and 14 of 21 AGE-12 pigs. The mean group PCV2 IHC scores ranged from 1.3 to 1.4, with no significant differences among age groups. When the effect of PCV2 antibody at the time of inoculation was evaluated, it was found that seropositive AGE-2 pigs (16 of 21) had significantly lower overall lymphoid score (0.7 ± 0.2) (P < .05) and PCV2 IHC score (0.9 ± 0.3) (P < .05) than the five of 21 seronegative pigs that had mean overall lymphoid score and PCV2 IHC scores of 2.1 ± 0.8 and 2.4 ± 0.7, respectively. When the effect of vaccination was evaluated, no significant differences were observed between pigs vaccinated or not vaccinated against M hyopneumoniae.
Discussion
Porcine circovirus associated diseases can affect pigs of widely varying ages. When investigating veterinary diagnostic laboratory case trends over time, it is important to realize that submission behaviors can change over time as veterinary practitioners and producers become accustomed to the different clinical manifestations of PCVAD. All known manifestations of PCVAD, including PMWS, respiratory disease, PDNS, enteric disease, and reproductive failure, were described as early as 2003, but it is certainly possible that some PCVAD forms were initially not recognized by some submitting veterinarians or pathologists in charge of case investigations and may have been misclassified. Also, it is possible that new disease definitions of PCVAD and submission biases during 2003–2010 affected the age distribution; however, we used the numbers of submissions instead of individual pigs to minimize the possible effects of large numbers in a few submissions.
On the basis of review of 3565 PCVAD cases submitted to the ISU-VDL in the midwestern United States between 2003 and 2010, pigs between 12 and 16 weeks of age appear to be most susceptible to PCVAD, and the age distribution was unchanged following the introduction and widespread use of PCV2 vaccination during 2007-2010. Limitations of this work comprising data from submissions to the ISU-VDL include the following: certain states such as Iowa may be over-represented, with higher numbers than other states due to proximity of the ISU-VDL to pig producers; multiple cases are likely to have come from individual facilities; a portion of the cases included multiple pigs; and samples from states outside the Midwest, such as Texas, North Carolina, Oklahoma, and others, were also included. A recent surveillance study for PMWS conducted in Spain assessing PCVAD cases between 1998 and 2008 revealed an older mean age (approximately 14 weeks) for PCVAD cases in 2005.33 Considering that the PCVAD epidemic in Spain started 4 to 5 years before it started in the United States, there are certainly some similarities between trends found in the Spanish VDL and in the ISU-VDL, such as the peak in age in the Spanish study in 2005, which was observed 5 years after the peak in cases in 2000, and the peak in age observed in the current study in 2008, which was also observed after the peak in cases in 2006. However, the mean age of PCVAD-diagnosed pigs appeared to decline from 2008 to 2010 in this study. After PCV2 vaccines became widely used, 98% of all pigs that reach the market in the United States are thought to be vaccinated against PCV2 (oral communication, Dr Edgar Diaz, Boehringer Ingelheim Vetmedica, 2010), and the mean age of pigs affected by PCVAD decreased by 2.4 weeks between 2008 and 2010. Much more needs to be learned about PCVAD, but among other facts, this observation raises concern that a lower level and duration of passive immunity is being transferred to piglets. Also not currently approved in the United States, regimens to vaccinate breeding herds to enhance levels of passively acquired antibodies in growing pigs may need to be considered in the few herds that do not currently vaccinate growing pigs for PCV2 or that have ongoing or recurring PCVAD problems in vaccinated growing pigs. Alternatively, other recently re-emerging pathogens such as rotavirus or Brachyspira species could be interacting with PCV2 and increasing replication earlier in life. In addition, the observation of age-dependent susceptibility to PCV2 could also be partially due to changes in submission practices.
Under experimental conditions, we found that AGE-12 pigs had significantly higher levels of PCV2 viremia than AGE-2 and AGE-7 pigs after PCV2 challenge. This suggests that 12-week-old pigs are more susceptible to PCV2 infection than younger pigs. This finding was also consistent with the predominance of pigs 12 to 16 weeks of age among the laboratory-submitted cases. There is much debate among practitioners on the significance of PCV2 viremia on an individual pig level and on a herd level, and more clarification on the impact of PCV2 viremia is needed. It appears that higher levels of PCV2 could increase the risk of secondary infections due to immune suppression or immune modulation. Additionally, PCV2 transmission within a population could be enhanced, but further studies on this are needed. Some PCV2 vaccines have been shown to reduce or even prevent PCV2 viremia under experimental conditions.14-16
There are contradictory reports on the efficacy of maternally derived anti-PCV2 antibodies. While clinical investigation suggested that low PCV2 antibody titers in sows were positively related to mortality in their offspring,34 virological and serological surveys showed that high levels of antibody to PCV2 in sows does not relate to a decrease in PCV2 viremia in their piglets.35 Some studies suggested that passive immunity was able to prevent the development of PCVAD, but not subclinical PCV2 infection.36 In this study, five of 21 pigs in the AGE-2 group were negative for PCV2 viremia at both 7 and 14 DPI. It is possible that these pigs had a sterilizing immunity, and the passively acquired antibodies in these pigs prevented PCV2 replication entirely. It is also possible that PCV2 viremia was very short in these pigs and with the 7-day sampling interval used in this study, we were unable to detect viremia. Since we selected piglets with lower S:P ratios at the beginning of the study, this may not be entirely representative of the field situation and the results may differ in production systems where there are piglets with high anti-PCV2 antibody levels.
It has also been suggested that the protection conferred by maternal antibodies may be titer dependent.37 In the present study, PCV2-seropositive AGE-2 pigs at the time of challenge had significantly lower levels of PCV2 viremia and overall lymphoid scores, as well as less PCV2 antigen in tissues compared with seronegative AGE-2 pigs after challenge, suggesting that the maternally derived antibody provided protection. This is in agreement with a previous study36 that suggested that dissemination and persistence of PCV2 may depend on the level of PCV2 antibodies at the time of inoculation. Moreover, since AGE-12 pigs had significantly lower PCV2 ELISA S:P ratios than AGE-2 and AGE-7 pigs at the time of inoculation, we speculate that the lower antibody levels in the AGE-12 group at that time may have been responsible for their increased susceptibility, as defined by the higher PCV2 viremia levels of this group. However, we did not observe lower levels of PCV2 viremia in seropositive AGE-7 and AGE-12 pigs than in their seronegative counterparts. This may be due to the uneven distribution of the pigs, the different immunological status of pigs of different ages, or additional unknown factors.
Immune activation has been suggested as an important triggering factor of PCVAD under experimental conditions using 1-day old gnotobiotic piglets38 or 7- to 10-week-old conventional pigs.32 However, there are also studies showing that immunostimulation does not seem to play a critical role in development of PCVAD.39-41 In the present study, in order to investigate the effect of immunostimulation on PCV2 infection in pigs of different ages, both M hyopneumoniae-vaccinated and nonvaccinated pigs were present in all three age groups. Interestingly, only AGE-12 vaccinated pigs had significantly higher PCV2 DNA loads at 7 DPI compared to nonvaccinated pigs, whereas in the other age groups, there were no significant differences between the vaccinated and nonvaccinated pigs. Possible explanations for this finding could be differences in the interval between vaccine administration and challenge (1 week for AGE-2, 6 weeks for AGE-7, and 11 weeks for AGE-12) and the age of the pigs at PCV2 challenge. However, since low numbers of pigs were used in the present study and they were vaccinated with only one product, this finding should be further investigated using larger groups of pigs and different vaccines.
In summary, assessment of diagnostic data from PCVAD cases submitted to a midwestern United States VDL from 2003 to 2010 indicated that PCVAD was most commonly observed in 12- to 16-week-old pigs. Under experimental conditions, AGE-12 pigs had significantly lower PCV2 antibody levels than AGE-2 and AGE-7 pigs at the time of inoculation and had significantly higher PCV2 viremia levels than AGE-2 and AGE-7 pigs after PCV2 inoculation. The effect of passively acquired antibodies protecting against PCV2 challenge was less evident in older pigs.
Implications
• Under field conditions in the United States, 12- to 16-week-old pigs are most commonly affected by PCVAD.
• Under experimental conditions, 12-week-old pigs are more susceptible to PCV2 infection than are 2- and 7-week-old pigs.
Acknowledgement
We thank Dr Chong Wang for assistance with the statistical analysis of this study.
References
1. Meehan BM, McNeilly F, Todd D, Kennedy S, Jewhurst VA, Ellis JA, Hassard LE, Clark EG, Haines DM, Allan GM. Characterization of novel circovirus DNAs associated with wasting syndromes in pigs. J Gen Virol. 1998;79:2171–2179.
2. Harms PA, Halbur PG, Sorden SD. Three cases of porcine respiratory disease complex associated with porcine circovirus type 2 infection. J Swine Health Prod. 2002;10:27–30.
3. Kim J, Chung HK, Chae C. Association of porcine circovirus 2 with porcine respiratory disease complex. Vet J. 2003;166:251–256.
4. Rosell C, Segalés J, Ramos-Vara JA, Folch JM, Rodríguez-Arrioja GM, Durán CO, Balasch M, Plana-Durán J, Domingo M. Identification of porcine circovirus in tissues of pigs with porcine dermatitis and nephropathy syndrome. Vet Rec. 2000;146:40–43.
5. Kim J, Ha Y, Jung K, Choi C, Chae C. Enteritis associated with porcine circovirus 2 in pigs. Can J Vet Res. 2004;68:218–221.
6. Kim J, Jung K, Chae C. Prevalence of porcine circovirus type 2 in aborted fetuses and stillborn piglets. Vet Rec. 2004;155:489–492.
7. Quintana J, Segalés J, Rosell C, Calsamiglia M, Rodríguez-Arrioja GM, Chianini F, Folch JM, Maldonado J, Canal M, Plana-Durán J, Domingo M. Clinical and pathological observations on pigs with postweaning multisystemic wasting syndrome. Vet Rec. 2001;149:357–361.
8. Sorden SD. Update on porcine circovirus and postweaning multisystemic wasting syndrome (PMWS). Swine Health Prod. 2000;8:133–136.
9. Segalés J, Domingo M. Postweaning multisystemic wasting syndrome (PMWS) in pigs. A review. Vet Q. 2002;24:109–124.
10. Walker IW, Konoby CA, Jewhurst VA, McNair I, McNeilly F, Meehan BM, Cottrell TS, Ellis JA, Allan GM. Development and application of a competitive enzyme-linked immunosorbent assay for the detection of serum antibodies to porcine circovirus type 2. J Vet Diagn Invest. 2000;12:400–405.
11. Zhou JY, Chen QX, Ye JX, Shen HG, Chen TF, Shang SB. Serological investigation and genomic characterization of PCV2 isolates from different geographic regions of Zhejiang province in China. Vet Res Commun. 2006;30:205–220.
12. Calsamiglia M, Segalés J, Quintana J, Rosell C, Domingo M. Detection of porcine circovirus types 1 and 2 in serum and tissue samples of pigs with and without postweaning multisystemic wasting syndrome. J Clin Microbiol. 2002;40:1848–1850.
13. Opriessnig T, Meng XJ, Halbur PG. Porcine circovirus type 2 associated disease: update on current terminology, clinical manifestations, pathogenesis, diagnosis, and intervention strategies. J Vet Diagn Invest. 2007;19:591–615.
14. Fort M, Sibila M, Allepuz A, Mateu E, Roerink F, Segalés J. Porcine circovirus type 2 (PCV2) vaccination of conventional pigs prevents viremia against PCV2 isolates of different genotypes and geographic origins. Vaccine. 2008;26:1063–1071.
15. Opriessnig T, Madson DM, Prickett JR, Kuhar D, Lunney JK, Elsener J, Halbur PG. Effect of porcine circovirus type 2 (PCV2) vaccination on porcine reproductive and respiratory syndrome virus (PRRSV) and PCV2 coinfection. Vet Microbiol. 2008;131:103–114.
16. Shen HG, Beach NM, Huang YW, Halbur PG, Meng XJ, Opriessnig T. Comparison of commercial and experimental porcine circovirus type 2 (PCV2) vaccines using a triple challenge with PCV2, porcine reproductive and respiratory syndrome virus (PRRSV), and porcine parvovirus (PPV). Vaccine. 2010;28:5960–5966.
17. Segalés J, Urniza A, Alegre A, Bru T, Crisci E, Nofrarias M, López-Soria S, Balasch M, Sibila M, Xu Z, Chu HJ, Fraile L, Plana-Durán J. A genetically engineered chimeric vaccine against porcine circovirus type 2 (PCV2) improves clinical, pathological and virological outcomes in postweaning multisystemic wasting syndrome affected farms. Vaccine. 2009;27:7313–7321.
18. Horlen KP, Dritz SS, Nietfeld JC, Henry SC, Hesse RA, Oberst R, Hays M, Anderson J, Rowland RR. A field evaluation of mortality rate and growth performance in pigs vaccinated against porcine circovirus type 2. JAVMA. 2008;232:906–912.
19. Allan GM, Ellis JA. Porcine circoviruses: a review. J Vet Diagn Invest. 2000;12:3–14.
*21. Smith WJ, Thomson JR, Done S. Dermatitis/nephropathy syndrome of pigs [letter]. Vet Rec. 1993;132:47.
22. Ritchie PA, Anderson IL, Lambert DM. Evidence for specificity of psittacine beak and feather disease viruses among avian hosts. Virology. 2003;306:109–115.
23. Pearson GR, McNulty MS. Pathological changes in the small intestine of neonatal pigs infected with a pig reovirus-like agent (rotavirus). J Comp Pathol. 1977;87:363–375.
24. Tzipori S, Chandler D, Makin T, Smith M. Escherichia coli and rotavirus infections in four-week-old gnotobiotic piglets fed milk or dry food. Aust Vet J. 1980;56:279–284.
25. Nagy B, Casey TA, Whipp SC, Moon HW. Susceptibility of porcine intestine to pilus-mediated adhesion by some isolates of piliated enterotoxigenic Escherichia coli increases with age. Infect Immun. 1992;60:1285–1294.
26. Thanawongnuwech R, Thacker EL, Halbur PG. Influence of pig age on virus titer and bactericidal activity of porcine reproductive and respiratory syndrome virus (PRRSV)-infected pulmonary intravascular macrophages (PIMs). Vet Microbiol. 1998;63:177–187.
27. van der Linden IF, Voermans JJ, van der Linde-Bril EM, Bianchi AT, Steverink PJ. Virological kinetics and immunological responses to a porcine reproductive and respiratory syndrome virus infection of pigs at different ages. Vaccine. 2003;21:1952–1957.
28. Nawagitgul P, Harms PA, Morozov I, Thacker BJ, Sorden SD, Lekcharoensuk C, Paul PS. Modified indirect porcine circovirus (PCV) type 2-based and recombinant capsid protein (ORF2)-based enzyme-linked immunosorbent assays for detection of antibodies to PCV. Clin Diagn Lab Immunol. 2002;9:33–40.
29. Fenaux M, Halbur PG, Gill M, Toth TE, Meng XJ. Genetic characterization of type 2 porcine circovirus (PCV-2) from pigs with postweaning multisystemic wasting syndrome in different geographic regions of North America and development of a differential PCR-restriction fragment length polymorphism assay to detect and differentiate between infections with PCV-1 and PCV-2. J Clin Microbiol. 2000;38:2494–2503.
30. Opriessnig T, Thacker EL, Yu S, Fenaux M, Meng XJ, Halbur PG. Experimental reproduction of postweaning multisystemic wasting syndrome in pigs by dual infection with Mycoplasma hyopneumoniae and porcine circovirus type 2. Vet Pathol. 2004;41:624–640.
31. Sorden SD, Harms PA, Nawagitgul P, Cavanaugh D, Paul PS. Development of a polyclonal-antibody-based immunohistochemical method for the detection of type 2 porcine circovirus in formalin-fixed, paraffin-embedded tissue. J Vet Diagn Invest. 1999;11:528–530.
32. Opriessnig T, Yu S, Gallup JM, Evans RB, Fenaux M, Pallares F, Thacker EL, Brockus CW, Ackermann MR, Thomas P, Meng XJ, Halbur PG. Effect of vaccination with selective bacterins on conventional pigs infected with type 2 porcine circovirus. Vet Pathol. 2003;40:521–529.
33. Segalés J, Cortey M. Changes in age at diagnosis of PMWS in pigs in Spain, 1998 to 2008. Vet Rec. 2010;167:940–941.
34. Calsamiglia M, Fraile L, Espinal A, Cuxart A, Seminati C, Martín M, Mateu E, Domingo M, Segalés J. Sow porcine circovirus type 2 (PCV2) status effect on litter mortality in postweaning multisystemic wasting syndrome (PMWS). Res Vet Sci. 2007;82:299–304.
35. Shen H, Wang C, Madson DM, Opriessnig T. High prevalence of porcine circovirus viremia in newborn piglets in five clinically normal swine breeding herds in North America. Prev Vet Med. 2010;97:228–236.
36. Ostanello F, Caprioli A, Di Francesco A, Battilani M, Sala G, Sarli G, Mandrioli L, McNeilly F, Allan GM, Prosperi S. Experimental infection of 3-week-old conventional colostrum-fed pigs with porcine circovirus type 2 and porcine parvovirus. Vet Microbiol. 2005;108:179–186.
37. McKeown NE, Opriessnig T, Thomas P, Guenette DK, Elvinger F, Fenaux M, Halbur PG, Meng XJ. Effects of porcine circovirus type 2 (PCV2) maternal antibodies on experimental infection of piglets with PCV2. Clin Diagn Lab Immunol. 2005;12:1347–1351.
38. Krakowka S, Ellis JA, McNeilly F, Ringler S, Rings DM, Allan G. Activation of the immune system is the pivotal event in the production of wasting disease in pigs infected with porcine circovirus-2 (PCV-2). Vet Pathol. 2001;38:31–42.
39. Ladekjær-Mikkelsen AS, Nielsen J, Stadejek T, Storgaard T, Krakowka S, Ellis J, McNeilly F, Allan G, Bøtner A. Reproduction of postweaning multisystemic wasting syndrome (PMWS) in immunostimulated and non-immunostimulated 3-week-old piglets experimentally infected with porcine circovirus type 2 (PCV2). Vet Microbiol. 2002;89:97–114.
40. Loizel C, Blanchard P, Grasland B, Dory D, Oger A, Nignol AC, Cariolet R, Jestin A. Effect of granulocyte-macrophage colony-stimulating factor on post-weaning multisystemic wasting syndrome in porcine circovirus type-2-transfected piglets. Int J Exp Pathol. 2005;86:33–43.
41. Resendes A, Segalés J, Balasch M, Calsamiglia M, Sibila M, Ellerbrok H, Mateu E, Plana-Durán J, Mankertz A, Domingo M. Lack of an effect of a commercial vaccine adjuvant on the development of postweaning multisystemic wasting syndrome (PMWS) in porcine circovirus type 2 (PCV2) experimentally infected conventional pigs. Vet Res. 2004;35:83–90.
*Non-refereed reference.