Bates RO, Edwards DB, Ernst CW, et al. Influence of finishing environment on pig growth performance and carcass merit. J Swine Health Prod. 2011;19(2):86–93
| Original research | Peer reviewed |
SummaryObjective: To compare growth, carcass merit, and meat-quality traits for pigs of similar genetic merit finished in two different environments. Materials and methods: Pigs (n = 973) from an F2 Duroc × Pietrain resource population were finished in either an environmentally controlled modified open-front (MOF) building or a non-environmentally controlled test station (TS) building. Serial body weight, tenth-rib backfat thickness and longissimus muscle area, and last-rib backfat thickness were collected every 3 weeks from 10 through 22 weeks of age. After slaughter, carcass, meat-quality, and eating-quality data were collected. Results: Growth rate was not significantly different from 10 to 22 weeks of age for pigs raised in the two different finishing environments. However, pigs raised in the MOF had a greater fat accretion rate through 22 weeks of age, compared to those raised in TS. This difference persisted through harvest. After harvest, the longissimus muscle from pigs raised in the MOF had higher 45-minute pH and lower Warner-Bratzler shear-force values than loins from pigs raised in the TS. Implications: Under the conditions of this study, finishing environment alters performance, carcass merit, and meat quality of pigs of similar genetic merit. Farms with differing types of finishing facilities should account for finishing environment in the development of their finishing management protocols. The appropriate diet formulation, ideal age and weight at marketing, and packer buying program may differ due to the impact of finishing environment on pig performance and carcass merit.
| ResumenObjetivo: Comparar las características del crecimiento, el valor de la canal, y la calidad de la carne de cerdos de valor genético similar, finalizados en dos medioambientes diferentes. Materiales y métodos: Se finalizaron cerdos (n = 973) F2 Duroc × Pietrain, en un edificio de frente abierto modificado con medio ambiente controlado (MOF por sus siglas en inglés) ó en una estación de prueba con medioambiente no controlado (TS por sus siglas en inglés). Cada 3 semanas, de la semana 10 a 22 de edad, se tomaron muestras seriadas de peso corporal, espesor de grasa dorsal de la décima costilla, área del músculo longissimus, y espesor de la grasa dorsal de la última costilla. Después del sacrificio, se colectó información de la canal, la calidad de la carne, y la calidad para consumo. Resultados: El índice de crecimiento no fue significativamente diferente de 10 a 22 semanas de edad en los cerdos criados en los dos diferentes medioambientes de finalización. Sin embargo, los cerdos criados en el MOF presentaron un mayor índice de adición de grasa durante las 22 semanas de edad comparados con los cerdos criados en TS. Esta diferencia fue consistente en todos los cerdos del grupo. Después del sacrificio, el músculo longissimus de los cerdos criados en el MOF tuvo un mayor pH de 45 minutos y valores más bajos de fuerza de corte de Warner-Bratzler que los lomos de cerdos criados en el TS. Implicaciones: Bajo las condiciones de este estudio, el medio ambiente de finalización altera el desempeño, el valor de la canal, y la calidad de la carne de cerdos con valor genético similar. Las granjas con instalaciones de finalización de diferentes tipos deberían considerar el medio ambiente de finalización en la preparación de sus protocolos de manejo de finalización. La formulación adecuada de la dieta, peso y edad ideal de venta y el programa de compra de la empacadora pueden diferir debido al impacto del medioambiente de finalización en el desempeño del cerdo y el valor de la canal. Résumé –
| ResuméObjectif: Comparer la croissance, le mérite de la carcasse, et la qualité de la viande de porcs ayant un mérite génétique similaire mis dans deux environnements différents. Matériels et méthodes: Des porcs (n = 973) provenant d’une population F2 Duroc × Piétrain ont été placés dans un bâtiment avec une façade ouverte modifiée contrôlée par l’environnement (MOF) ou dans un bâtiment d’une station d’épreuve non-contrôlé par l’environnement (TS). À chaque 3 semaines, débutant à la semaine d’âge 10 jusqu’à la semaine 22, on nota le poids corporel, l’épaisseur du gras dorsal vis-à-vis la dixième et la dernière côte ainsi que la surface du muscle longissimus. Après l’abattage, les données sur la carcasse, la qualité ainsi que l’appétence la viande ont été notées. Résultats: Aucune différence significative n’a été notée durant la période d’étude entre les porcs élevés dans les deux environnements différents. Toutefois, les porcs élevés en MOF avait un taux d’acquisition de gras plus élevé jusqu’à 22 semaines comparativement à ceux élevés en TS. Cette différence a persisté tout au long de la cueillette. Quarante-cinq minutes après abattage, le muscle longissimus des porcs élevés en MOF avait une valeur de pH plus élevée et des valeurs de force de déchirement de Warner-Bratzler plus faibles que les longes des porcs élevés en TS. Implications: Dans les conditions de la présente étude, l’environnement de finition altéra les performances, le mérite de la carcasse, et la qualité de la viande de porcs ayant un mérite génétique similaire. Les fermes avec différents types d’installation de finition devraient tenir compte de l’environnement de finition dans le développement de leurs protocoles de gestion des animaux en finition. La formulation appropriée de la ration, l’âge et le poids idéal pour la mise en marché, et le programme d’achat de l’acheteur peuvent différer dû à l’impact de l’environnement en période de finition sur les performances des animaux et le mérite des carcasses. |
Keywords: swine, environment, growth, meat quality
Search the AASV web site
for pages with similar keywords.
Received: February 2, 2010
Accepted: August 18, 2010
Growth performance can depend on environmental and housing differences, with animals of similar genetic merit showing differing patterns of growth performance due to differences in management.1 It has been reported2 that differences can occur in growth and meat-quality traits between pigs reared indoors and outdoors. To determine the influence that differing finishing environments may have on pig growth, a genetic resource population with known pedigrees can be an ideal study group. With known pedigrees and similar genetic merit among animals in a resource population, those genetic factors can be accounted for and actual differences in finishing environments can be determined.
Body weight and composition traits can be measured serially over time and modeled through the use of random regression.3 These procedures allow (co)variance matrices to be estimated that model the relationship of the same trait with itself at different points throughout the measurement period. Previous reports have demonstrated the usefulness of random regression modeling of weight data in pigs.4,5 The objective of this study was to compare growth, composition, and meat-quality traits for pigs of similar genetic merit finished in two different finishing environments.
Materials and methods
Pigs were managed according to Michigan State University swine farm operating procedures as approved by the All University Committee for Animal Use and Care.
Animal management
Pigs used in this study were from the F2 generation of a Duroc × Pietrain resource population6,7 and were produced across 11 farrowing groups. All pigs were managed similarly during the farrowing and nursery stages. At 10 weeks of age, pigs were placed into either a naturally ventilated modified open-front building (MOF; n = 521 pigs) with supplemental winter heat, or a naturally ventilated, non-heated test station (TS; n = 465 pigs). The MOF represented an environmentally controlled facility with performance expectations similar to that of the majority of environmentally controlled facilities within the industry. The TS represented a finishing environment in which pigs were subjected to greater extremes in environmental conditions.
In the eight-pen MOF, four pens differed in size from the other four; however, pigs had a minimum of 0.74 m2 of floor space regardless of pen size. The four larger pens (2.03 m × 6.91 m) each had one two-space feeder and were targeted to contain 16 pigs. The four smaller pens (1.42 m × 6.91 m) each had a one single-space feeder and were targeted to contain 12 pigs. Each pen had a two-thirds solid, one-third slatted floor and wet-dry feeders. The TS pens had solid floors, were bedded with straw or wood shavings, and had one single-space dry feeder and one cup drinker per pen. During winter, a 1.42 × 1.25-m wooden hover covered in bales of straw, without heat, was placed over each pen, and pigs were bedded in straw. Pens (1.42 m × 4.93 m) were targeted to contain four pigs with a minimum of 1.75 m2 of floor space per pig. All diets fed were Michigan State University standard swine farm diets that met or exceeded National Research Council8 requirements for all nutrients at each production stage. Pigs in both facilities had ad libitum access to feed and water.
Trait collection
Live-animal traits collected included body weight (BW) at 10, 13, 16, 19, and 22 weeks of age. An average daily body weight gain (ADG) from 10 to 22 weeks of age was calculated. Additionally, β-mode ultrasound (Pie Medical 200SLC; Classic Medical Supply, Inc, Tequesta, Florida) estimates of tenth-rib backfat and longissimus muscle area at the tenth rib, along with last-rib backfat, were recorded at 10, 13, 16, 19, and 22 weeks of age. At each of these time points, estimates of fat-free total lean and total body-fat tissue were calculated using prediction equations.9 Pigs were weighed prior to leaving the farm for harvest. Not all pigs placed into the facilities were harvested. Pigs not harvested either died or were too light to be sent to the harvest facility and were marketed into light-pig market channels, and no further data were recorded on these individuals.
At harvest, pigs were transported to two abattoirs. A total of 176 pigs were harvested at the Michigan State University Meats Laboratory (East Lansing, Michigan) to facilitate tissue collection for future studies, and the remainder of the pigs were transported approximately 162 km to a small commercial abattoir in western Michigan. Pigs harvested at different plants were slaughtered on different days. Harvest age was 165.8 ± 9.2 days. All groups were fasted and allowed to rest overnight with access to water. Hot carcass weight was measured. In addition, pH in the longissimus muscle was measured adjacent to the last rib at 45 minutes and 24 hours post mortem with a portable pH meter (Model 1140; Mettler-Toledo, Woburn, Massachusetts) equipped with a puncture-type combination pH electrode (Lot 406-M6-DXK-57/25; Mettler-Toledo). Temperature of the longissimus muscle was recorded adjacent to the last rib 45 minutes and 24 hours post mortem using a hand-held thermocouple thermometer (Model 33032; Atkins Technical, Inc, Gainesville, Florida).
After overnight chilling, measurements were taken according to National Pork Producers Council guidelines10 and included midline first-rib backfat, last-rib backfat, last-lumbar backfat, number of ribs, and carcass length. Carcass length was measured from the first rib to the split portion of the pelvis bone. During dissection of the carcass into wholesale cuts, tenth-rib backfat and longissimus muscle area were measured with rib intact using a ruler to measure backfat and a grid to estimate longissimus muscle area. A tenth-to-last-rib loin section was retained for further meat-quality analysis. After deboning, two 2.54-cm chops were cut and exposed to air for 10 minutes. Data collected included International Committee on Illumination (CIE)11 measurements of light reflectance (L*), red-green color spectrum (a*), and blue-yellow color spectrum (b*); subjective color score on a 1 to 6 scale,10 where 1 was pale pinkish gray and 6 was dark purplish red; subjective marbling score on a 1 to 10 scale10 which approximated intramuscular fat percentage; and firmness on a 1 to 5 scale,12 where 1 indicated very soft and watery and 5 was very firm and dry. Furthermore, drip loss (muscle fluid loss)13 was recorded for 24 hours on these two chops hung individually in sealed plastic bags for 24 hours at 4°C. In addition, longissimus intramuscular fat percentage was estimated by proximate analysis. The remaining section of the longissimus muscle was vacuum packaged, aged 7 days at 4ºC (similar to time from harvest to commercial display within the industry), and frozen for further meat-quality tests of cook yield and Warner-Bratzler13 shear force (WBS), a measure of tenderness.
In addition, sensory taste panel analyses were conducted on all chops. From frozen loin sections, 2.54-cm thick chops were cut for cook yield, WBS, and sensory analysis. For cook yield and WBS measurements, two chops per loin section were thawed for 24 hours at 2.6ºC, weighed, cooked to 71ºC internal temperature on a Taylor clamshell grill (Model QS24; Taylor Co, Rockton, Illinois), cooled to room temperature, and weighed again. Temperature was monitored by inserting a copper constantan thermocouple, 0.05-cm diameter, 15.2 cm length (Omega Engineering Inc, Stamford, Connecticut) or 0.089-cm diameter, 5.72-cm length (Cole-Parmer, Vernon Hills, Illinois), into the geometric center of each pork chop. From these chops, six cores (three from each chop) were collected parallel to the muscle-fiber direction using a drill-press-mounted corer. Cores were sheared perpendicular to muscle fibers using a Warner-Bratzler head on a TA-HDi texture analyzer (Texture Technologies Corp, Scarsdale, New York). The cross-head speed was 3.30 mm per second.
For sensory analysis, chops were prepared as described above. A trained panel of seven healthy adults (ages 20 to 65) was utilized to determine specific sensory attributes of each loin (longissimus) chop. The sensory panel was trained14,15 and had previous meat-product evaluation experience. Each sample was evaluated for juiciness, muscle-fiber mouth feel and overall tenderness, connective tissue, and off-flavor using an 8-point hedonic scale. Higher scores were more favorable in each of the first four categories and indicated extremely juicy, extremely tender, or no connective tissue for each of these attributes, respectively, while lower scores for off-flavor were indicative of less off-flavor.
For the carcass, meat-quality, and sensory analysis data, the number of observations for each trait varied, for a variety of reasons. A few carcasses were lost in the harvest facility. In addition, malfunction of equipment (eg, pH meter) reduced the number of possible observations. Furthermore, some samples were misplaced in storage, which reduced the number of samples available for proximate and sensory analyses.
Statistical analysis
The 22-weeks-of-age off-test traits, carcass phenotypes, and meat-quality traits were analyzed using a mixed model that included the fixed effects of sex and finishing environment (MOF or TS) and the random effects of farrowing group, finishing pen nested within farrowing group, litter, and the individual animal effect. The animal variance-covariance matrix was augmented by the relationship between animals. In addition, covariates were included as deemed appropriate. For off-test traits, age at measurement was included as a covariate. Harvest age was used as a covariate for the analyses of off-farm and carcass weight, dressing percentage, subjective marbling and color scores, and drip loss percentage. For all pH and temperature traits, as well as all carcass measures of backfat thickness, carcass weight was included as a covariate. For the CIE L*, a*, and b* measurements, along with cook yield, shear force, and the sensory traits, covariates were not included in the models. An F test was used to determine finishing-environment treatment differences, and values of P < .05 were considered significant.
Serial data analysis
Serial BW and ultrasound estimates from 10 to 22 weeks of age were used to generate random regression equations to model pig BW, tenth-rib backfat thickness, loin muscle area, last-rib backfat thickness, fat-free total lean, and total body-fat tissue on age at measurement. Age at measurement was modeled as week on-test, calculated as age in weeks minus 10 (ie, 0, 3, 6, 9, and 12), and included in the models as distinct covariate values. A random intercept for each animal and a linear regression on age for each animal were included in each model. Table 1 lists the polynomial order of week of age and interactions that were significant and that were used in the models for these six traits, as determined by log likelihood tests of significance. Higher order polynomials were included in the model by forward selection if they significantly contributed to the model at P < .05.
Table 1: Order of polynomial for week of age, sex × week interaction, and finisher × week interaction terms for random regression analyses of serial growth data*
* Pigs used in this study were an F2 Duroc × Pietrain cross. At 10 weeks of age, pigs were placed into either a naturally ventilated modified open-front building (MOF; n = 521 pigs) with supplemental winter heat or a naturally ventilated, non-heated test station (TS; n = 465 pigs). Pigs were provided a minimum of 0.74 m2 of floor space in the MOF, with 12 to 16 pigs per pen. Pigs were provided with 1.75 m2 of floor space in the TS, with four pigs per pen. At approximately 22 weeks of age, 176 pigs were harvested at a nearby research abattoir, while the remaining 782 pigs were transported to a commercial abattoir 162 km away. All pigs were fasted and rested overnight at the abattoir. Body weight was determined before the pigs were transported. Significant (P < .05) terms as determined by F test were included in the final model. |
The following model was used: Yijklmno = μ + Σweekiφ + sexj + Σ(sex×weekφ)j + fink + S(fin×weekφ)k + grp(fin)kl + pen(grp)lm + litn + go + αo×Zi + eijklmno
in which
Yijklmno = record on the oth pig within jth sex, kth finisher, lth group, mth pen, and nth litter regressed on φth polynomial week i,
μ = overall mean of trait,
weekiφ = fixed regression coefficient for polynomial terms φ (1 to 4) of week i,
sexj = fixed effect of sex of animal j (barrow or gilt),
fink = fixed effect of finisher k (MOF or TS building),
grp(fin)kl = random effect of farrowing group l (1 to 11) nested within finisher, where grp ~N(0, Iσgrp2),
pen(grp)lm = random effect of pen m (1 to 25) nested within farrowing group, where pen ~N(0, Iσpen2),
litn = random effect of litter n (1 to 142), where lit ~N(0, Iσlit2),
go = random intercept for animal o,
αo = random linear regression coefficient on age for animal o,
Zi = week on test as a covariate, and
eijklmno = random error.
The distributional assumptions on g ={go} and α = {αo} were such that:
is the intercept genetic variance for the individuals, σ2 is the linear age by animal genetic variance, σgα is the genetic covariance between the intercept and linear term for each animal, and A is the numerator relationship matrix among animals. To account for residual variances across serial measurements and the relationship between time points, the e = {eijklmno} was specified as normally distributed with a general (co)variance structure calculated from the data and specified within and across weeks, with five variance and 10 covariance terms. These analyses were performed utilizing the ASREML software package (New South Wales, Orange, Australia).
Results
Off-test, carcass composition, and meat quality
From 10 to 22 weeks of age, ADG did not differ between pigs in the two different finishing environments, but at 22 weeks of age, pigs in the MOF had more tenth-rib and last-rib backfat thickness than pigs raised in the TS (Table 2). At harvest, BW was significantly higher in animals from the MOF than from the TS (Table 3). Pigs raised in the MOF also had more tenth-rib, last-rib, and last-lumbar backfat at slaughter than pigs raised in the TS (Table 3). The 45-minute pH and the decline in pH from 45 minutes to 24 hours post slaughter were greater among carcasses from pigs raised in the MOF versus those raised in the TS (Table 3).
Table 2: Number of observations, least squares means, standard error of the mean (SEM), and P value, as determined by F test, of the difference between finisher means for off-test traits in pigs at 22 weeks of age*
* Pigs and housing described in Table 1. MOF = modified open-front building; TS = test station building; ADG = average daily gain. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||
Table 3: Number of observations, least squares means, standard error of the mean (SEM), and P value, as determined by F test, of the difference between finisher means for carcass and meat-quality traits†
† Pigs and housing described in Table 1. Numbers of observations varied because of loss of carcasses and of samples in storage, and missing data due to malfunction of equipment. ‡ Color score of 1 (pale pinkish gray) to 6 (dark purplish red). § Marbling score of 1 to 10 indicated approximate intramuscular fat percentage. ¶ Firmness score of 1 (very soft and watery) to 5 (very firm and dry). †† L*: Higher values indicate greater light reflectance; a*: higher values indicate greater red intensity; b*: higher values indicate greater yellow intensity. ‡‡ Higher scores indicate more favorable attributes. §§ Lower scores indicate more favorable attribute. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Warner-Bratzler shear force was influenced by finishing environment. Pigs raised in the MOF yielded loin chops that had lower shear-force values than chops from pigs raised in the TS (Table 3).
Serial data results
Serial growth analyses revealed differences in growth patterns between the MOF-housed and TS-housed pigs. The six serial traits were plotted against week of age with standard error bars included (Figures 1 through 6). For traits in which the finishing-environment-by-week model term was significant (Table 1), point estimates of treatment means on each graph in which the standard error intervals did not overlap were considered significant. Figure 1 shows how BW change differed between MOF- and TS-housed pigs. Pigs in the MOF were heavier at 13 and 19 weeks of age, but BW did not differ at 22 weeks of age.
Figure 1: Body weight means with standard errors for pigs 10 to 22 weeks of age raised in a modified open-front, environmentally controlled building (MOF) versus a test station building (TS) with no supplemental heat, as described in Table 1. Significant terms in the model (*; P < .05) were determined by F test. 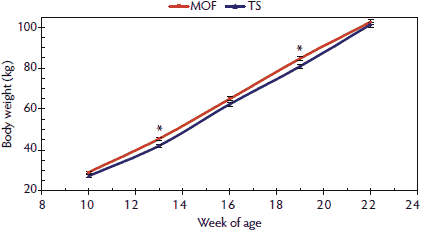 |
The loin muscle area (Figure 2) exhibited a pattern similar to that reported for BW in this study. Numerical differences occurred in the shape of the loin muscle area growth curve between pigs raised in the MOF and those raised in the TS; however, these differences were not significant. Pigs reared in the MOF had more tenth-rib backfat (Figure 3) than those reared in the TS, and this difference in backfat was observed through harvest, as indicated by the significant differences reported in the carcass data. The pattern for last-rib backfat (Figure 4) was similar to that of the tenth-rib backfat over time, with pigs housed in the MOF having more last-rib backfat than pigs housed in the TS. These differences in backfat thickness were significant at 22 weeks of age, and the harvest data suggested that the differences between pigs raised in the MOF and those raised in the TS were maintained at harvest.
Figure 2: Longissimus muscle area means with standard errors for pigs 10 to 22 weeks of age raised in a modified open-front building (MOF) versus a test station building (TS), as described in Table 1. Means did not differ significantly (P > .05) as determined by F test. 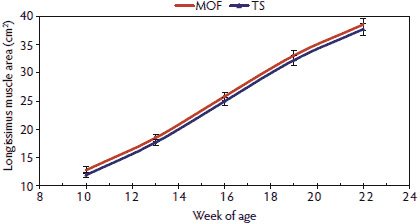 |
Figure 3: Tenth-rib backfat means with standard errors for pigs 10 to 22 weeks of age raised in a modified open-front building (MOF) versus a test station building (TS), as described in Table 1. Significant terms in the model (*; P < .05)were determined by F test. 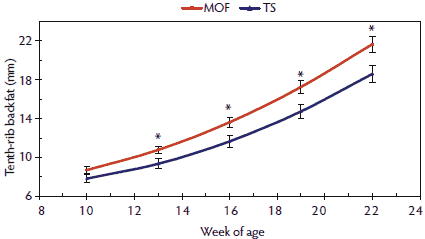 |
Figure 4: Last-rib backfat means with standard errors for pigs 10 to 22 weeks of age raised in a modified open-front building (MOF) versus a test station building (TS), as described in Table 1. Significant terms in the model (*; P < .05) were determined by F test. 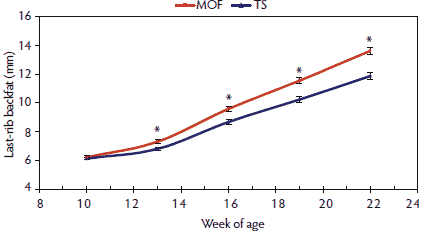 |
When the differences in backfat measurements were combined with BW and loin muscle area measurements, there were no differences, except at 13 weeks of age, between MOF- and TS-raised pigs for fat-free total lean accretion (Figure 5). Conversely, differences in total body-fat tissue continued to increase as the finishing period progressed from 10 to 22 weeks of age (Figure 6). The differences in tenth-rib backfat and last-rib backfat thickness contributed to the differences in total body-fat tissue, where the MOF-reared pigs had increasingly more total body-fat tissue than the TS-reared pigs.
Figure 5: Fat-free total lean means with standard errors for pigs 10 to 22 weeks of age raised in a modified open front building (MOF) versus a test station building (TS), as described in Table 1. Significant terms in the model (*; P < .05) were determined by F test. 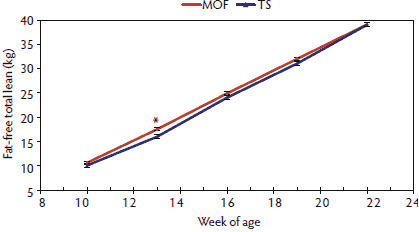 |
Figure 6: Total body-fat tissue means with standard errors for pigs 10 to 22 weeks of age raised in a modified open front building (MOF) versus a test station building (TS), as described in Table 1. Significant terms in the model (*; P < .05) were determined by F test. 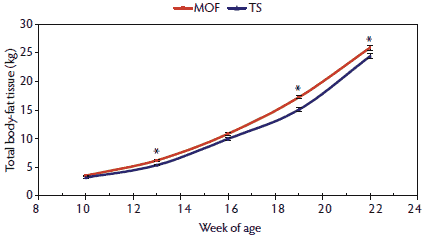 |
Discussion
Differences found between pigs raised in the MOF and those raised in the TS conflicted somewhat with those of a previous study2 reporting a higher fat deposition in pigs being finished in a deep-bedded system than in a slatted-floor system. Pigs finished in the deep-bedded facility were fatter than pigs raised on slatted floors, in contrast to the results of the present study. However, in the earlier study,2 the initial pH measurement (1 hour) in the longissimus muscle was lower in pigs raised in deep bedding than in pigs housed on slats, which is similar to the results of the current study. Furthermore, hot carcass weight was not significantly different for pigs from the MOF than for pigs from the TS, whereas the previous study2 reported heavier cold carcass weights in pigs in the bedded facility versus those on slatted floors. The differences in backfat thickness associated with housing environment reported in the present study were consistent with those in a study16 in which pigs housed in bedded hoop buildings were leaner at harvest than those housed in conventional finishing facilities. However, overall growth rate in the present study did not differ between finishing environments, whereas the growth rate of pigs housed in conventional finishing facilities16 was better than that of pigs housed in bedded hoop buildings.
The observed differences in fat measurements between pigs raised in the MOF and those raised in the TS may be a result either of differing feeder types in the two buildings or changes in maintenance energy requirements. Pigs consuming feed from wet-dry feeders have been reported17 to have more subcutaneous fat than pigs fed from dry feeders. Since the MOF was equipped with wet-dry feeders, this might explain part of the differences observed in our study. It has been reported that feed intake of pigs is greater from ad libitum wet-dry feeders than from dry feeders,18,19 and in one study, pigs grew significantly faster during the finishing period when fed from wet-dry feeders than from dry feeders.18 However, in a more recent report,20 average daily feed intake and ADG during the finishing phase did not differ for pigs consuming feed ad libitum from dry or wet-dry feeders. Feed disappearance was not recorded in this study. If feed intake was greater for pigs housed in the MOF in the present study, this might have resulted in greater backfat thickness. However, this was not true in other studies that observed greater feed intake among pigs using wet-dry feeders than dry feeders.19,20
The amount of climate control inherent to the MOF and TS finishers created an environmental difference between the buildings. The MOF was naturally ventilated and had supplemental heat in the winter. While the TS had a curtain, wind blocks, and hovers in winter, it did not have supplemental heat, and pigs were more exposed to the ambient temperature. Considering these environmental differences, pigs in the TS may have had less fat because they may have had a greater maintenance energy requirement in the TS environment than those raised in the MOF. Since the energy requirement of the pigs raised in the MOF may have been less, the excess energy may have been stored as fat.
Changes in body growth and composition are more evident over time when considering the serial data comparison between the pigs raised in the MOF and those raised in the TS. Body-weight serial data analyses indicated some differences in the shape of the growth curve between the two finishers, with the TS pigs growing more slowly in the early stages, but compensating and equaling body weight of those in the MOF by the end of the trial period. The additional exposure to climatic changes for the pigs raised in the TS may have been partially responsible for these observed data trends. Serial traits with the greatest differences were tenth-rib and last-rib backfat thickness. The differences between finisher observations for these measures increased with weeks in the finishers. In agreement with the off-test data, the carcass results indicated that pigs raised in the MOF had more backfat at the tenth rib, last rib, and last lumbar vertebra than did those raised in the TS. Environmental differences between the two housing types did influence pig performance, carcass merit, and meat-quality traits within genetically similar pigs.
Implications
• Under the conditions of this study, finishing environment alters performance, carcass merit, and meat quality of pigs of similar genetic merit.
• Management protocols for finishing pigs raised in different finishing environments should consider the environmental implications as they relate to diet formulation, age and weight at market, and packer buying programs.
• As finishing environment can influence meat quality and tenderness, persons marketing pigs through pork chains with meat quality and sensory specifications should consider the ramifications of the finishing environment on market specifications and determine if finishing environment can be altered to improve the product provided to the targeted market.
Acknowledgements
The authors acknowledge the technical assistance of R. J. Tempelman in data analysis and W. N. Osburn and C. S. Quinlan for sensory analysis. This research was financially supported by the Michigan State University Department of Animal Science, the Michigan Agricultural Experiment Station, and a Michigan Animal Initiative Coalition Grant.
References
1. Hamilton DN, Ellis M, Wolter BF, Schinckel AP, Wilson ER. The growth performance of the progeny of two swine sire lines reared under different floor space allowance. J Anim Sci. 2003;81:1126–1135.
2. Gentry JG, McGlone JJ, Blanton JR Jr, Miller MF. Alternative housing systems for pigs: Influences on growth, composition, and pork quality.
J Anim Sci. 2002;80:1781–1790.
3. Meyer K. Scope for a random regression model in genetic evaluation of beef cattle for growth. Livest Prod Sci. 2004;86:69–83.
4. Huisman AE, Veerkamp RF, van Arendonk JAM. Genetic parameters for various random regression models to describe the weight data of pigs. J Anim Sci. 2002;80:575–582.
5. Edwards DB, Tempelman RJ, Bates RO. Evaluation of Duroc- vs Pietrain-sired pigs for growth and composition. J Anim Sci. 2006;84:266–275.
6. Edwards DB, Ernst CW, Tempelman RJ, Rosa GJM, Raney NE, Hoge MD, Bates RO. QTL mapping in an F2 Duroc x Pietrain resource population: I. Growth traits. J Anim Sci. 2008;86:241–253.
7. Edwards DB, Ernst CW, Raney NE, Doumit ME, Hoge MD, Bates RO. QTL mapping in an F2 Duroc x Pietrain resource population: II. Carcass and meat quality traits. J Anim Sci. 2008;86:254–266.
8. National Research Council. Nutrient Requirements of Swine. 10th rev ed. Washington, DC: National Academy Press; 1998.
9. Wagner JR, Schinckel AP, Chen W, Forrest JC, Coe BL. Analysis of body composition changes of swine during growth and development. J Anim Sci. 1999;77:1442–1466.
10. National Pork Producers Council. Pork Composition and Quality Assessment Procedures. 1st ed. Des Moines, Iowa: National Pork Producers Council; 2000.
11. CIE, International Commission on Illumination. Colorimetry: Official Recommendations of the International Commission on Illumination. Publication CIE No. 15 (E-1.3.1). Paris, France: Bureau Central de la CIE; 1976.
12. National Pork Producers Council. Procedures to Evaluate Market Hogs. 3rd ed. Des Moines, Iowa: National Pork Producers Council; 1991.
13. Honikel KO. Reference methods for the assessment of physical characteristics of meat. Meat Sci. 1998;49:447–457.
14. Meilgaard M, Civille GV, Carr BT. Sensory Evaluation Techniques. 1st ed. Boca Raton, Florida: CRC Press Inc; 1999.
15. American Meat Science Association. Research Guidelines for Cookery, Sensory Evaluation and Instrumental Measurements of Fresh Meat. Chicago, Illinois: American Meat Science Association and National Livestock and Meat Board; 1995.
16. Patton BS, Huff-Lonergan E, Honeyman MS, Crouse JD, Kerr BJ, Lonergan SM. Effects of deep-bedded finishing system on market pig performance, composition and pork quality. Animal. 2008;2:459–470.
*17. Barnes DJ, Snedegar JA, Rozeboom DW. Comparison of Farmweld model CR42SAS0 and Crystal Spring model WR3050 early-wean feeders. MSU Pork Q. 1999;4(4):1–3.
18. Brumm MC, Dahlquist JM, Heemstra JM. Impact of feeders and drinker devices on pig performance, water use and manure volume. J Swine Health Prod. 2000;8:51–57.
19. Botermans JAM, Svendsen J. Effect of feeding environment on performance, injuries and behavior in growing-finishing pigs: Group-based studies. Acta Agric Scand Sect A Anim Sci. 2000;50:237–249.
20. Magowan E. McCann MEE, O’Connell NE. The effect of feeder type and change of feeder type on growing and finishing pig performance and behavior. Anim Feed Sci Tech. 2008;142:133–143.
* Non-referred reference.