Vilalta C, Alcalá T, López-Jimenez R, et al. Clinical efficacy of acetylsalicylic acid as an adjunct to antibacterial treatment of porcine respiratory disease complex. J Swine Health Prod. 2012;20(1):10–16
| Original research | Peer reviewed |
SummaryObjective: To investigate the efficacy of acetylsalicylic acid (ASA) as an adjunct to antibiotic treatment of pigs with fever during an outbreak of porcine respiratory disease complex (PRDC). Materials and methods: The animals were divided into two groups. The experimental group received doxycycline hyclate and ASA in the drinking water for 5 consecutive days at doses of 10 mg per kg and 100 mg per kg of body weight, respectively, whereas the control group received only doxycycline hyclate (10 mg per kg). Clinical efficacy was investigated by comparing the synergistic or antagonistic effects of ASA administered with an antibiotic versus use of the antibiotic alone to reduce fever or clinical signs or both. Results: Results showed a significant decrease in fever in the group that received ASA and antibiotic versus the values observed in the group that received only antibiotic. No synergistic effect between ASA and doxycycline hyclate was observed to decrease respiratory signs. Implication: Acetylsalicylic acid is efficacious, at least to reduce fever, as an adjunct to antibacterial treatment of PRDC. | ResumenObjetivo: Investigar la eficacia del ácido acetilsalicílico (ASA por sus siglas en inglés) como un complemento al tratamiento antibiótico de cerdos con fiebre durante un brote del complejo de enfermedad respiratoria porcina (PRDC por sus siglas en inglés). Materiales y métodos: Los animales fueron divididos en dos grupos. El grupo experimental recibió hiclato de doxiciclina y ASA en el agua para beber por cinco días consecutivos en dosis de 10 mg por kg y 100 mg por kg de peso corporal, respectivamente; mientras que el grupo control recibió solo hiclato de doxiciclina (10 mg por kg). Se investigó la eficacia clínica al comparar los efectos sinérgicos ó antagonistas del ASA administrado con un antibiótico contra el uso de un antibiótico solo para reducir la fiebre ó lo signos clínicos ó ambos. Resultados: Los resultados mostraron una disminución significativa en la fiebre en el grupo que recibió el ASA y el antibiótico contra los valores observados en el grupo que recibió solamente el antibiótico. No se observó un efecto sinérgico entre ASA y el hiclato de doxiciclina para disminuir los signos respiratorios. Implicacion: El ácido acetilsalicílico es eficaz, al menos para reducir la fiebre, como un complemento al tratamiento antibacteriano de PRDC. | ResuméObjectif: Étudier l’efficacité de l’acide acétylsalicylique (ASA) en tant qu’ajout au traitement aux antibiotiques de porcs avec de la fièvre durant une poussée de cas du complexe respiratoire porcin (PRDC). Matériels et méthodes: Les animaux ont été répartis en deux groupes. Le groupe expérimental a reçu de l’hyclate de doxycycline et de l’ASA dans l’eau de boisson pendant 5 jours consécutifs à des doses de 10 mg/kg et 100 mg/kg de poids corporel, respectivement, alors que le groupe témoin n’a reçu que de l’hyclate de doxycycline (10 mg/kg). L’efficacité clinique a été étudiée en comparant les effets synergiques ou antagonistes de l’ASA administré avec un antibiotique versus l’utilisation d’un antibiotique seul pour réduire la fièvre ou les signes cliniques, ou les deux. Résultats: Les résultats ont permis de constater une diminution significative de la fièvre dans le groupe recevant de l’ASA et l’antibiotique comparativement aux valeurs observées dans le groupe ne recevant que de l’antibiotique. Aucun effet synergique entre l’ASA et l’hylate de doxycycline n’a été observé pour réduire les signes respiratoires. Implication: L’ASA est efficace, à tout le moins pour réduire la fièvre, en tant qu’ajout au traitement antibactérien du PRDC.
|
Keywords: swine, acetylsalicylic acid, fever, porcine respiratory disease complex, PRDC
Search the AASV web site
for pages with similar keywords.
Received: January 19, 2011
Accepted: July 4, 2011
Porcine respiratory disease complex (PRDC) is characterized clinically by dyspnea, coughing, acute depression, anorexia, fever, and nasal discharge, primarily affecting growing to finishing pigs.1 This complex disease is most often due to interaction of multiple factors. Both viral and bacterial organisms play a role, as well as the environment and various management practices employed by producers. When in the right combination, these factors can compromise respiratory defense mechanisms, resulting in severe respiratory disease.2 The most common viral pathogens associated with PRDC are porcine reproductive and respiratory syndrome virus (PRRSV), swine influenza virus, pseudorabies virus, and porcine respiratory coronavirus. The prevalence of pseudorabies virus is extremely low in Spain due to a national eradication program presently in effect. Thus, the relevance of this virus is decreasing quickly as an etiologic agent of PRDC in Spain. The most common bacterial pathogens associated with this complex include Mycoplasma hyopneumoniae, Actinobacillus pleuropneumoniae, Bordetella bronchiseptica, Pasteurella multocida, Haemophilus parasuis, Streptococcus suis, Arcanobacterium pyogenes, Salmonella enterica serovar Choleraesuis, and Actinobacillus suis.3
Diseases of the respiratory tract are common in domestic animals. Infection and environmental conditions make inflammation a frequent manifestation of respiratory disease that decreases the ability of the lungs to exchange gases. Moreover, the pathogenesis of the lung lesions is associated with an excessive inflammatory response.4 Thus, the ability to control the inflammatory response may be critical in the treatment of pneumonias in livestock. The benefits of therapeutic intervention with several classes of anti-inflammatory drugs are the result of their properties as inhibitors of the action, synthesis, or release of inflammatory mediators. One of the most important groups of anti-inflammatory drugs belongs to the non-steroidal anti-inflammatory drug (NSAID) class, that also has antipyretic and analgesic actions.5,6 NSAIDs are safely employed by humans in many inflammatory conditions with well-known side-effects.7 In bovine medicine, therapy is normally based on antimicrobial and NSAID treatment for symptomatic improvement in the treatment of acute respiratory disease.8,9 On the other hand, therapy of porcine respiratory disease complex is normally based on antimicrobials, and NSAIDs are not universally used as adjuncts to this treatment under field conditions.
Acetylsalicylic acid (ASA), also known as aspirin, is commonly used as an analgesic, antipyretic, and anti-inflammatory drug in animals,10 although the Food and Drug Administration Center for Veterinary Medicine has never approved ASA for these purposes.11 However, the regulatory framework is different in Europe, where ASA is approved as an NSAID to be used in swine. It is an anti-inflammatory, analgesic, and antipyretic drug which irreversibly inhibits cyclooxygenase (COX)-1 more than COX-2, limiting prostaglandin and thromboxane synthesis.7 Baert et al12 observed a dose-dependent attenuation of the fever response in chickens treated with salicylate, using a lipopolysaccharide injection model to reproduce fever. To our knowledge, there is no peer-reviewed information concerning the antipyretic effect of ASA in pigs. Thus, there is only one report available describing the pharmacokinetic parameters of ASA in pigs after oral administration in drinking water.13 The aim of this study was to investigate the clinical efficacy of ASA as an adjunct to antibacterial treatment of PRDC. Clinical efficacy was investigated by comparing the synergistic or antagonistic effects of ASA with an antibiotic versus the use of the antibiotic alone to reduce fever or clinical signs or both under field conditions.
Materials and methods
All animals were fed, housed, and handled with due concern for their welfare. The facility operated under the guidelines of the animal care and use committee of the Universidad Autónoma de Barcelona that approved this procedure.
Animals and experimental design
A total of 142 three-month-old crossbred pigs were used in this study. These animals were housed in a finishing unit of 1000 pigs in northeastern Spain. Pigs were allocated to 77 pens in which males and females were not mixed, with 13 pigs per pen and a space allowance of 0.75 m2 per pig. The building was equipped with manual mechanisms to control ventilation. Feed and water were provided ad libitum. Feed was distributed in hoppers (one per pen) and water was supplied through an automated system. All pigs included in the study received the commercial nonmedicated feed normally provided in this farm, which met the requirements established by the National Research Council.14 Two 1000-L tanks with independent water distribution systems allowed two different treatments to be administered to the pens included in the trial and daily water intake to be recorded for all pens receiving the same treatment. Water samples were collected from nipples before medications were added, and ASA concentration in the water was determined in three pens of each study group twice a day as previously described.15
The inclusion criteria for this study were pyrexia (body temperature ≥ 39.7ºC) in pigs with respiratory disease clinically characterized by dyspnea, coughing, and anorexia. Clinical signs began 1 day before beginning the clinical trial as a consequence of natural infection. Pigs were ear-tagged and weighed at the beginning of the trial. Each pen that contained enrolled animals was assigned a number from 1 to 40. Pens containing enrolled animals were distributed haphazardly in the barn, with three or four enrolled animals allocated to each pen and all enrollments made on the same day. Pens containing enrolled animals were randomly distributed into two treatment groups, the ASA + Doxycycline group (72 pigs) and the Doxycycline group (70 pigs), balanced by weight and sex and randomized to treatment. For welfare reasons, animals were not moved from one pen to another in order to obtain balanced groups at the beginning of the experiment. However, whole pens were changed from one group to another in order to create balanced groups before beginning the trial. The enrolled pigs remained in pens among non-enrolled pigs, so that all pigs in the finisher (both enrolled and non-enrolled) were treated because the drug was administered in the drinking water.
The study was designed as a controlled, masked (blinded), parallel group (1:1), randomized trial with a positive control. The ASA + Doxycycline group received doxycycline hyclate and ASA in the drinking water at doses of 10 mg per kg body weight (Doxiporc; Laboratorios Polichem, Spain) and 100 mg per kg body weight (Fiebrina porcino; Laboratorios SYVA, Spain), respectively, for 5 consecutive days. The Doxycycline group received only doxycycline hyclate at 10 mg per kg. The two products were mixed in the same tank for the ASA + Doxycycline group. Daily intake of water was measured twice daily (morning and late afternoon) for each experimental group (average for all the included pigs in each group) and the concentration of each product was adjusted, taking into account the daily water intake in each group and the recommendations established by the authorized marketing holder of Fiebrina porcino (Laboratorios SYVA, Spain). Thus, the amount of commercial product to be added in the water was calculated as follows: g medicinal product per L drinking water = [dose of active substance (mg per kg body weight per day) × mean body weight (kg)] ÷ [mg of active substance per g of medicinal product × water consumed (L)].
Mean weight of the animals in each group was known because all included animals were weighed at the beginning of the trial. However, mean water consumption (L per day) at the beginning of the trial was estimated, taking into account initial body weight and expecting daily water intake to be approximately 8% of body weight. Finally, the dispenser (who mixed the medications in the water tanks) and the clinician (who performed clinical examinations) were different persons to ensure blinding of the clinician to treatment.
Before the trial was begun, three animals with evident respiratory symptoms were sacrificed for culture and isolation of organisms in the lungs, focused on the bacteria commonly involved in PRDC (M hyopneumoniae, B bronchiseptica, and P multocida). Organisms were isolated and identified by microbiological methods,16 and their antimicrobial susceptibilities to doxycycline were determined following accepted standard procedures. Briefly, a broth microdilution procedure was used.17 In addition, blood samples were collected from the three euthanized animals to test for the PRRSV genome by reverse-transcriptase polymerase chain reaction (RT-PCR) as previously described.18 Tissue samples (lung, superficial inguinal lymph node, spleen, kidney, and liver) were submitted to the histopathology department, Universidad Autónoma de Barcelona (Barcelona, Spain) for histopathological examination and for circovirus type 2 (PCV2) infection by in situ hybridization.19
Clinical examination and data recording system
Animals were clinically examined by a single observer on Day 1 before treatment was applied, during treatment on Days 2 through 5, and then on Day 6, 1 day after treatment ended. Rectal temperature, abdominal breathing, cough, and depression were scored using a scale of 0 = normal, 1 = slight or moderate, and 2 = severe20 (Table 1). Rectal temperature was taken using a calibrated thermometer (rectal thermometer; Testo 110, Cabrils, Spain). Body condition was scored 0 = normal, 1 = slightly thin,
2 = moderately thin, and 3 = wasted or emaciated. This classification, based on the individual assessment of the pelvis, vertebrae, and ribs, was modified from that described by Straw et al21 (Table 2). Thus, five parameters per evaluation time contributed to two combined clinical scores. The higher the score, the worse the respiratory clinical signs or the clinical condition, respectively. Respiratory clinical score = (cough score + abdominal breathing score) ÷ 2. General clinical score = ([depression score + cough score + fever score + abdominal breathing score] ÷ 4) + body condition score.
Table 1: Description of the depression, cough, fever, and abdominal breathing scoring system20 applied in 142 three-month-old crossbred pigs in a study comparing effects of treating pigs with PRDC with either ASA plus doxycycline or doxycycline alone*
* Animals were clinically examined before applying the treatments at Day 1 and at Days 2, 3, 4, 5, and 6 by a single observer blinded to treatment. The two treatment groups were balanced by weight and sex and pigs were randomized to treatment (72 pigs in the ASA + Doxycycline group and 70 in the Doxycycline group). The ASA + Doxycycline group received doxycycline hyclate and ASA in the drinking water at doses of 10 mg/kg (Doxiporc; Laboratorios Polichem, Spain) and 100 mg/kg body weight (Fiebrina porcino; Laboratorios SYVA, Spain), respectively, for 5 consecutive days, and the Doxycycline group received only doxycycline hyclate (10 mg/kg). PRDC = porcine respiratory disease complex; ASA = acetylsalicylic acid |
Table 2: Description of the pelvic bones, vertebrae, and ribs scoring system21 applied in 142 three-month-old crossbred pigs in a study comparing effects of treating pigs with PRDC with either ASA plus doxycycline or doxycycline alone*
* Animals were scored before applying treatment at Day 1 and at Days 2, 3, 4, 5, and 6 by a single observer blinded to treatment. Animals and treatments described in Table 1. |
|||||||||||||||||||||||
Statistical analysis
Body temperature, respiratory clinical score, and general clinical score were compared for the ASA + Doxycycline and Doxycycline groups every day, with the pig as the experimental unit. A t test was used for the normally distributed variable (temperature) and the Mann-Whitney U test for the non-normally distributed variables (all other variables). The percentage of animals with a temperature value < 39.7ºC (threshold value for fever) at the end of the trial (Day 6) was compared between groups using a chi-square test. Animals with body temperature < 39.7 and ≥ 39.7 were classified as 0 or 1, respectively, for this statistical analysis. In order to discard a possible pen effect to decrease fever, a linear mixed model comparing both treatments simultaneously, with the variable “pen” as a random factor, was also performed. All statistical analysis was performed using the statistical software SPSS System v15 (SPSS, Inc, Chicago, Illinois). The alpha level used for determination of significance for all analyses was P < .05, with statistical tendencies reported when P < .10.
Results
ASA analyses
Acetylsalicylic acid was not detected in any Doxycycline group water samples. Average ASA concentration for the 36 samples of drinking water for the ASA + Doxycycline group was 1.1 g ASA per L throughout the trial, range 0.82 to 1.21 g per L. Variability in ASA concentration was extremely low every day between pens (measured at nipple). The variation coefficient for ASA concentration was 1.5% to 2.4% throughout the trial.
Body weight, water intake, and bacteriological results
Mean weight of the included animals was 31.3 ± 3.8 kg and 31.6 ± 4.1 kg for the ASA + Doxycycline and Doxycycline groups, respectively.
No adverse reactions were observed in either treatment throughout the trial. Daily intake of water was very similar in the ASA + Doxy-cycline group (3.03 L per kg) and the Doxycycline group (2.89 L per kg).
Infections with both PRRSV (serum samples PCR-positive) and PCV2 (large amount of PCV2 identified in lymphoid organs by histopathologic examination) were diagnosed in the three euthanized pigs. Mycoplasma hyopneumoniae and P multocida were isolated from the lungs of all three animals. The MIC for doxycycline was 0.08 μg per mL for M hyopneumoniae and 0.05 μg per mL for P multocida.
Fever
At the beginning of the trial, mean body temperature was 40.49 ± 0.50ºC in the ASA + Doxycycline group and 40.48 ± 0.49ºC in the Doxycycline group, with no significant differences between groups (P = .94). After applying the two treatments, mean temperature was always lower in the ASA + Doxycycline group than in the Doxycycline group (Figure 1); these differences were statistically significant at Days 2, 5, and 6 of the experiment (P < .05). The percentage of animals with body temperature < 39.7ºC was significantly higher (P < .001 ) in the ASA + Doxycycline group (67.6%) than in the Doxycycline group (21.7%) at Day 6 of the trial.
Figure 1: Mean body temperature (± standard deviation) in the ASA + Doxycycline and Doxycycline groups before treatment began on Day 1, and on Days 2, 3, 4, 5, and 6. Pigs and treatments described in Table 1. Asterisks indicate statistical differences between treatment groups (P < .05; t-test). 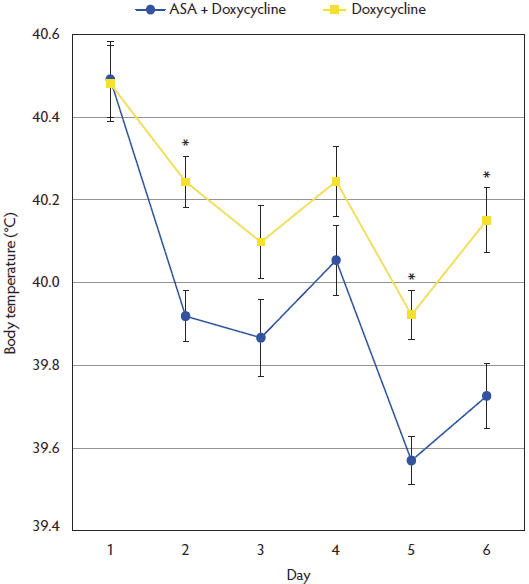 |
Respiratory clinical score
Mean respiratory scores (RS) for animals in the ASA + Doxycycline and Doxycycline groups were 0.31 and 0.22, respectively, at Day 1 of the trial. The RS decreased in both groups throughout the trial and reached a minimum value of 0.03 at Day 4 in the ASA + Doxycycline group (Figure 2). The evolution of the RS was similar in both groups and no significant differences were observed between groups throughout the trial (P > .05).
Figure 2: Mean (± standard deviation) for respiratory clinical score (A) and general clinical score (B) in the ASA + Doxycycline and Doxycycline groups before treatment began on Day 1 and on Days 2, 3, 4, 5, and 6. Pigs, treatments, and scoring systems described in Tables 1 and 2. Respiratory clinical score = (cough score + abdominal breathing score) ÷ 2. General clinical score = ([depression score + cough score + fever score + abdominal breathing score] ÷ 4) + body condition score. No statistically significant differences in either respiratory clinical score or general clinical score were observed during the study (Mann-Whitney U test; P > .05). 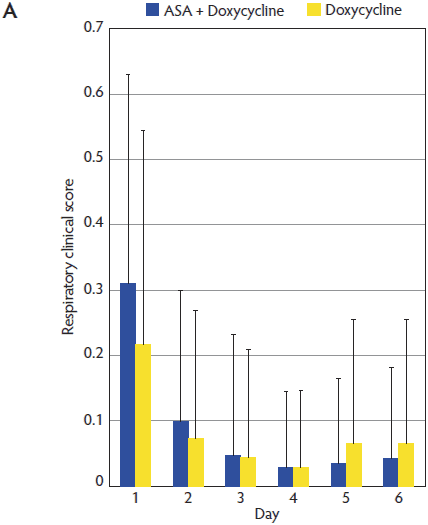 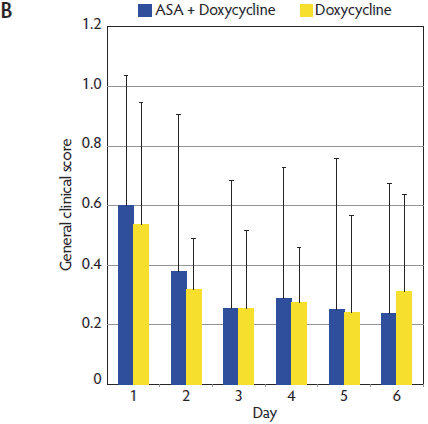 |
General clinical score
General clinical score (GS) for animals in the ASA + Doxycycline and Doxycycline groups were 0.60 and 0.53, respectively, at Day 1. The evolution of the GS was very similar in both groups (Figure 2), but the GS was numerically lower in the ASA + Doxycycline group at the end of the trial (Day 6). No significant differences in GS were observed between treatment groups throughout the trial (P > .05).
Discussion
In this respiratory outbreak, the viral pathogens associated with PRDC were PRRSV and PCV2, and the bacterial pathogens were M hyopneumoniae and P multocida. Thus, this clinical case is representative of respiratory cases observed in Spain under field conditions.22 It was surprising that no mortality was observed in the animals included in the trial. However, mortality in respiratory outbreaks is extremely variable and depends on the microorganisms involved, their virulence, and other factors such as management and ventilation. Moreover, it must be taken into account that all animals in the finisher were treated with an antibiotic at the beginning of the respiratory outbreak. Finally, the MIC values observed for the two isolated bacteria in this case were lower than the threshold values for clinical efficacy described for these organisms.23,24 Thus, treatment with doxycycline in this case should have been efficacious, and was, as the clinical status of the study animals improved after doxycycline was administered. It should be noted that doxycycline hyclate is approved for this use in swine in the EU, but not in all other countries. Fortunately, other antibiotics are available to treat respiratory outbreaks worldwide.
Two main effects of ASA are rapid reduction of pyrexia and its anti-inflammatory effect, blocking production or effects of inflammatory mediators and modulators or both.7 Results showed a significant decrease in fever in the ASA + Doxycycline group versus the fever decrease observed in the Doxycycline group. Thus, these results support the idea that ASA is an effective adjunct for treatment of PRDC, at least to decrease fever. No synergistic effect was observed to decrease respiratory clinical signs in animals receiving both ASA and doxycycline.
In this study, ASA was administered via the drinking water system. Obviously, this method of administration is very useful under field conditions, but has the disadvantage that it is impossible to be sure that each pig receives the correct dose. In this controlled study, ASA concentration at the water nipples was measured many times in several pens distributed homogenously in the finisher to ensure that the two drugs were available at pen level. An average daily drug intake could be calculated to assure the correct dose of the drug was available at population level. It may be argued that sick animals drink less than healthy ones, and that the actual dose for sick animals might be lower than for healthy ones and lower than the average value calculated in this trial. Thus, a type II error may have been introduced in this experimental design (ie, low potency of the ASA because of variability in daily drug intake in sick animals). However, temperatures were lower in the ASA + Doxycycline group than in the Doxycycline group.
Baert et al12 observed a dose-dependent attenuation of the fever response in chickens treated with salicylate using a lipopolysaccharide injection model to reproduce fever. These data agree with our results, confirming that ASA is an effective antipyretic agent. Glew et al25 demonstrated that the overall recovery period from pyrexia, depression, and anorexia was shorter in cats treated with antibiotics and ketoprofen (another NSAID) than in cats treated only with antibiotics. Moreover, Bednarek et al26 reported that calves treated with a combination of oxytetracycline and meloxicam (another NSAID) showed a significantly faster return to normal body temperature than calves treated only with oxytetracycline. Thus, ketoprofen and meloxicam were useful adjuncts in the treatment of pyretic cats and calves with respiratory disease, respectively.
The acute inflammatory component of pneumonia results in impaired gas exchange, and the aim of modulating pulmonary inflammation by the use of NSAIDs is to block the production or the effects of inflammatory mediators and modulators or both, which have a deleterious effect on alveolar exchange of gases.27 In cattle production, the use of NSAIDs plus antibiotics in bovine respiratory disease can minimize the extent of the lesions and improve performance in the treated animals more than in cattle receiving only antibiotic treatment, although only one peer-reviewed report is available to support this affirmation.28 On the other hand, the clinical efficacies of flunixin, carprofen, and ketoprofen as adjuncts to antibacterial treatment (ceftiofur) of bovine respiratory disease have been compared.8 There were no statistically significant differences between the four groups with respect to depression, illness scores, dyspnea, or coughing, but there was less lung consolidation in the three groups treated with an NSAID than in the animals treated with ceftiofur alone. Thus, it seems that observation of respiratory clinical signs (cough and abdominal breathing) is not sensitive enough to observe differences between animals receiving NSAIDs and antibiotics versus animals receiving only antibiotics, even when significant differences between experimental groups are observed in the percentage of the lung with pneumonic lesions.8 In our study, a synergistic effect on respiratory signs in animals treated with ASA and antibiotic was not observed according to the parameters studied. Taking into account the results from the study in calves,8 an estimation of the lung lesions in both groups at different time points may be required to detect synergistic effects between ASA and doxycycline in relation to pneumonia in pigs with PRDC. Further studies are required to estimate pneumonia extension in animals that receive ASA and antibiotic versus animals receiving only antibiotic to cope with PRDC.
Implications
• Acetylsalicylic acid is efficacious, at least to decrease fever, as an adjunct to antibacterial treatment of PRDC.
• NSAIDs are accepted as an adjunct therapy for respiratory disease in other veterinary species and should be considered for treatment of PRDC in pigs.
Acknowledgement
This work was partly funded by a project of Laboratorios SYVA SA, León, Spain
References
1. Dee SA. The PRDC: Are subpopulations important? Swine Health Prod 1996;4:147.
2. Thacker EL. Lung inflammatory responses. Vet Res. 2006;37:469–486.
3. Christensen G, Sorensen V, Mousing J. Diseases of the respiratory system. In: Straw BA, D’Allaire S, Mengeling WL, Taylor DJ, eds. Diseases of Swine. 8th ed. Ames, Iowa: Iowa State University Press; 1999:913–941.
4. Ramirez-Romero R, Brogden KA. The potential role of the Arthus and Shwartzman reactions in the pathogenesis of pneumonic pasteurellosis. Inflamm Res. 2000;49:98–101.
5. Lees P, Giraudel J, Landoni MF, Toutain PL. PK–PD integration and PK–PD modelling of non-steroidal anti-inflammatory drugs: principles and applications in veterinary pharmacology. J Vet Pharmacol Ther. 2004;27:491–502.
6. Curry SL, Cogar SM, Cook JL. Nonsteroidal antiinflammatory drugs: a review. J Am Anim Hosp Assoc. 2005;41:298–309.
7 Süleyman H, Demircan B, Karagöz Y. Anti-inflammatory and side effects of cyclooxygenase inhibitors. Pharmacol Rep. 2007;59:247–258.
8. Lockwood PW, Johnson JC, Katz TL. Clinical efficacy of flunixin, carprofen and ketoprofen as adjuncts to the antibacterial treatment of bovine respiratory disease. Vet Rec. 2003;152:392–394.
9. Elitok B, Elitok OM. Clinical efficacy of carprofen as adjunct to the antibacterial treatment of bovine respiratory disease. J Vet Pharmacol Ther. 2004;27:317–320.
10. Davis LE. Clinical pharmacology of salicylates. JAVMA. 1980;176:65–66.
11. Langston C, Apley MD, Boothe DM, Clark TP, Davidson GF, Dowling P, Kemp DT, Papich MG, Riddle MG, Riviere JE, Tubbs RC, Wilke JR. USP Veterinary Pharmaceutical Information Monographs – Anti-inflammatories. J Vet Pharmacol Ther. 2004;27(S1):4–14.
12. Baert K, Duchateau L, De Boever S, Cherlet M, De Backe P. Antipyretic effect of oral sodium salicylate after an intravenous E. coli LPS injection in broiler chickens. Br Poult Sci. 2005;46:137–143.
13. Patterson AR, Karriker LA, Apley MD, Imerman PM. Plasma concentration of sodium salicylate in nursery pigs treated orally. J Swine Health Prod. 2007;15:146–151.
14. Subcommittee on Swine Nutrition, Committee on Animal Nutrition, National Research Council. Nutrient Requirements of Swine: 10th rev ed. Washington, DC: National Academy Press; 1998. Available at: http://www.nap.edu/openbook.php?record_id=6016&page=31. Accessed 19 October 2011.
15. McMahon GP, Kelly MT. Determination of aspirin and salicylic acid in human plasma by column switching-chromatography using on-line solid phase extraction. Anal Chem. 1998;70:409–414.
16. Paton R. Vitek automated identification and susceptibility testing system: Introduction into a busy clinical bacteriology laboratory. Br J Biomed Sci. 2000;57:307–312.
17. Clinical and Laboratory Standards Institute. Performance standards for antimicrobial disk and dilution susceptibility test for bacteria isolated from animals. 3rd ed. Vol 28, No. 8. Available at: http://www.clsi.org/source/orders/free/m31-a3.pdf. Accessed 24 Oct 2011.
18. Mateu E, Martín M, Vidal D. Genetic diversity and phylogenetic analysis of glycoprotein 5 of European-type porcine reproductive and respiratory virus strains in Spain. J Gen Virol. 2003;84:529–534.
19. Rosell C, Segalés J, Plana-Durán J, Balasch M, Rodríguez-Arrioja GM, Kennedy S, Allan GM, McNeilly F, Latimer KS, Domingo M. Pathological, immunohistochemical, and in-situ hybridization studies of natural cases of postweaning multisystemic wasting syndrome (PMWS) in pigs. J Comp Pathol. 1999;120:59–78.
20. Moore GM, Basson RP, Tonkinson LV. Clinical field trials with tilmicosin phosphate in feed for the control of naturally acquired pneumonia caused by Actinobacillus pleuropneumoniae and Pasteurella multocida in swine. Am J Vet Res. 1986;57:224–228.
21. Straw BE, Meuten DJ, Thacker BJ. Physical examination. In: Straw BA, D’Allaire S, Mengeling WL, Taylor DJ, eds. Diseases of Swine. 8th ed. Ames, Iowa: Iowa State University Press; 1999:3–18.
22. Fraile L, Alegre A, López-Jiménez R, Nofrarías M, Segalés J. Risk factors associated with pleuritis and cranio-ventral pulmonary consolidation in slaughter-aged pigs. Vet J. 2010;184:326–333.
23. Bousquet E, Morvan H, Aitken I, Morgan JH. Comparative in vitro activity of doxycycline and oxytetracycline against porcine respiratory pathogens. Vet Rec. 1997;141:37–40.
24. Prats C, El Korchi G, Giralt M, Cristòfol C, Peña J, Zorrilla I, Saborit J, Pérez B. PK and PK/PD of doxycycline in drinking water after therapeutic use in pigs. J Vet Pharmacol Therap. 2005;28:525–530.
25. Glew A, Aviad AD, Keister DM, Meo NJ. Use of ketoprofen as an antipyretic in cats. Can Vet J. 1996;37:222–225.
26. Bednarek D, Zdzisinska B, Kondracki M, Kandefer-szerszen M. Effect of steroidal and non-steroidal anti-inflammatory drugs in combination with long-acting oxytetracycline on non-specific immunity of calves suffering from enzootic bronchopneumonia. Vet Microbiol. 2003;96:53–67.
27. Van de Weerdt ML, Lekeux P. Modulation of lung inflammation in the control of bovine respiratory disease. Bov Pract. 1997;31:19–30.
28. Friton GM, Cajal C, Ramirez-Romero R. Long-term effects of meloxicam in the treatment of respiratory disease in fattening cattle. Vet Rec. 2005;156:809–811.