Roberts E, Hammer JM, Lechtenberg K, et al. Investigation of tiamulin hydrogen fumerate in-feed antibiotic for the control of porcine respiratory disease complex that includes Mycoplasma hyopneumoniae. J Swine Health Prod. 2011;19(4):218–225
| Original research | Peer reviewed |
SummaryObjective: To evaluate tiamulin hydrogen fumerate (THF) as an in-feed antibiotic for control of porcine respiratory disease complex (PRDC) that includes infection with Mycoplasma hyopneumoniae (M hyo). Materials and methods: Two studies were performed to evaluate PRDC-associated clinical signs and pneumonia lesion resolution in pigs treated with THF (Denagard 10; Novartis Animal Health US, Greensboro, North Carolina) at 137.5 and 165.0 ppm. Study One evaluated nursery pigs multi-sourced from two herds affected by PRDC. In Study Two, single-sourced naive pigs were challenged with M hyo. Results: In Study One, nursery pigs exhibiting clinical signs of PRDC and treated with THF at 165.0 ppm showed significantly better treatment success than did controls, defined as body temperature < 40ºC and respiratory and depression scores < 2 on Day 17 (P < .001), with lower total percent pneumonia lesions Implication: In-feed THF produces a clinical benefit in pigs experiencing PRDC or experimentally challenged with M hyo. | ResumenObjetivo: Evaluar la tiamulina hidrogeno fumarato (THF por sus siglas en inglés) como un antibiótico en alimento para el control del complejo respiratorio porcino (PRDC por sus siglas en inglés) que incluye la infección con Mycoplasma hyopneumoniae (M hyo por sus siglas en inglés). Materiales y métodos: Se realizaron dos estudios para evaluar los signos clínicos asociados con el PRDC y la resolución de las lesiones de neumonía en cerdos tratados con el THF (Denagard 10; Novartis Animal Health US, Greensboro, North Carolina) a 137.5 y 165.0 ppm. El Estudio Uno evaluó cerdos en destete de fuentes múltiples de dos hatos afectados con PRDC. En el Estudio Dos, cerdos de una sóla fuente y libres de la enfermedad fueron retados con M hyo. Resultados: En el Estudio Uno, los cerdos de destete que presentaban signos clínicos de PRDC y fueron tratados con THF a 165.0 ppm, mostraron un resultado al tratamiento significativamente mejor que los controles, definido como temperatura corporal < 40ºC, calificación de depresión y signos respiratorio < 2 en el Día 17 (P < .001), con un porcentaje total menor de lesiones de neumonía (P < .001) y mortalidad (P < .05). En el grupo de 137.5 ppm de THF, el porcentaje total de lesiones de neumonía (P < .001) y mortalidad (P < .05) fueron menores que en los controles. El éxito del tratamiento no difirió entre el grupo de 137.5 ppm de THF y los controles. En el Estudio Dos, el porcentaje total de lesiones de neumonía fue menor en los grupos de 165.0 ppm (11.76%; P < .001) y 137.5 ppm de THF (16.02%; P < .05) que en los controles (22.35%). Implicacione: La THF en alimento produce un beneficio clínico en cerdos retados experimentalmente con el M hyo. | ResuméObjectif: Évaluer l’effet de l’ajout de fumerate d’hydrogène de tiamuline dans l’aliment sur la maîtrise des maladies du complexe respiratoire porcin (PRDC) incluant l’infection par Mycoplasma hyopneumoniae (M hyo). Matériels et méthodes: Deux études ont été réalisées afin d’évaluer la résolution des signes cliniques associés au PRDC et des lésions de pneumonie chez les porcs traités avec du THF (Denagard 10; Novartis Animal Health US, Greensboro, North Carolina) à des concentrations de 137.5 et 165.0 ppm. L’Étude Un a permis d’évaluer des porcs en pouponnière provenant de sources multiples de deux troupeaux affectés par PRDC. Dans l’Étude Deux, des porcs naïfs d’une source unique ont été challengés avec M hyo. Résultats: Dans l’Étude Un, les porcs en pouponnière montrant des signes cliniques de PRDC et traités avec du THF à 165.0 ppm ont présenté un succès du traitement significativement meilleur que les témoins, tel que déterminé par la température corporelle < 40ºC, des pointages de dépression et respiratoire < 2 au Jour 17 (P < .001), un pourcentage total inférieur de lésions de pneumonie (P < .001) et de mortalité (P < .05). Dans le groupe THF 137.5 ppm le pourcentage total de lésions pulmonaires (P < .001) et la mortalité (P < .05) étaient inférieurs aux valeurs des animaux témoins. Le succès du traitement n’a pas différé entre le groupe THF 137.5 et le groupe témoin. Dans l’Étude Deux, le pourcentage total de lésions pulmonaires était inférieur dans les groupes THF 165.0 ppm (11.76%, P < .001) et 137.5 ppm (16.02%; P < .05) que chez les témoins. Implication: Du THF dans l’aliment entraîne des bénéfices cliniques chez les porcs avec PRCD ou subissant un challenge expérimental avec M hyo. |
Keywords: swine, tiamulin hydrogen fumerate, porcine respiratory disease complex, Mycoplasma hyopneumoniae, PRDC
Search the AASV web site
for pages with similar keywords.
Received: March 17, 2010
Accepted: October 7, 2010
Porcine respiratory disease complex (PRDC) is a mixed viral and bacterial respiratory infection commonly seen in commercial pig production. Viral pathogens associated with PRDC typically include one or more of the following: porcine reproductive and respiratory syndrome virus (PRRSV), swine influenza virus (SIV), and porcine circovirus type 2 (PCV2). Bacterial components may include Mycoplasma hyopneumoniae (M hyo) and other pathogens such as Actinobacillus pleuropneumoniae, Bordetella bronchiseptica, Haemophilus parasuis, Pasteurella multocida, Actinobacillus suis, and Streptococcus suis.1 Finishing pigs (14 to 20 weeks of age) are most likely to be severely affected. Clinical respiratory disease associated with PRDC is characterized by decreased rate of growth, decreased feed efficiency, anorexia, fever, cough, dyspnea, and mortality.2 Mycoplasma hyopneumoniae, in particular, is a highly prevalent, insidious component of PRDC.3 Viral and bacterial pathogens in conjunction with M hyo can cause increased morbidity and mortality that result in poor performance and associated higher costs of production.4,5
Antibiotics can be applied to manage PRDC. Although viral initiators are not responsive to antibiotics, the bacterial components of PRDC can be minimized, resulting in less severe clinical signs, better lesion resolution, and ultimately, better performance.6 The objective of these studies was to evaluate tiamulin hydrogen fumerate (THF) administered alone at experimental doses of 137.5 ppm or 165.0 ppm in feed for 14 days for the control of PRDC that includes M hyo.
Materials and methods
These studies were approved by Novartis Animal Health’s Internal Animal Care and Use Committee.
Two studies were performed, each of 17 days duration. Study One evaluated the effect of treatment with THF (Denagard 10; Novartis Animal Health, Greensboro, North Carolina) on the clinical signs associated with PRDC and pneumonia lesion resolution, while Study Two examined similar parameters in naive pigs experimentally challenged with M hyo.
Study One
In Study One (Figure 1), 219 nursery pigs approximately 6 to 8 weeks of age from two herds known to have clinical PRDC were transported to an environmentally controlled barn with partial solid concrete and expanded-wire flooring, where they were group-housed. Feed (free of antibiotics) and water were provided ad libitum. Respiratory disease was evaluated using a four-point categorical scale: 0, normal; 1, mild; 2, moderate; and 3, severe (Table 1). Once 5% of the pigs demonstrated clinical signs consistent with respiratory disease (as characterized by depression and respiratory scores ≥ 2) and a body temperature ≥ 40ºC, the animals were randomized to treatment. Clinical scoring was performed daily until the 5% threshold was obtained prior to treatment (Day 0) and again at study termination (Day 17).
Figure 1: Timeline for Study One. Pigs from two herds that had experienced outbreaks of porcine respiratory disease complex (PRDC) were comingled to evaluate the effect of in-feed tiamulin hydrogen fumerate (THF) treatment on the clinical signs associated with PRDC and pneumonia-lesion resolution. The pigs were group-housed in one barn. Once 5% of the pigs demonstrated clinical signs of respiratory disease, four clinically ill pigs (as defined by the clinical scoring system) were humanely euthanized and necropsied to confirm PRDC. Following confirmation of pneumonic lesions consistent with those of PRDC, pigs were blocked by illness status (clinically ill or non-clinically ill), weight, and sex and randomly allocated to pens within treatment (THF at 0 ppm, 137.5 ppm, or 165 ppm). Clinical scores, individual body weights, and feed consumption were measured. On Day 17, pigs were necropsied to calculate total percent pneumonia lesions. 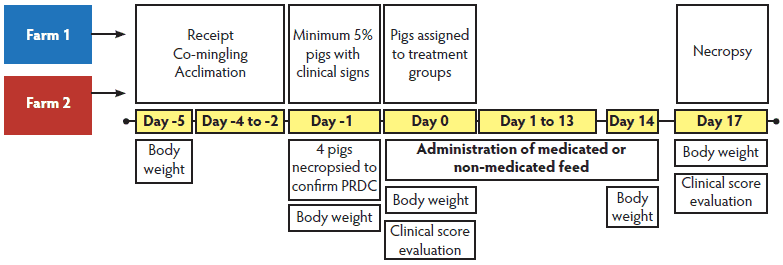 * Study One: pigs and procedures described in Figure 1. Clinical scoring was performed daily until the 5% threshold was obtained prior to treatment (Day 0) and again at study termination (Day 17). Study Two: 6-week-old pigs sourced from a Mycoplasma hyopneumoniae (M hyo)-free herd were housed together and challenged endotracheally with 10 mL of an M hyo lung homogenate containing 109 color changing units per mL. Three days post challenge, pigs were ranked by weight within sex and each pig was allocated to one of the three treatment groups: tiamulin hydrogen fumerate in feed at 0 ppm (control), 137.5 ppm, or 165 ppm. Clinical assessments (depression score, respiratory score, body temperature, and cough score) were performed on Day 0 prior to treatment and on Day 17 prior to euthanasia for necropsy. † Cough scores performed only on Day 17 of Study Two. Cough assessed only as Absent, Moderate, or Severe. ‡ Body temperature measured but not scored categorically. PRDC = porcine respiratory disease complex; NA = not applicable |
Table 1: Clinical parameter scoring system used to characterize respiratory disease in nursery pigs that either spontaneously developed PRDC (Study One) or were challenged with Mycoplasma hyopneumoniae (Study Two)*
|
To confirm PRDC infection prior to treatment administration, four clinically ill pigs (as defined by the clinical scoring system) were humanely euthanized and necropsied 4 days post receipt (Day -1). Following confirmation of pneumonic lesions consistent with PRDC, 180 pigs were blocked by illness status (clinically ill or non-clinically ill), weight, and sex and randomly allotted to pens within treatment to obtain 10 pens per treatment and six pigs per pen. Treatment groups included THF provided as an in-feed antibiotic at 0 ppm (control), 137.5 ppm, or 165.0 ppm, with approximately equal numbers of male and female pigs per group (0 ppm: 29 males, 31 females; 137.5 ppm: 30 males, 30 females; 165.0 ppm: 31 males, 29 females).
Clinically ill pigs were assigned to pens first to ensure equal representation within each treatment group. When all clinically ill pigs had been assigned, the non-clinically ill pigs were assigned to pens so that each treatment contained pens having a mixture of clinically ill and non-clinically ill pigs. Tiamulin hydrogen fumerate was administered as a medicated feed for 14 days starting Day 0, 5 days after receipt (Figure 1). The clinical investigator (KL) was blinded to treatment to eliminate potential for bias during clinical scoring. General health observations were performed once daily from receipt through study termination (Day 17). Body weights were obtained at receipt, Day -1 (prior to randomization), Day 0 (prior to treatment administration), Day 14, and Day 17. Feed consumption was measured by feed removed on Days 14 and 17 and feed added as needed within pens during the study. All pigs were necropsied on Day 17.
Procedures and tests
Samples collected at necropsy included bronchoalveolar lavage fluid for polymerase chain reaction (PCR) to evaluate the presence of M hyo and PRRSV infections. In addition, swab samples of lung lesions were collected and cultured to determine the presence of A pleuropneumoniae, B bronchiseptica, P multocida, H parasuis, S suis, and A suis, including determination of minimum inhibitory concentrations (data not reported). Samples of lung and lymph nodes were collected for immunohistochemistry (IHC) to diagnose PCV2. All samples were submitted to the University of Minnesota Diagnostic Laboratory (St Paul, Minnesota).
The primary variable to assess efficacy was percent treatment success. Each pig was classified as a treatment success or failure on the last day of the study (Day 17). A treatment success was defined as a pig having a body temperature < 40ºC in conjunction with respiratory and depression scores < 2. Secondary efficacy parameters included total percent pneumonia lesions at necropsy, calculated as the sum of the lung lesion percent in the separate six lobes (left cranial, right cranial, left caudal, right caudal, right middle, and accessory) multiplied by the approximate volume that each lung lobe contributed to the entire lung;7 percent mortality; and production parameters (average daily gain [ADG], average daily feed intake [ADFI], and feed conversion efficiency [FCE; ADFI ÷ADG]) from the initiation of treatment (Day 0) to the end of the study (Day 17). Average daily gain, defined as the average amount of weight gained per pig over the course of the experiment, was calculated by dividing the total weight gain by the total number of pig-days per pen. Pig-days were defined as the sum of the number of days each pig was alive per pen. Average daily feed intake, defined as the average amount of feed consumed per pig over the course of the study, was calculated by dividing total feed consumption (total feed consumed – total feed removed) by the number of pig-days per pen.
Study Two
In Study Two, 180 six-week-old pigs were sourced from a documented M hyo-free herd (Figure 2). The pigs were housed together in an environmentally controlled barn with solid concrete flooring and solid PVC pen dividers. Feed (free of antibiotics) and water were provided ad libitum. After a 5-day acclimation period all pigs, which were confirmed serologically negative for M hyo (Idexx M. hyo Ab Test; Idexx Laboratories, Westbrook, Maine), were endotracheally challenged with 10 mL of an M hyo lung homogenate containing 109 color changing units (CCU) per mL. The M hyo isolate originated from a clinical case of respiratory disease from a pig farm located in Minnesota (2004) and was provided by Midwest Veterinary Services, Inc (Oakland, Nebraska). Prior to challenge, the isolate was propagated in two pigs, and a pooled lung homogenate was used as the challenge material. To quantify the number of M hyo organisms per mL (109) in the lung homogenate, 5 mL of lung homogenate was passed through a 0.45-micron filter and added to a mycoplasma medium as previously described.8 Three days post challenge, 168 pigs were ranked by weight within sex and each pig was allocated to one of three treatment groups (THF at 0 ppm [control], 137.5 ppm, or 165.0 ppm), with eight pens per group and six pigs per pen and equal numbers of male and female pigs per group, or to a fourth, non-treated group consisting of four pens with six pigs per pen. The fourth group was maintained to substantiate the disease outbreak prior to treatment. On the basis of clinical evaluation of the challenged pigs, a pen of pigs from the non-treated group was selected for necropsy and gross pneumonic lung lesions were assessed (weighted left cranial 20%, left caudal 27.5%, right cranial 10%, right middle 10%, right caudal 27.5%, and accessory 5%). When a minimum of four of six pigs from a pen in the non-treated group showed weighted gross pneumonic lesions of at least 5% consistent with M hyo infection, the treatment phase was initiated (Day 0; 11 days post challenge). All pigs from two non-treated pens were necropsied 7 and 10 days post challenge to determine when to initiate treatment. Tiamulin hydrogen fumerate was then administered in feed for 14 days. The clinical investigator (KL) was blinded to treatment to eliminate potential for bias during clinical scoring. Clinical assessments (depression score, respiratory score, body temperature, and cough score) were performed on Day 0 prior to treatment and on Day 17 prior to euthanasia for necropsy. Body weights were obtained at receipt, prior to randomization, Day 0 (prior to treatment administration), Day 14, and Day 17. Feed consumption was measured by feed in as needed and feed out within pens on Days 14 and 17. General health observations, including an assessment of coughing, were conducted twice daily (at least 4 hours between assessments) from time of receipt until the end of the study (Day 17) at approximately the same times each day. All pigs were necropsied on Day 17.
Figure 2: Timeline for Study Two. Pigs challenged with Mycoplasma hyopneumoniae (M hyo) were evaluated to determine the effect of tiamulin hydrogen fumerate (THF) treatment on clinical signs associated with M hyo pneumonia lesions. The pigs were group-housed in one barn and challenged endotracheally with 10 mL of an M hyo lung homogenate containing 109 color changing units per mL. Post challenge, pigs were ranked by weight within sex and each pig was allocated to a treatment group (THF at 0 ppm, 137.5 ppm, or 165 ppm or a non-treated disease-confirmation group). When challenged pigs showed clinical signs of respiratory disease, pigs in the confirmation group were necropsied, and gross pneumonic lung lesions were assessed. When a minimum of four of six pigs within a pen showed weighted gross pneumonic lesions affecting at least 5% of the lung, consistent with an M hyo infection, the treatment phase was initiated. Clinical scores, body weight, and feed consumption were measured. On Day 17, pigs were necropsied to calculate total percent pneumonia lesions. 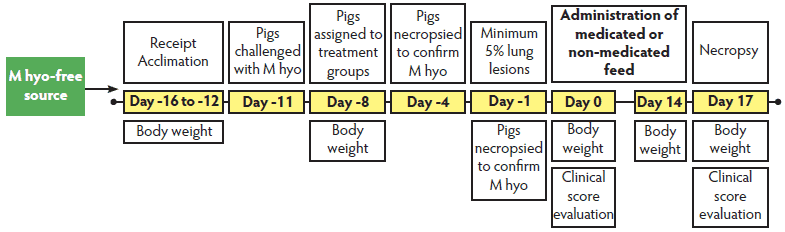 |
Procedures and tests
At study conclusion (Day 17), pneumonic lung-tissue samples from each pig were collected in sterile tubes containing Friis medium8 for Mycoplasma culture, isolation, and identification. Individual colonies were confirmed as M hyo by PCR. Isolation and PCR were conducted at Central States Research Center Veterinary Diagnostic Laboratory (Oakland, Nebraska). In addition, jugular blood samples were collected from all pigs prior to treatment administration and at study termination. All sera were stored at -80°C until submitted after the trial’s completion to the University of Minnesota Veterinary Diagnostic Laboratory (St Paul, Minnesota) for ELISA for M hyo antibodies (Idexx M. hyo Ab Test; Idexx Laboratories).
The primary efficacy variable for this study was total percent pneumonia-lesions score (as described in Study One) performed at necropsy (Day 17). Secondary efficacy evaluations included percent treatment success, percent mortality, and production parameters (ADG, ADFI, and FCE) as defined in Study One. In addition, percent abnormal clinical scores on Day 17 (depression, respiratory, and cough) were assessed using the same four-point categorical scale for depression and respiratory changes as described previously for Study One, with the addition of a three-point categorical scale for cough (0, absent; 2, moderate; and 3, severe; Table 1).
Statistical analysis
The pen was the experimental unit in both studies. All analyses were performed using SAS/STAT software (SAS Institute Inc, Cary, North Carolina). The primary efficacy parameter was percent treatment success in Study One and total percent pneumonia lesions in Study Two. All variables were averaged by pen and analyzed using analysis of variance (ANOVA) utilizing SAS PROC MIXED with “Treatment” as the fixed effect. Percentage data (treatment success, total lung lesions percent, mortality, and percent abnormal clinical scores [depression and respiratory score > 1 and cough score > 1 were considered abnormal]) were transformed using an arcsine square-root transformation prior to analysis. An additional analysis was performed in Study Two data regarding the number of pigs coughing per pen during the morning and evening over the course of the study. Morning (am) observations were performed before noon, and afternoon (pm) observations were performed after noon, with am and pm observations performed at least 4 hours apart. The count data was transformed (am data transformed using power = 2; pm data natural log transformed) to linearize it and it was then analyzed using regression analysis (SAS PROC REG). The am and pm data were analyzed separately. All hypotheses were tested at a two-sided .05 level of significance.
Results
In Study One, 14% of the study population was deemed clinically ill on study Day -1. The pigs in this study had viral respiratory disease, with some pigs’ infections complicated with bacterial pneumonia (Table 2). By study conclusion, the necropsy data and clinical parameter changes (depression score, respiratory score, and body temperature) supported the establishment of an adequate clinical manifestation of PRDC, as both viral and bacterial pathogens consistent with PRDC were identified in two or more pigs in 22 of 30 pens (73%). Pigs treated with THF at 165.0 ppm had numerically lower body temperatures when compared to the untreated control animals. Pigs treated with THF at 165.0 ppm and 137.5 ppm appeared normal to only mildly lethargic compared to control animals.
Table 2: Microbial analyses results collected at necropsy confirming exposure of grow-finish pigs to PRDC (Study One)*
* Study One pigs and procedures described in Figure 1. Bronchoalveolar lavage fluid was collected at necropsy for polymerase chain reaction to evaluate M hyo and PRRSV infection. Swab samples of lung lesions were cultured to determine the presence of Actinobacillus pleuropneumoniae, Bordetella bronchiseptica, Pasteurella multocida, Haemophilus parasuis, Streptococcus suis, and Actinobacillus suis. Samples of lung and lymph nodes were collected for immunohistochemistry to identify PCV2. All samples submitted to the University of Minnesota Diagnostic Laboratory (St Paul, Minnesota). PRDC = porcine respiratory disease complex; THF = tiamulin hydrogen fumerate; M hyo = Mycoplasma hyopneumoniae; PRRSV = porcine reproductive and respiratory syndrome virus; PCV2 = porcine circovirus type 2 |
The clinical outcomes and production parameters for Study One are summarized in Table 3. Treatment success, total percent pneumonia lesions, and mortality were significantly better in pigs treated with THF at 165.0 ppm than in the controls. In the group treated with THF at 137.5 ppm, total percent pneumonia lesions and mortality were significantly lower than in the controls, but treatment success did not differ. In the groups treated with THF, production parameters (ADG, ADFI, and FCE) were numerically but not significantly better than those of the controls over the 17-day study.
Table 3: Clinical and production parameters during a PRDC outbreak in grow-finish pigs treated with tiamulin hydrogen fumerate (THF) in feed for 14 days or not treated (Control) and necropsied 72 hours after the last treatment day (Day 17) (Study One)*
* Ten pens per treatment, 6 pigs per pen. The primary variable to assess efficacy was percent treatment success on the last day of the study (Day 17). Treatment success = a pig having a body temperature < 40°C in conjunction with respiratory and depression scores < 2 (scoring system described in Table 1). Secondary efficacy parameters: total percent pneumonia lesions at necropsy, percent mortality, and production parameters (ADG, ADFI, and FCE, with FCE = ADFI ÷ ADG) Days 0 to 17. All variables averaged by pen and analyzed using analysis of variance with “Treatment” as the fixed effect. Percentage data (treatment success, total lung lesions percent, mortality, and percent abnormal clinical scores) were transformed using an arcsine square-root transformation prior to analysis. a Value in a row differed significantly from group treated with THF at 165.0 ppm (P < .05). b Value in a row differed significantly from Control (P < .05). c Value in a row differed significantly from Control (P < .01). PRDC = porcine respiratory disease complex; ADG = average daily gain; ADFI = average daily feed intake; FCE = feed conversion efficiency |
In Study Two, all pigs were positive for M hyo infection by bacterial isolation (Table 4) after M hyo challenge and necropsy. Total percent pneumonia lesions was lower in both groups treated with THF than in the controls (Table 4). There were no significant differences in treatment success, percent mortality, or clinical assessment scores performed on Day 17. An analysis of general health observations showed that the number of pigs coughing in the morning (am) differed from the number coughing in the evening (pm). Figure 3 illustrates the statistically significant differences between the am regression line slopes for the THF 137.5 and 165.0 ppm treatment groups and for the control group, with the THF groups having a lower rate of increase in the number of morning coughs over time. The rate of increase was significantly lower in the THF 165.0 ppm treatment group than in the THF 137.5 ppm group. The rate of increase in pm coughs over time did not differ among the groups (data not shown). Average daily gain, ADFI, and FCE were numerically but not statistically better in the THF groups than in the control group over the 17-day study (Table 4).
Figure 3: Regression analysis of the number of pigs coughing in the morning (am) for each treatment group in Study Two (described in Figure 2) from Day 0 (M hyo challenge) to Day 17 (study termination). Morning observations were performed before noon, and afternoon (pm) observations were performed after noon, with am and pm observations ≥ 4 hours apart. The am data were transformed using power = 2 and pm data were natural log transformed to linearize the data, and then were analyzed separately (regression analysis). Rate of increase in am coughs over time differed between tiamulin hydrogen fumerate (THF) at 137.5 ppm and Controls (0 ppm THF; P < .01), between THF at 165.0 ppm and Controls (P < .05), and between THF at 165.0 ppm and THF at 137.5 ppm (P < .01). Rate of increase in pm coughs over time did not differ among the groups. 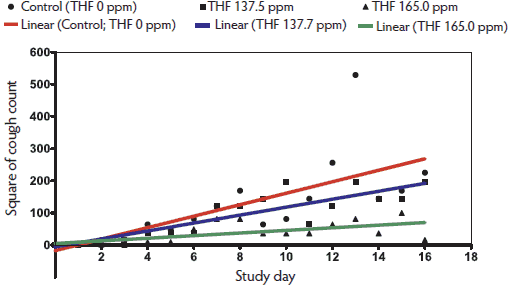 |
Table 4: Microbial confirmation, efficacy variables, and production parameters of grow-finish pigs challenged by endotracheal inoculation with Mycoplasma hyopneumoniae (M hyo) and treated with tiamulin hydrogen fumerate (THF) for 14 days (Study Two)*
* Six-week old pigs from an M hyo-free herd (8 pens per group and 6 pigs per pen) were challenged with M hyo as described in Figure 2. Clinical assessments (depression score, respiratory score, body temperature, and cough score, described in Table 1) were performed on Day 0 (prior to treatment), and on Day 17 prior to euthanasia for necropsy. At necropsy, pneumonic lung-tissue samples were collected for bacterial culture, isolation, and identification. Individual colonies were confirmed as M hyo by PCR, and Day 0 and 17 serum samples were tested for M hyo antibodies by ELISA (Idexx M. hyo Ab Test; Idexx Laboratories, Westbrook, Maine). All variables were averaged by pen and analyzed using analysis of variance with “Treatment” as the fixed effect. Percentage data (treatment success, total lung lesions percent, mortality, and percent abnormal clinical scores) were transformed using an arcsine square-root transformation prior to analysis. † Treatment success = body temperature < 40ºC and respiratory and depression scores < 2 on Day 17. a Within a row, value differs significantly from Control (P < .05). b Within a row, value differs significantly from Control (P < .01). |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Discussion
In Study One, all pigs treated with THF survived to study termination, while three pigs in the non-medicated control group died (5% mortality). Previous reports show that untreated pigs infected with PRDC experience mortality rates ranging from 2% to 20%, further evidence that the PRDC outbreak in Study One was typical of that in commercial swine settings.9
The positive clinical results from Study One are consistent with the pharmacokinetic and pharmacodynamic data reported for tiamulin hydrogen fumerate.10,11 This antibiotic, when used as an in-feed application, suggests a beneficial impact on grow-finish pigs infected with PRDC by mitigating the clinical effects of the respiratory disease. In addition, a triple challenge study by Stipkovits et al12 showed that THF used as an in-feed antibiotic reduced bacterial colonization and pneumonia-lesion development in 6-week-old piglets experimentally infected with M hyo, P multocida, and A pleuropneumoniae. Subsequent necropsy results for Study One correlated well with these findings by showing that pigs treated with THF also had a significantly lower total percentage of pneumonia lesions than did unmedicated controls.
In Study Two, bacterial isolation at necropsy confirmed that all pigs were infected with M hyo. Bacteriological culture from lung tissue is considered the “gold standard” diagnostic technique for M hyo diagnosis.1 Clinically, pigs had mild to moderate coughing and abnormal breathing across treatment groups, which is consistent with M hyo-associated respiratory disease. No mortalities or other serious clinical signs were noted, which is also consistent with previous reports that infection is characterized by high morbidity and low mortality.1 Mycoplasma hyopneumoniae infection is further characterized by colonization of the ciliated epithelium of the bronchial tree, which damages the mucociliary escalator, predisposing animals to secondary bacterial pneumonia.12 Indeed, coughing associated with the impaired mucociliary escalator is the primary clinical sign associated with M hyo infection. While the cough score (evaluated on Day 17) was not significantly different among groups, general health observations, performed twice daily on Days 0 to 17 for clinical response to infection and treatment, differed between treated and untreated pigs. Pigs that received THF had a lower rate of increase in the number of am coughs over time than did control animals, with the 165.0 ppm THF group showing greater clinical resolution than the 137.5 ppm THF group. Interestingly, the number of pm coughs among treatment groups did not differ even though subsequent necropsy results showed that pigs were adequately challenged with M hyo and that total percent pneumonia lesions was lower in treated pigs than in control pigs. The difference between morning and evening coughs may be attributed to the observation that pigs clinically ill with M hyo are more likely to cough once they are roused following a significant resting period (ie, night).13
The production parameters ADG, ADFI, and FCE were not statistically different between groups for pigs infected with PRDC or experimentally challenged with M hyo. However, pigs treated with THF in both studies had numerically better performance compared to non-medicated pigs with respiratory disease. Both studies were designed so that the pigs received treatment with THF (or no treatment) Days 0 to 14, with necropsy examination on Day 17. This timeframe was intended to evaluate treatment success as defined in Study One and total percent pneumonia lesions in Study Two after 14 days of THF treatment. Production parameters were evaluated as secondary evaluation parameters. However, the 17-day timeframe appeared too short to detect production differences among treatments. Statistically significant differences might have been observed if these production parameters had been assessed through to market weight (120 kg), a timeframe that would have allowed the pigs to more fully recover from the respiratory disease.
Implication
Under the conditions of this study, in-feed tiamulin hydrogen fumerate produces a positive clinical benefit in pigs experiencing PRDC or experimentally challenged with M hyo.
Acknowledgement
The authors wish to thank Eric Smith and Krissy Utter for their significant contributions in the completion of these studies.
References
1. Thacker EL. Mycoplasmal disease. In: Straw BE, Zimmermann JJ, D’Allaire S, Taylor DJ, eds. Diseases of Swine. Iowa State University Press: Ames, Iowa; 2006:701–717.
3. Sibila M, Pieters M, Molitor T, Maes D, Haese-brouck F, Segalés J. Current perspectives on the diagnosis and epidemiology of Mycoplasma hyopneumoniae infection. Vet J. 2009;1818:221–231.
4. Gillespie J, Opriessnig T, Meng XJ, Pelzer K, Buechner-Maxwell V. Porcine circovirus type 2 and porcine circovirus-associated disease. J Vet Intern Med. 2009;23:1151–1163.
5. Neumann EJ, Kliebenstein JB, Johnson CD, Mabry JW, Bush EJ, Seitzinger AH, Green AL, Zimmerman JJ. Assessment of the economic impact of porcine reproductive and respiratory syndrome on swine production in the United States. JAVMA. 2005;227:385–392.
6. Vicca J, Maes D, Jonker L, de Kruif A, Haese-brouck F. Efficacy of in-feed medication with tylosin for the treatment and control of Mycoplasma hyopneumoniae infections. Vet Rec. 2005;156:606–610.
7. Halbur PG, Paul PS, Frey ML, Landgraf J, Eernisse K, Meng XJ, Lum MA, Andrews JJ, Rathje JA. Comparison of the pathogenicity of two US porcine reproductive and respiratory syndrome virus isolates with that of the Lelystad virus. Vet Path. 1995;32:648–660.
8. Friis N. Some recommendations concerning primary isolation of Mycoplasma suipneumoniae and Mycoplasma flocculare. A Survey. Nord Vet Med. 1975;27:337–339.
*10. Anderson MD, Strohl SL, Rogers S. Tiamulin (Denagard) activity in certain swine tissues following oral and intramuscular administration. Proc AASP. 1994;115.
*11. Mills J, Apley M, Dau D, Bustamante A. In-vitro antimicrobial activity of tiamulin and chlortetracycline against field swine pathogens. Proc AASV. 2008;253.
12. Stipkovits L, Miller D, Glavits R, Fodor L, Burch D. Treatment of pigs experimentally infected with Mycoplasma hyopneumoniae, Pasteurella multocida, and Actinobacillus pleuropneumoniae with various antibiotics. Can J Vet Res. 2001;65:213–222.
13. Respiratory diseases of pigs: Mycoplasmal pneumonia. In: Kahn CM, ed. The Merck Veterinary Manual. 9th ed. Philadelphia, Pennsylvania: National Publishing, Inc; 2005:1225–1226.
* Non-refereed references.