Jacela JY, Dritz SS, DeRouchey JM, et al. Field evaluation of the effects of a porcine circovirus type 2 vaccine on finishing pig growth performance, carcass characteristics, and mortality rate in a herd with a history of porcine circovirus-associated disease. J Swine Health Prod. 2011;19(1):10–18.
| Original research | Peer reviewed |
SummaryObjective: To evaluate porcine circovirus type 2 (PCV2) vaccine influence on growth performance and mortality rate of finishing pigs. Materials and methods: The study treatments (vaccinated for PCV2 or nonvaccinated control and barrow or gilt) were arranged as a 2 × 2 factorial. Pigs (PIC L337 × 1050) were randomly assigned to Vaccinated or Control treatments within gender. In Experiment One, pigs were vaccinated at 9 and 11 weeks of age, and in Experiment Two, pigs were vaccinated at 5 and 7 weeks of age. Performance data were then collected during the finisher on-test period beginning when pigs were 11 weeks of age. Pig weights and feed intake were recorded on a pen basis. Results: In Experiment One, average daily gain (ADG), average daily feed intake, gain-to-feed ratio, and mortality were better (P < .05) in Vaccinated pigs than in Controls. In Experiment Two, there were vaccine-by-gender interactions for ADG (P < .01) and final weight (P < .05), as ADG was greater in Vaccinated barrows than in Vaccinated gilts (P < .01). Overall, greater ADG resulted in Vaccinated pigs being 2.9 kg heavier (P < .01) than Controls at market. Mortality rates of Vaccinated pigs were lower (P < .05) than those of Controls (2.8 percentage units in Experiments One and 6.2 percentage units in Experiment Two). Implications: The superior growth performance and lower mortality after vaccination confirmed the efficacy of the commercial PCV2 vaccine used in this study.
| ResumenObjetivo: Evaluar la influencia de la vacuna del circovirus porcino tipo 2 (PCV2 por sus siglas en inglés) en el desempeño del crecimiento y porcentaje de mortalidad de cerdos de finalización. Materiales y métodos: Los tratamientos de estudio (vacunado contra PCV2 ó control no vacunado y macho ó hembra) se organizaron como un factorial de 2 × 2. Los cerdos (PIC L337 × 1050) se asignaron al azar a tratamientos Vacunación o Control dentro de género. En el Experimento Uno, los cerdos se vacunaron a las 9 y 11 semanas de edad, y en el Experimento Dos, los cerdos se vacunaron a las 5 y 7 semanas de edad. Los registros de desempeño se colectaron durante el periodo de prueba de finalización iniciando cuando los cerdos tenían 11 semanas de edad. El consumo de alimento y los pesos de los cerdos se registraron en base al corral. Resultados: En el Experimento Uno, la ganancia diaria promedio (ADG por sus siglas en inglés), consumo de alimento diario promedio, proporción ganancia-alimento, y la mortalidad fueron mejores (P < .05) en los cerdos Vacunados que en los Controles. En el Experimento Dos, hubo interacciones de vacunación por género para la ADG (P < .01) y peso final (P < .05), siendo mayor la ADG en los machos Vacunados que en las hembras Vacunadas (P < .01). En general, se obtuvo una mayor ADG en los cerdos Vacunados ya que pesaron 2.9 kg más (P < .01) que los Controles al momento de la venta. El porcentaje de mortalidad en cerdos Vacunados fue menor (P < .05) que en los Controles (2.8% en el Experimento Uno y 6.2% en el Experimento Dos). Implicaciones: El desempeño de crecimiento superior y la mortalidad más baja después de la vacunación, confirmaron la eficacia de la vacuna comercial PCV2 utilizada en este estudio. | ResuméObjectif: Évaluer l’influence d’un vaccin contre le circovirus porcin de type 2 (PCV2) sur les performances de croissance et le taux de mortalité de porcs en période de finition. Matériels et méthodes: Les traitements étudiés (vaccinés pour PCV2 ou témoins non-vaccinés et cochettes ou castrats) étaient organisés selon un modèle factoriel 2 × 2. Les porcs (PIC L337 × 1050) étaient distribués au hasard à l’intérieur de leur genre aux traitements Vaccinés ou Témoins. Dans l’Expérience 1, les porcs ont été vaccinés à 9 et 11 semaine d’âge, et dans l’Expérience 2, les porcs ont été vaccinés à 5 et 7 semaine d’âge. Les données de performance ont été notées durant la période de finition qui a débuté lorsque les porcs ont atteint l’âge de 11 semaines. Le poids des animaux et la quantité de nourriture consommée ont été enregistrés sur une base d’enclos. Résultats: Dans l’Expérience 1, le gain quotidien moyen (ADG), la consommation journalière moyenne de nourriture, le rapport gain-nourriture, et la mortalité étaient meilleurs (P < .05) chez les Vaccinés comparativement aux Témoins. Dans l’Expérience 2, il y avait une interaction vaccin-genre pour l’ADG (P < .01) et le poids final (P < .05), étant donné que l’ADG était plus élevé chez les castrats Vaccinés que chez les cochettes Vaccinées (P < .01). Globalement, un ADG plus élevé a fait que les Vaccinés pesaient 2.9 kg de plus (P < .01) que les Témoins au moment de la mise en marché. Les taux de mortalité des Vaccinés étaient inférieurs (P < .05) à celui des témoins (2.8 unités de pourcentage lors de l’Expérience 1 et 6.2 unités de pourcentage dans l’Expérience 2). Implications: Les meilleures performances de croissance et la mortalité plus faible après la vaccination confirment l’efficacité du vaccin commercial PCV2 utilisé dans la présente étude. |
Keywords: swine, growth, porcine circovirus type 2, vaccine
Search the AASV web site
for pages with similar keywords.
Received: November 12, 2009
Accepted: April 28, 2010
Porcine circovirus-associated disease (PCVAD) principally affects finishing pigs, but was first described in younger pigs in Canadian herds in 1996.1 It has since been identified in almost every country involved in pig production and has become one of the most economically important diseases affecting pigs worldwide.2 The disease is caused by porcine circovirus type 2 (PCV2), a circular, single-stranded DNA virus.3 Before the introduction of commercial vaccines, PCV2 was difficult to control4 because of the resistance of the virus to inactivation even at low pH, its stability at high temperatures,5 and its resistance to common disinfectants.6,7 In addition, the virus is highly transmissible via direct contact,8 feces, and oro-nasal secretions.9 Recent research has shown that the virus also may be spread through sow’s milk,10 potentially through semen,11 and even through fresh pig tissues such as muscle.12 Once infected, the pig may either appear normal or show various clinical syndromes involving different body systems, hence, the more general term PCVAD is used. The appearance of different clinical syndromes has been attributed to possible immunosuppressive properties of PCV2, which predispose the infected animal to other infections.13 Among the clinical presentations of PCVAD, postweaning multisystemic wasting syndrome (PMWS), characterized by progressive weight loss, respiratory signs, and enlargement of lymph nodes in growing-finishing pigs, is the most common.14 Clinical disease can lead to high death loss and increased cull rates in growing and finishing pigs, resulting in huge losses in income.4 However, PCV2 was originally not thought to greatly affect growth performance in subclinically infected pigs. Nevertheless, losses associated with clinical disease have required the need for effective control measures.
Approaches for PCVAD control have focused on improving production practices and minimizing coinfections, but results may still remain unsatisfactory.4 Initial results in the early stage of experimental PCV2 vaccine development have shown positive results in terms of reducing incidence of PMWS.15 Beginning in 2006, PCV2 vaccines for growing-finishing pigs became commercially available in the United States. Several studies have since been conducted to evaluate the efficacy of these vaccines by using various criteria, including mortality rate, viremia, co-infections, and growth rate.16-20 However, with its positive effect on growth rate, the impact of PCV2 vaccination on feed efficiency and carcass quality has not been investigated. Because of PMWS and possible subclinical effects of PCVAD on pig growth, quantifying the impact of PCV2 vaccination on finishing-pig performance under field conditions is also needed to justify the cost of vaccination. Therefore, this trial was conducted to evaluate the effects of a commercial PCV2 vaccine on growth performance, feed efficiency, carcass characteristics, and mortality in a commercial finishing facility.
Materials and methods
Experimental procedures used in the experiments were approved by the Kansas State University Institutional Animal Care and Use Committee.
Herd
Two experiments were conducted in a commercial swine research finishing facility in southwestern Minnesota that had documented cases of PCVAD. Porcine circovirus type 2-associated disease had been documented by clinical signs of PMWS in finishing groups prior to the initiation of the studies. Also, evidence of histopathologic lesions characterized by lymphocyte depletion and macrophage infiltration of germinal centers were present in necropsied pigs. Immunohistochemical staining had confirmed the presence of PCV2 antigen in these lesions. Also, at the finisher site, occasionally pigs were affected by porcine dermatitis and nephropathy syndrome (PDNS), primarily noted as skin lesions in the perineal area and on the hind limbs. The herd was positive for porcine reproductive and respiratory syndrome (PRRS) and had a historical finishing mortality rate of 6% before the implementation of PCV2 vaccination. However, prior to these experiments, no rise in mortality was observed that was sufficient to meet the case definition for PCVAD set forth by the American Association of Swine Veterinarians.21
Pigs used in each study were weaned over a 3-day period from three sow herds at approximately 21 days of age and comingled in a single nursery room. Two nurseries, each having two rooms, were located on the site. At the end of the nursery phase (Day -7 for Experiment One and Day -6 for Experiment Two), pigs were transported 3 km to the research finishing facility for collection of the production data. Pigs were then housed in one of the four barns at the research finisher site.
Pigs and management
A total of 1291 pigs, initially 24.3 kg, in groups of approximately 27 pigs per pen, were used in Experiment One, and a total of 1253 pigs, initially 5.5 kg, in groups of approximately 28 pigs per pen, were used in Experiment Two, which was conducted after Experiment One ended. All pigs included in the study (PIC 337 × 1050) were evaluated physically before each experiment to ensure that only pigs free of any physical defect were included.
Pigs in both experiments were housed in pens measuring 5.5 × 3.0 m. The barns were managed in an all in-all out system and were double curtain-sided with completely slatted flooring and a deep pit for manure storage. Each pen contained one self-feeder and one cup waterer. In accordance with the production system’s vaccination program, pigs included in the study were all vaccinated against Mycoplasma hyopneumoniae using a 2-dose commercial vaccine (RespiSure; Pfizer Animal Health, New York, New York). All pigs in each of the two experiments were fed similar diets based on corn and soybean meal in a phase-feeding scheme based on body weight and formulated to meet or exceed NRC22 recommendations for swine. Ractopamine HCl (Paylean; Elanco Animal Health, Greenfield, Indiana) was added to the diet for the last 21 days in both experiments. In each of the experiments, both treatment groups were switched to the ractopamine HCl-containing diets at the same time. Each barn at the research site used a robotic feeding system capable of delivering feed and providing data on feed amount delivered on an individual pen basis (FeedPro; Feedlogic Corporation, Willmar, Minnesota). Pigs were weighed every 2 weeks during the course of the experiments. Pens were observed daily to ensure feeders and waterers were working properly and to check the health status of pigs, as determined by physical appearance, pen activity, and absence or presence of abnormal clinical signs. Pigs that were sick or appeared to be lagging behind in growth and were deemed to have very little chance of catching up with their pen mates, which could compromise their welfare, were removed from the study. Weights of pigs removed from the study (died or culled) were recorded at the time of removal. Seven days before the end of the test period, pigs visually identified as the heaviest in the pen (three per pen in Experiment One, two per pen in Experiment Two) were weighed and marketed in accordance with the normal marketing procedures of the farm.
PCV2 vaccine
A commercially available killed bacculovirus-expressed capsid-protein-derived vaccine (Circumvent; Intervet Inc, Millsboro, Delaware) was administered according to label directions (2 mL per dose; IM in the neck muscle). Pigs in Experiment One were vaccinated at 9 and 11 weeks of age; pigs in Experiment Two were vaccinated earlier, at 5 and 7 weeks of age. The timing of vaccination in the first experiment was due to vaccine availability.
Experimental design
Experiments in this study were conducted using a completely randomized design with pen as the experimental unit.
In Experiment One, pigs were housed in single-gender pens at placement in the nursery. Just prior to the first PCV2 vaccination, pigs (650 barrows and 650 gilts) were individually weighed and ear-tagged for identification. Pigs were then ranked within gender on the basis of body weight and randomly allotted within weight-rank pairs to two treatments, Vaccinated and Control (not vaccinated). Thus, before the first vaccination 15 days prior to starting the pigs on test, average weight did not differ between Vaccinated and Control pigs. Nine pigs died before the growth-performance monitoring period in the finisher. Thus, a total of only 1291 pigs were included in the performance data-collection period. Vaccinated and Control pigs were comingled in single-gender pens until transport to the finishing barn. Pens in the finishing barn were randomly assigned to treatments. After arrival at the finishing site (Day -7), pigs were housed in single-gender pens of Vaccinated and Control pigs to allow for collection of feed intake data, with 12 barrow and 12 gilt pens for each Vaccinated and Control treatment. Vaccinated groups were administered two doses of the PCV2 vaccine (2 mL per dose at 9 and 11 weeks of age), 15 days and 1 day, respectively, before data collection started for the 96-day finishing period.
In Experiment Two, gilts and barrows were allocated to separate pens in the nursery. Pens were then ranked within gender on the basis of body weight and randomly allotted within weight-rank pairs to two treatments, Vaccinated and Control, to ensure the same average pig weight for both treatments at the time of the first vaccination. Vaccinated groups were administered two doses of the PCV2 vaccine (2 mL per dose at 5 and 7 weeks of age), 41 and 27 days, respectively, before data collection started for the 105-day finishing period. Control pigs were left unvaccinated. Pigs were vaccinated in the nursery phase and then were moved to the commercial research finishing site, commingled within treatment group and gender, and randomly placed into their respective pens. Finisher pens were randomly assigned to treatment and gender, with 11 barrow and 11 gilt pens for the Control treatment and 12 barrow and 11 gilt pens for the Vaccinated treatment. Vaccinated and Control pigs in each of the experiments were housed in the same barn throughout the study.
At the end of Experiment Two, pigs were individually tattooed according to pen number to allow for carcass data collection at the packing plant and data retrieval by pen. Pigs were transported to JBS Swift and Company (Worthington, Minesota) for processing and carcass data collection. Standard carcass criteria of loin and backfat depth, hot carcass weight, percentage lean, and yield were collected.
Postmortem examination
In Experiment One on Days 14, 25, and 54, and in Experiment Two on Day 21, one Vaccinated and one Control pig were selected for necropsy and further laboratory evaluation of tissues to document PCVAD lesions by histological examination and PCV2 infection using immunohistochemistry. Pigs in poor body condition were selected.
Serology and polymerase chain reaction (PCR)
In Experiment One, blood samples were collected from 10 randomly selected pigs (one pig per pen) from each treatment group (20 pigs total) on Days -9, 14, 42, and 75 to determine serological status relative to PCV2, Mycoplasma hyopneumoniae (M hyo), PRRS virus (PRRSV), and H1N1 and H3N2 swine influenza virus (SIV). These 20 pigs were identified by a second tag, thus ensuring that serial blood samples were collected from the same pigs. Serum samples were submitted to the Iowa State University Veterinary Diagnostic Laboratory and tested for antibodies using enzyme-linked immunosorbent assays (ELISAs) for PCV2, M hyo, and PRRSV, and a hemagglutination inhibition assay for SIV (H1N1 and H3N2). Five of the 10 serum samples for each treatment group were combined to create two pooled samples per treatment for detection of PRRSV and PCV2 nucleic acids using PCR. For ELISA results, sample-to-positive (S:P) ratios of ≥ 0.3, > 0.4, and ≥ 0.4 were considered positive for antibodies against PCV2, M hyo, and PRRSV, respectively. Geometric mean antibody titers > 3.2 (log2 transformed) were considered positive for antibodies against H1N1 and H3N2 SIV.
Serologic and PCR testing were not performed in Experiment Two.
Calculations and statistical analysis
In both experiments, pen weights were obtained every 2 weeks during the data-collection period to determine average daily gain (ADG; calculated by dividing weight gain by the number of pig-days on test). Data from the feed-delivery report generated by the automated feeding system were used to determine average daily feed intake (ADFI; calculated by dividing the total feed consumption per pen by the number of pig-days on test). Gain-to-feed ratio (G:F) was calculated by dividing ADG by ADFI. Pigs that died during the finishing phase were recorded, and mortality rate was calculated as the number of deaths divided by the initial number of pigs placed on test. Overall data for growth performance and carcass data were analyzed as a completely randomized design. Analysis of variance was conducted on all data by using the GLIMMIX procedure of SAS version 9.1 (SAS Institute, Inc, Cary, North Carolina) with the pen as the experimental unit in both experiments. The fixed effects of the statistical model were the effects of PCV2 vaccination (Vaccinated or Control), gender (barrow or gilt), and their interaction. Transformed serological and PCR data were analyzed by repeated measures ANOVA using the MIXED procedure of SAS. Values are presented as least squares means, and all standard errors reported are pooled standard errors of the mean. Alpha level was set at .05 to assess significance among least squares means.
Results
Postmortem examination
Clinical signs and histopathologic lesions consistent with PCVAD were found in necropsied pigs from both experiments. Although there was evidence of bacterial-origin pathology in some pigs evaluated, no clinically significant bacterial pathogens were isolated from any tissues submitted for laboratory evaluation.
Serology
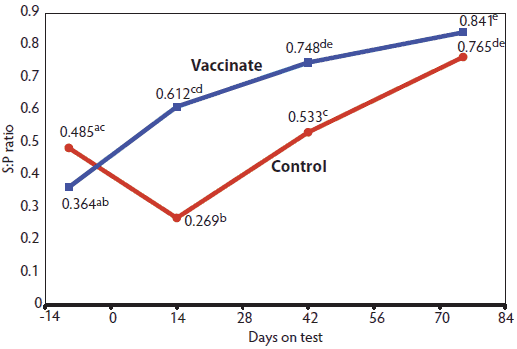
In Experiment One, 9 days before pigs were put on test in the finisher, both treatment groups were seropositive for PCV2, and there was no difference (P > .05) in antibody titers (Figure 1). There was a vaccine-by-day interaction (P < .01) detected for PCV2; antibody titers of the Vaccinated pigs increased as the trial progressed, whereas antibody titers of the Control pigs showed a decrease that reached levels below the 0.3 S:P ratio cut-off on Day 14. Increased titers were observed on Days 42 and 75. On Days 14 and 42, antibody titers against PCV2 were higher (P < .05) in Vaccinated pigs than in Control pigs.
Figure 1: Antibody titers of pigs vaccinated or not vaccinated against porcine circovirus type 2 (PCV2), as determined by enzyme-linked immunosorbent assay (Experiment One). A total of 1291 pigs were randomly assigned to two treatments (Vaccinated and Control) within barrows and gilts. A commercial PCV2 vaccine (Circumvent; Intervet Inc, Millsboro, Delaware; 2 mL per dose) was administered to the Vaccinated pigs at 9 and 11 weeks of age (Day -15 and Day -1) and production data were collected for 96 days beginning Day 0. Serum samples were collected from 10 randomly selected pigs from each treatment (20 pigs total) on days -9, 14, 42, and 75. Samples with sample-to-positive (S:P) ratios ≥ 0.3 were considered serologically positive for PCV2. Each mean is the average S:P ratio from 10 pigs for each day on test. Transformed serological data were analyzed by repeated measures ANOVA using the MIXED procedure of SAS to compare the means within and between treatments. Vaccine-by-day interaction, P < .01. Values with no common superscript differ (P < .05).
 |
Both treatment groups were seropositive for M hyo. At all time points, M hyo antibody titers did not differ between treatment groups (P > .05) and remained constant within treatment group (P > .05). Both treatment groups tested seropositive for PRRS, and there was no difference between treatment groups. Antibodies to H1N1 SIV were not detected (data not shown). Several pigs tested positive for H3N2 at Day -9. However, the average geometric mean of antibody titers for both groups at Day -9 was below the positive cutoff point because of undetectable titers in a few pigs. Geometric mean SIV antibody titers increased between Day -9 and Day 14, then decreased to negative levels at succeeding sampling time points.
PCR
Results of PCR testing showed that all pools were positive for PRRSV at Days -9 and 14 and negative on Days 42 and 75. All pools were positive for PCV2 at all time points, except for one negative pool on Day -9 and one negative pool on Day 75 (Table 1).
Table 1: Porcine circovirus type 2 (PCV2) and porcine reproductive and respiratory syndrome virus (PRRSV) status of vaccinates and nonvaccinated controls as determined by polymerase chain reaction (PCR) (Experiment One)*
* Pigs in the Vaccinated group were vaccinated with a commercial PCV2 vaccine at 9 and 11 weeks of age. Control pigs were not vaccinated. The 96-day production-data collection period began 1 day after the second dose of vaccine (Day 0). Serial serum samples were collected from 10 randomly selected pigs. Five samples were pooled to form two pools of serum for each treatment group, which were tested for PCV2 and PRRSV nucleic acids by PCR. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Mortality
In Experiment One, there was no vaccine-by-gender interaction (P > .05) for mortality rate. However, mortality rate was lower (P < .05) in the Vaccinated group (3.1%) than in the Control group (5.9%; Table 2). In Experiment Two, there also was no vaccine-by-gender interaction (P > .05) detected, but mortality rate in the Vaccinated group (3.0%) was significantly lower (P < .001) than in the Control group (9.2%; Table 3). As shown in Figure 2, cumulative mortality rate among the Controls showed a sudden increase after day 28 and continued increasing until day 84. As in Experiment One, mortality rate was higher (P < .05) in barrows than in gilts (7.7% versus 4.5%).
Table 2: Effects of gender on the efficacy of a porcine circovirus type 2 vaccine (Experiment One)*
* A total of 1291 pigs were randomly assigned to two treatments (Vaccinated and Control) within barrows and gilts. Commercial PCV2 vaccine (Circumvent; Intervet Inc, Millsboro, Delaware; 2 mL per dose) was administered to Vaccinated pigs at 9 and 11 weeks of age; Control pigs were not vaccinated. There were 12 barrow and 12 gilt pens for each Vaccinated and Control treatment. † Data were analyzed as a completely randomized design by ANOVA using the GLIMMIX procedure of SAS (SAS Institute, Inc, Cary, North Carolina), with pen as the experimental unit. The fixed effects of the statistical model were the effects of PCV2 vaccination (vaccinated or not vaccinated), gender (barrow or gilt), and their interaction. There was no significant vaccine-by-gender interaction detected for any measured criteria (P > .05). ‡ Market weight was the average weight of pigs marketed 7 days before the end of the trial (Day 89) and the pigs remaining at the end of the trial (Day 96). On Day 89, pigs visually identified as the heaviest in the pen (three per pen) were weighed and marketed in accordance with normal farm procedures.
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Figure 2: Effect of porcine circovirus type 2 vaccination on cumulative mortality rate in pigs either vaccinated or not vaccinated against porcine circovirus type 2 (PCV2) (Experiment Two). Production data was collected from Day 0 to Day 105. A total of 1253 pigs were randomly assigned to two treatments (Vaccinated and Control) within barrows and gilts. There were 12 barrow and 11 gilt pens for the Vaccinated treatment and 11 barrow and 11 gilt pens for the Control treatment. A commercial PCV2 vaccine (Circumvent; Intervet Inc, Millsboro, Delaware; 2 mL per dose) was administered to the Vaccinated pigs at 5 and 7 weeks of age (Day -41 and Day -27, respectively). Cumulative mortality differed at day 105 (ANOVA; P < .001). 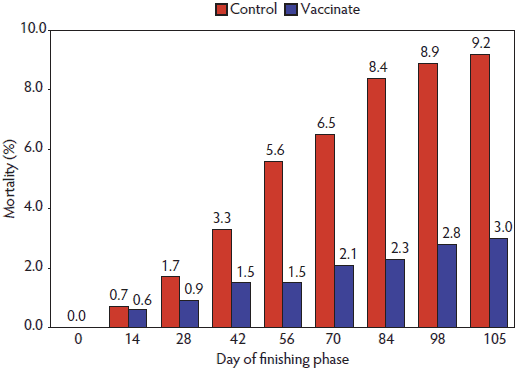 |
Table 3: Effects of gender on the efficacy of a porcine circovirus type 2 vaccine (Experiment Two )*
* Commercial PCV2 vaccine (Circumvent; Intervet Inc, Millsboro, Delaware; 2 mL per dose) administered at 5 and 7 weeks of age to the Vaccinated treatment group (41 and 27 days before being placed on test in the finisher). Controls were not vaccinated. A total of 1253 pigs (initially 5.5 kg) were assigned randomly by nursery pen average weight to two treatments within barrows and gilts before administration of the first vaccine dose. There were 12 barrow and 11 gilt pens for the Vaccinated treatment and 11 barrow and 11 gilt pens for the Control treatment. † Data were analyzed as a completely randomized design by ANOVA using the GLIMMIX procedure of SAS (SAS Institute, Inc, Cary, North Carolina) with pen as the experimental unit. The fixed effects of the statistical model were the effects of PCV2 vaccination (Vaccinated or Control), gender (barrow or gilt), and their interaction. ‡ Seven days before the end of the trial (Day 98), pigs visually identified as the heaviest in the pen (two per pen) were weighed and marketed in accordance with normal farm procedures. Market weight was the average weight of pigs marketed Day 98 and those remaining at the end of the trial (Day 105). § Values were adjusted to a common carcass weight by using carcass weight as a covariate in the model. ab Values within a row with different superscripts differ significantly (P < .05).
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Growth performance
There were no vaccine-by-gender interactions for any growth criteria in Experiment One (Table 2). However, ADG was significantly greater (P < .001) in Vaccinated pigs (952 ± 4.9 g) than in Control pigs (920 ± 4.9 g). This difference was due to the greater ADFI (2.40 ± 0.01 kg versus 2.36 ± 0.01 kg) and better G:F (0.396 ± 0.002 versus 0.390 ± 0.002) in Vaccinated pigs than in Control pigs, respectively (P < .05). As expected, barrows exhibited greater ADG (952 g versus 920 g; P < .001) and ADFI (2.47 kg versus 2.30 kg; P < .001) than gilts. Efficiency of gain, however, was poorer (P < .01) in barrows than in gilts (0.386 versus 0.401).
In contrast to Experiment One, there was a vaccine-by-gender interaction (P < .01) in Experiment Two for ADG, as ADG improved with PCV2 vaccination (P = .01) in barrows but not in gilts (Table 3). Overall, ADG was greater in Vaccinated pigs than Control pigs (920 ± 5.0 g versus 887 ± 5.0 g; P < .001). The difference in ADG between Control and Vaccinated pigs was numerically greatest between Day 15 and Day 42 on test (Figure 3). There were no significant differences in ADFI (P > .05) and G:F (P > .05) between Vaccinated and Control groups. As in Experiment One, feed intake was greater in barrows than gilts (2.35 kg versus 2.19 kg; P < .001), but G:F was poorer in barrows than in gilts (0.39 versus 0.41; P < .05).
Figure 3: Average daily gain (ADG) during the data-collection period for Vaccinated and Control pigs (described in Figure 2) (Experiment Two; Days 0 to 105). 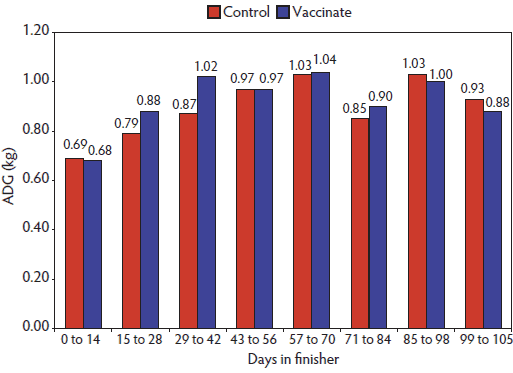 |
Weight and carcass traits
In Experiment One, there were no vaccine-by-gender interactions for average weight, but barrows were 2.5 kg heavier (P < .01) than gilts at the end of the trial. However, Vaccinated pigs tended (P < .10) to be heavier than Control pigs at Day 89 (118.4 kg versus 117.0 kg) and at market (119.2 kg versus 117.9 kg), respectively. This is worth noting because vaccinated pigs were 0.8 kg lighter (P < .05) than Control pigs (35.8 kg versus 35.0 kg) when placed on test in the finisher on Day 0 just after the second dose of vaccine was administered.
There were vaccine-by-gender interactions (P < .05) for weight on Day 98 on test and at market in Experiment Two. Vaccination against PCV2 was associated with heavier weights among Vaccinated barrows at Day 98 on test and at market than in Control barrows, but this was not seen in Vaccinated versus Control gilts. Overall, the greater ADG resulted in Vaccinated pigs being 2.7 kg and 2.9 kg heavier (P < .01) than Control pigs at Day 98 and at market. With the better growth performance among Vaccinated pigs in Experiment One, we decided to collect carcass data in Experiment Two to assess the impact of vaccination on carcass quality. Because of the heavier average body weight, carcass weights were also heavier in Vaccinated pigs than in Control pigs (92.4 kg versus 90.9 kg; P < .05), but percentage yield did not differ (P > .05) between the two groups. Although carcass weights were heavier in Vaccinated pigs, no differences were detected between groups (P > .05) for percentage lean, loin depth, and backfat after adjusting to a common carcass weight.
Discussion
Since the commercial introduction of PCV2 vaccines in 2006, several studies have been published that documented these vaccines’ efficacy in reducing mortality due to PCVAD.16-20,23-26 However, production data measuring growth rate and feed efficiency of PCV2-vaccinated pigs is limited. The killed, bacculovirus-expressed, capsid-protein-derived vaccine used in this study proved effective at minimizing the negative effects of PCVAD, as indicated by the lower mortality and better growth performance and feed efficiency of vaccinated pigs. Vaccination against PCV2 was associated with 47% lower mortality (5.9% versus 3.1%) in Experiment One and 67% lower mortality (9.2% versus 3.0%) in Experiment Two. The lower mortality rate in vaccinated pigs was consistent with that reported in previous studies.16-18 Although interactions between vaccine and gender were not significant for mortality rate, mortality rate was numerically higher in barrows than gilts in both experiments. Previous research reported similar results, wherein gilts showed a relative risk reduction for finishing mortality of 76%, compared with only 46% for barrows, when vaccinated against PCV2.16 This suggests that even though vaccination was able to reduce the negative effects of PCVAD, barrows remained more susceptible to the disease than gilts. However, in contrast to the results of Horlen et al,16 a lower mortality rate among barrows was observed in our study and was consistent across the two experiments.
In the present study, there was a marked increase in PCV2 antibodies from 4 to 8 weeks after vaccination (Days 14 and 42 on test) among vaccinated pigs. Thus, the vaccine was successful at stimulating an immune response and inducing production of antibodies against PCV2. Fachinger et al17 noted an increase in antibody titers in vaccinated pigs 4 and 8 weeks after vaccination that provided protection for at least 15 weeks. They also noted that efficacy of the vaccine was not affected by the relatively high level of maternal antibodies present at the time of vaccination; this is similar to the observations made by Kixmoller et al18 and Opriessnig et al,25 who used bacculovirus-expressed PCV2 open reading frame 2 and PCV1-PCV2 chimeric vaccines, respectively. In the present study, Control pigs had antibodies against PCV2 nine days before the start of the trial. These were possibly maternal antibodies, as evidenced by the declining titer when pigs were sampled at the subsequent time point (23 days later). However, antibody levels in Control pigs increased at the succeeding sampling points, suggesting that pigs were being actively infected. In the case of Vaccinated pigs, no further increase in antibody titers occurred after Day 42. This agrees with a previous PCV2 vaccine study,17 in which an increase in the antibody titer postvaccination resulted in a decrease in serologic response in vaccinated animals after exposure to PCV2. The observed increase in antibody titers in Vaccinated pigs 4 weeks after vaccination (Day 14 on test) suggests that the vaccine used in this study was effective even in the possible presence of maternal antibodies.
Among syndromes associated with PCV2, PMWS is considered the most economically important.2 One objective of the present study, aside from determining the effect of PCV2 on mortality, was to evaluate vaccine efficacy in terms of eliminating or minimizing PMWS and improving growth performance of susceptible pigs. We measured ADG and feed efficiency of pigs as indicators of vaccine efficacy. Vaccinating against PCV2 was associated with greater ADG, and ADG was greater in barrows than in gilts. Thus, aside from being more protective in barrows than gilts on the basis of mortality rate, PCV2 vaccination appears to be more beneficial in barrows than gilts from a growth performance standpoint.
Horlen et al,16 using the same vaccine evaluated in the present study, reported a 9.3% greater ADG among vaccinated pigs that resulted in an 8.8-kg difference between vaccinated and nonvaccinated pigs at market. Another study reported an 18-g per day greater ADG among PCV2-vaccinated pigs than in placebo-treated pigs that reduced days to market by 5.6 days.17 In a third study, use of a single-dose PCV2 vaccine resulted in a 4.70-kg greater weight gain in vaccinated pigs than in placebo-treated pigs.18 In the present study, Vaccinated pigs were 1.3 and 2.9 kg heavier at market than nonvaccinated (Control) pigs in Experiments One and Two, respectively. These results indicate the consistent efficacy of PCV2 vaccination in terms of growth-performance improvement.
Growth-rate differences between Vaccinated and Control pigs peaked between the second and sixth week on test. The lower ADG in Control pigs preceded the observed rise in mortality, and the greatest difference in cumulative mortality between Vaccinated and Control pigs was noted between the sixth and 12th week on test. The period in which increased mortality occurred in this trial was consistent with the study of Horlen et al,16 who observed an increased mortality rate between the sixth and 14th week of the finishing phase. Ractopamine HCl was added to the diets of both treatment groups 3 weeks before market, which explains the observed increase in ADG from Day 84 to 98. Ractopamine HCl is a ß-adrenergic agonist commonly used as a feed additive in pig diets during the last 3 to 4 weeks before market to improve pig growth performance and carcass leanness.27 It should be noted that previous research28,29 indicates that increased pen space per pig is associated with increased growth rate. Although the increased space per pig in the Control pens because of the removal of dead or culled pigs was associated with improved growth rate in the remaining pigs, higher growth rate and survival rate were observed in the Vaccinated pens. Therefore, our estimates of difference in performance between treatment groups will be conservative.
Feed efficiency is another production parameter that could be used to evaluate improvement in overall herd health, because feed intake and growth rate of pigs is negatively affected during disease conditions.30 To our knowledge, this study was the first to use both ADG and G:F in addition to mortality rate to evaluate the efficacy of a commercially available vaccine under field conditions. The significantly better feed efficiency exhibited by Vaccinated pigs in Experiment One was a clear indicator of better health status of the Vaccinated group. Although not statistically significant, the same magnitude of difference in G:F was also observed in Vaccinated pigs compared with Control pigs in Experiment Two. Chronic infection with pathogenic microorganisms causes negative metabolic effects with subsequently reduced feed intake, inefficient utilization of nutrients, and, ultimately, poor growth performance.31,32 It is possible that the protection conferred by vaccination may have led to a more efficient use of energy for growth and lower amounts of energy and nutrients being spent on eliminating infectious agents and repairing tissue damage.
Implications
• The PCV2 vaccine used in this study is effective in reducing mortality rate and improving growth performance of pigs in a PCV2-infected herd as indicated by heavier weights and better feed efficiency in the vaccinated group.
• The positive effects of PCV2 vaccination on growth performance observed in this study further validate the role of PCV2 as the main pathogenic organism in PCVAD.
Acknowledgements
Appreciation is expressed to New Horizon Farms for use of pigs and facilities and Richard Brobjorg, Cal Hulstein, and Marty Heintz for technical assistance.
References
1. Harding JCS, Clark EG. Recognizing and diagnosing postweaning multisystemic wasting syndrome (PMWS). J Swine Health Prod. 1997;5:201–203.
2. Segalés J, Allan GM, Domingo M. Porcine circovirus diseases. In: Straw BE, Zimmerman JJ, D’Allaire S, Taylor DJ, eds. Diseases of Swine. 9th ed. Oxford, England: Blackwell Publishing Ltd; 2006:299–307.
3. Allan GM, Ellis JA. Porcine circoviruses: a review. J Vet Diagn Invest. 2000;12:3–14.
*4. Henry SC, Tokach LM. Porcine circovirus diseases. Proc Allen D. Leman Swine Conference. St Paul, Minnesota. 2006;110–111.
5. Allan GM, Phenix KV, Todd D, McNulty MS. Some biological and physico-chemical properties of porcine circovirus. J Vet Med B. 1994;41:17–26.
6. Martin H, Le Potier MF, Maris P. Virucidal efficacy of nine commercial disinfectants against porcine circovirus type 2. Vet J. 2008;177:388–393.
7. Royer RL, Nawagitgul P, Halbur PG, Paul PS. Susceptibility of porcine circovirus type 2 to commercial and laboratory disinfectants. J Swine Health Prod. 2001;9:281–284.
8. Kristensen CS, Baekbo P, Bille-Hansen V, Bøtner A, kan Vigre H, Enøe C, Larsen LE. Induction of porcine post-weaning multisystemic wasting syndrome (PMWS) in pigs from PMWS unaffected herds following mingling with pigs from PMWS-affected herds. Vet Microbiol. 2009;138:244–250.
9. Segales J, Calsamiglia M, Olvera A, Sibila M, Badiella L, Domingo M. Quantification of porcine circovirus type 2 (PCV2) DNA in serum and tonsillar, nasal, tracheo-bronchial, urinary and faecal swabs of pigs with and without postweaning multisystemic wasting syndrome (PMWS). Vet Microbiol. 2005;111:223–229.
10. Ha Y, Ahn KK, Kim B, Cho KD, Lee BH, Oh YS, Kim SH, Chae C. Evidence of shedding of porcine circovirus type 2 in milk from experimentally infected sows. Res Vet Sci. 2009;86:108–110.
11. Madson DM, Ramamoorthy S, Kuster C, Pal N, Meng XJ, Halbur PG, Opriessnig T. Infectivity of porcine circovirus type 2 DNA in semen from experimentally-infected boars. Vet Res. 2009;40:10. doi:10.1051/vetres:2008048.
12. Opriessnig T, Patterson AR, Meng X, Halbur PG. Porcine circovirus type 2 in muscle and bone marrow is infectious and transmissible to naive pigs by oral consumption. Vet Microbiol. 2009;133:54–64.
13. Segales J, Mateu E. Immunosuppression as a feature of postweaning multisystemic wasting syndrome. Vet J. 2006;171:396–397.
14. Segales J, Rosell C, Domingo M. Pathological findings associated with naturally acquired porcine circovirus type 2 associated disease. Vet Microbiol. 2004;98:137–149.
15. Fenaux M, Opriessnig T, Halbur PG, Elvinger F, Meng XJ. A chimeric porcine circovirus (PCV) with the immunogenic capsid gene of the pathogenic PCV type 2 (PCV2) cloned into the genomic backbone of the nonpathogenic PCV1 induces protective immunity against PCV2 infection in pigs. J Virol. 2004;78:6297–6303.
16. Horlen KP, Dritz SS, Nietfeld JC, Henry SC, Hesse RA, Oberst R, Hays M, Anderson J,
Rowland RRR. A field evaluation of mortality rate and growth performance in pigs vaccinated against porcine circovirus type 2. JAVMA. 2008;232:906–912.
17. Fachinger V, Bischoff R, Jedidia SB, Saalmuller A, Elbers K. The effect of vaccination against porcine circovirus type 2 in pigs suffering from porcine respiratory disease complex. Vaccine. 2008;26:1488–1499.
18. Kixmoller M, Ritzmann M, Eddicks M, Saalmuller A, Elbers K, Fachinger V. Reduction of PMWS-associated clinical signs and co-infections by vaccination against PCV2. Vaccine. 2008;26:3443–3451.
19. Opriessnig T, Patterson AR, Madson DM, Pal N, Halbur PG. Comparison of efficacy of commercial one dose and two dose PCV2 vaccines using a mixed PRRSV-PCV2-SIV clinical infection model 2–3-months post vaccination. Vaccine. 2009;27:1002–1007.
20. Neumann E, Simpson S, Wagner J, Karaconji B. Longitudinal field study of the effect of a commercial porcine circovirus type 2 vaccine on postweaning mortality in New Zealand farms. J Swine Health Prod. 2009;17:204–209.
21. American Association of Swine Veterinarians. Porcine Circovirus Associated Disease (PCVAD) Case Definition. 2006. Available at http://aasp.org/aasv/position-PCVAD.htm. Accessed 4 October 2010.
22. National Research Council. Nutrient Requirements of Swine. 10th ed. Washington, DC: National Academic Press; 1998.
23. Fort M, Sibila M, Allepuz A, Mateu E, Roerink F, Segales J. Porcine circovirus type 2 (PCV2) vaccination of conventional pigs prevents viremia against PCV2 isolates of different genotypes and geographic origins. Vaccine. 2008;26:1063–1071.
24. Fort M, Sibila M, Perez-Martin E, Nofrarias M, Mateu E, Segales J. One dose of a porcine circovirus 2 (PCV2) sub-unit vaccine administered to 3-week-old conventional piglets elicits cell-mediated immunity and significantly reduces PCV2 viremia in an experimental model. Vaccine. 2009;27:4031–4037.
25. Opriessnig T, Madson DM, Prickett JR, Kuhar D, Lunney JK, Elsener J, Halbur PG. Effect of porcine circovirus type 2 (PCV2) vaccination on porcine reproductive and respiratory syndrome virus (PRRSV) and PCV2 coinfection. Vet Microbiol. 2008;131:103–114.
26. Opriessnig T, Meng XJ, Halbur PG. Porcine circovirus type 2 associated disease: update on current terminology, clinical manifestations, pathogenesis, diagnosis, and intervention strategies. J Vet Diagn Invest. 2007;19:591–615.
27. Apple JK, Rincker PJ, McKeith FK, Carr SN, Armstrong TA, Matzat PD. Review: Meta-analysis of the ractopamine response in finishing swine. Prof Anim Sci. 2007;23:179–196.
28. DeDecker JM, Ellis M, Wolter BF, Corrigan BP, Curtis SE, Parr EN, Webel DM. Effects of proportion of pigs removed from a group and subsequent floor space on growth performance of finishing pigs. J Anim Sci. 2005;83:449–454.
29. Black JL, Bray HJ, Giles LR. The thermal and infectious environment. In: Kyriazakis I, ed. A Quantitative Biology of the Pig. Wallingford, UK: CAB International; 1999:71–97.
30. Straw BE, Shin SJ, Yeager AE. Effect of pneumonia on growth-rate and feed-efficiency of minimal disease pigs exposed to Actinobacillus pleuropneumoniae and Mycoplasma hyopneumoniae. Prev Vet Med. 1990;9:287–294.
31. Escobar J, Van Alstine WG, Baker DH, Johnson RW. Decreased protein accretion in pigs with viral and bacterial pneumonia is associated with increased myostatin expression in muscle. J Nutr. 2004;134:3047–3053.
32. Williams NH, Stahly TS, Zimmerman DR. Effect of level of chronic immune system activation on the growth and dietary lysine needs of pigs fed from 6 to 112 kg. J Anim Sci. 1997;75:2481–2496.
* Non-referred reference.