Prickett JR, Cutler S, Kinyon JM, et al. Stability of porcine reproductive and respiratory syndrome virus and antibody in swine oral fluid. J Swine Health Prod. 2010;18(4):187–195
| Original research | Peer reviewed |
Cite as: Prickett JR, Cutler S, Kinyon JM, et al. Stability of porcine reproductive and respiratory syndrome virus and antibody in swine oral fluid. J Swine Health Prod. 2010;18(4):187–195.
Also available as a PDF.
SummaryObjective: To evaluate the stability of porcine reproductive and respiratory syndrome virus (PRRSV) and anti-PRRSV antibodies in oral fluid as a function of time and temperature. Materials and methods: A 4-L pool of swine oral fluid was collected from 16-week-old finisher pigs. To ensure uniform, quantifiable levels of virus and antibody over time, 4 mL of PRRSV isolate ISU-P containing 1 × 1012 RNA copies per mL and 10 mL of concentrated hyperimmune anti-PRRSV antibodies were added to the pool. The pool was then divided into three equal portions: no treatment, chlorhexidine digluconate at 0.01% by volume, and isothiazolinone at 3 ppm. Each treatment was tested in triplicate at each of five temperatures (-20°C, 4°C, 10°C, 20°C, and 30°C). Samples were removed at specific intervals (0, 12, 24, 48, 72, 144, 216, and 288 hours), stored at -80°C, and then assayed for PRRSV RNA; IgM, IgA, and IgG PRRSV-specific antibody; and culturable bacteria per mL. Results: The stabilities of anti-PRRSV antibody and detectable PRRSV were temperature-dependent, with antimicrobial treatment providing no improvements in stability at lower temperatures. Both virus and antibody were stable at ≤ 10°C over 12 days of storage. Implication: Conventional serum storage protocols (freezing or refrigeration at 4°C) preserves PRRSV and anti-PRRSV antibody in oral-fluid diagnostic samples without the use of preservatives. | ResumenObjetivo: Evaluar la estabilidad del virus del síndrome reproductivo y respiratorio porcino (PRRSV por sus siglas en inglés) y los anticuerpos anti-PRRSV en el fluido oral en función de tiempo y temperatura. Materiales y métodos: Se colectaron 4 L de fluido oral porcino de cerdos de finalización de 16 semanas de edad. Para asegurar niveles uniformes, cuantificables de virus y de anticuerpos al paso del tiempo, se agregaron al fluido 4 mL del aislado ISU-P de PRRSV con un contenido de 1 × 1012 copias RNA por mL y 10 mL de concentrado hiperinmune de anticuerpos anti-PRRSV. Se dividió la mezcla en tres porciones iguales: sin tratamiento, digluconato de clorhexidina al 0.01% por volumen, e isotiazolinona a 3 ppm. Cada tratamiento fue probado por triplicado a cada una de cinco temperaturas (-20°C, 4°C, 10°C, 20°C, y 30°C). Se retiraron muestras a intervalos específicos (0, 12, 24, 48, 72, 144, 216, y 288 horas), se almacenaron a -80°C, y luego se probaron en busca de RNA de PRRSV; anticuerpos IgM, IgA, e IgG PRRSV-específicos; y bacterias cultivables por mL. Resultados: La estabilidad del anticuerpo anti-PRRSV y PRRSV detectable fueron temperaturas dependientes, sin que el tratamiento antimicrobiano aportara mejoras en la estabilidad a temperaturas más bajas. Tanto el virus como el anticuerpo fueron estables a ≤ 10°C durante el periodo de almacenamiento de 12 días. Implicación: Los protocolos convencionales de almacenaje de suero (congelación ó refrigeración a 4°C) conservan el PRRSV y el anticuerpo anti-PRRSV en muestras diagnosticas de fluido oral sin la utilización de conservadores.. | ResuméObjectif: Évaluer la stabilité du virus du syndrome reproducteur et respiratoire porcin (PRRSV) et des anticorps anti-PRRSV dans le fluide oral en fonction du temps et de la température. Matériels et méthodes: Un total de 4 L de fluide oral a été récolté de porcs en période de finition âgés de 16 semaines. Afin d’assurer dans le temps des quantités uniformes et quantifiables de virus et d’anticorps, 4 mL de l’isolat ISU-P de PRRSV contenant 1 × 1012 copies d’ARN par mL et 10 mL d’anticorps hyperimmuns concentrés anti-PRRSV ont été ajoutés au pool de liquide. Ce pool a par la suite été divisé en trois parties égales: aucun traitement, 0.01% par volume de digluconate de chlorhexidine, et 3 ppm d’isothiazolinone. Chaque traitement a été testé en triplicata à chacune de 5 températures (-20°C, 4°C, 10°C, 20°C, et 30°C). Des échantillons ont été retirés à des intervalles spécifiques (0, 12, 24, 48, 72, 144, 216, et 288 heures), entreposés à -80°C, et testés pour déterminer, par mL, les quantités d’ARN du PRRSV; d’anticorps IgM, IgA, et IgG spécifiques à PRRSV; et de bactéries cultivables. Résultats: La stabilité des anticorps anti-PRRSV et de PRRSV détectables était dépendante de la température, un traitement antibactérien n’apportant aucune amélioration à la stabilité aux températures plus basses. Les virus et les anticorps étaient stables à des températures ≤ 10°C durant 12 jours d’entreposage. Implication: Les protocoles conventionnels d’entreposage (congélation ou réfrigération à 4°C) permettent de conserver, sans l’utilisation d’agent de préservation, le PRRSV de même que les anticorps anti-PRRSV contenus dans des échantillons diagnostiques de fluide oral. |
Keywords: swine, antibody, oral fluid, nucleic acid,
porcine reproductive and respiratory syndrome virus, PRRS, PRRSV
Search the AASV web site
for pages with similar keywords.
Received: September 23, 2009
Accepted: January 6, 2010
Oral-fluid specimens have been widely used in human medicine and forensics for diagnosis or detection of a variety infectious agents, hormones, toxins, and drugs.1-3 When used in the surveillance of infectious disease, oral-fluid testing facilitates the efficient collection of large numbers of diagnostic samples at low cost. For example, the New York City Department of Health and Mental Hygiene performed 166,058 oral-fluid rapid HIV antibody tests between March 2005 and May 2008 in 10 walk-in clinics.4 In the United Kingdom, oral-fluid samples were collected from 11,698 children at home and mailed to the laboratory for antibody testing.5
Oral fluids have not been widely exploited in veterinary diagnostic medicine, but detection of pathogens and antibodies in swine oral fluids, eg, porcine circovirus type 2 (PCV2),6,7 porcine reproductive and respiratory syndrome virus (PRRSV),7-9 transmissible gastroenteritis virus,10 and Actinobacillus pleuropneumoniae,11,12 suggests this approach could be used in the surveillance of these pathogens in commercial swine herds.
Oral fluids inherently contain endogenous antiviral and antibacterial factors that could theoretically affect the stability of polymerase chain reaction (PCR) targets.13-17 Furthermore, normal oral bacterial flora and environmental bacterial contaminants present in livestock facilities could produce bacterial proteases capable of enzymatic cleavage of immunoglobulins.18 Therefore, the objective of this research was to evaluate the stability of PCR-detectable PRRSV and anti-PRRSV antibody in oral fluids and develop handling and storage guidelines for porcine oral-fluid specimens.
Materials and methods
The commercial finisher pigs used in this study were managed under Pork Quality Assurance Plus (PQA Plus™) guidelines.19
Experimental design
The effects of antimicrobial treatment, storage temperature, and storage time were evaluated in respect to total detectable PRRSV RNA; total porcine IgM, IgA, and IgG; enzyme-linked immunosorbent assay- (ELISA-) detectable PRRSV-specific antibody; total bacteria; and pH. To conduct the experiment, 4 L of swine oral fluid was collected from 16-week-old commercial finisher pigs. After collection, a type 2 PRRSV isolate and anti-PRRSV antibodies were added to the pool. To test the effect of antimicrobials on diagnostic target stability, the pool was divided into three treatments: untreated control, chlorhexidine digluconate (0.01% by volume), and isothiazolinone (3 ppm). To ensure the uniform distribution of virus, antibody, and antimicrobials, the solution was stirred constantly using a magnetic mixer throughout the process of preparation and transfer. To test the effect of treatment by storage temperature and time, 2.5-mL volumes of each antimicrobial treatment were aliquoted into 5-mL tubes and held at each of five temperatures (-20°C, 4°C, 10°C, 20°C, and 30°C). Three tubes (replicates) from each antimicrobial treatment and temperature combination were removed at specific times (0, 12, 24, 48, 72, 144, 216, and 288 hours) and held at -80°C until tested. Thereafter, samples were randomized using a random-sequence generator (random.org) and tested for bacteria, PRRSV antibody, and PCR-detectable PRRSV. The results were evaluated for the effects of treatment, time, and temperature.
Porcine oral fluid
A pool of swine oral fluid (4 L) was obtained from a population of 16-week-old commercial finisher pigs housed in a 2400-head facility located in Iowa. Pigs were allowed to chew on cotton ropes, after which oral fluids were mechanically extracted, as previously described.8 The herd had been vaccinated for PCV2 and Mycoplasma hyopneumoniae at approximately 6 weeks of age and had seroconverted to PRRSV between 10 and 14 weeks of age (HerdChek 2XR PRRS ELISA, Idexx Laboratories, Inc, Westbrook, Maine).
PRRS virus
Four mL of PRRSV isolate ISU-P (1 × 1012 RNA copies per mL) were added to the pool of oral fluid. The isolate was initially recovered from lung tissues collected from young pigs in a herd experiencing an acute outbreak of porcine reproductive and respiratory syndrome (PRRS) in Illinois. As described elsewhere,20 the virus was initially isolated on porcine alveolar macrophage (PAM) cultures and then cloned by three rounds of limiting dilutions on PAMs, and two rounds of plaquing on MA-104 cells. The working stock of virus used in the present study represented the fourth passage in MA-104 cells.
Anti-PRRSV antibodies
Anti-PRRSV antibody (kindly provided by Dr Fernando Osorio) had been produced by hyperimmunizing 13 pigs against several PRRSV isolates as previously described.21 In brief, hyperimmunization produced neutralizing antibody titers of 1:32 to 1:128 in all 13 pigs. Thereafter, animals were euthanized and exsanguinated. Serum antibodies from each animal were precipitated and concentrated by ammonium sulfate ([NH4]2SO4) treatment. The concentrated antibodies used in this study represented a pool from all 13 animals. Prior to adding to the pool of oral fluids, the concentrated antibodies were reconstituted in 10 mL of phosphate-buffered saline (PBS).
Antimicrobial agents
The effect of antimicrobial preservatives on the stability of PRRSV and anti-PRRSV antibody was tested by adding chlorhexidine digluconate or isothiazolinone to oral fluids at concentrations of 0.01% and 0.003% (3 ppm), respectively. Biguanides (eg, chlorhexidine digluconate) act against non-sporulating bacteria and yeasts by disrupting cell membranes.22 Isothiazolinones inhibit metabolic pathways by reacting with thiol-containing enzymes, thereby leading to cell death.23 Pilot standard plate count (SPC) assays using a “checkerboard” of antimicrobial concentrations were conducted to select bacteriostatic, not virucidal, concentrations (data not shown).
Standard plate count
The total number of bacteria per mL in each sample was quantified using an SPC method.24 In brief, samples were thawed at 23°C for 20 minutes and then serially diluted in PBS from 1 × 10-1 through 1 × 10-7. To perform the assay, all dilutions, including the undiluted sample, were plated in 10-μL volumes on culture plates containing trypticase soy agar with 5% sheep blood. Plates were incubated in 5% CO2 at 35°C for 72 hours. Colonies were counted manually and the total bacteria per mL were estimated and reported in log10 values, with ≥ 1 × 108 considered the upper limit of detection.
PRRSV real-time RT-PCR
PRRS virus RNA for quantitative reverse transcriptase-PCR (qRT-PCR) amplification was extracted from oral-fluid samples using a commercially available viral RNA isolation kit (Applied Biosystems, Foster City, California). The manufacturer’s protocol was modified by increasing the initial volume of the sample from 50 μL to 175 μL. This volume of sample was added to 235 μL of lysis-binding solution (provided in the kit) to which carrier RNA had been added (provided in the kit) in a 96-well plate. The plate was sealed and placed onto an orbital plate shaker for 3 minutes (1000 rpm), after which cell debris was removed by centrifugation of the plate at 1500g for 6 minutes. Thereafter, 115 μL of the supernatant was transferred to each well of a 96-well plate containing 65 μL of 100% isopropanol in each well. The remainder of the RNA extraction protocol was performed according to the manufacturer’s instruction.
Real-time RT-PCR reactions were performed using a commercially available PRRSV PCR kit (Applied Biosystems) and analyzed according to the manufacturer’s recommendations. Positive and negative plate-control samples were tested concurrently with the oral-fluid samples. A standard curve was generated using the genomic copy standards provided in the kit (1 × 105 to 1 × 1010 genomic copies per mL) and the results were expressed as PRRSV genomic equivalents per mL (log10).
PRRS ELISA
All oral-fluid samples were tested for antibodies against PRRSV using a commercially available PRRSV antibody ELISA (HerdChek PRRS 2XR ELISA; Idexx Laboratories) and a modified protocol. Oral-fluid samples were diluted 1:3 using the kit diluent, the diluted oral-fluid samples were incubated on the ELISA plates overnight at 4°C, plates were brought to room temperature in the morning (30 minutes), and the remainder of the assay was completed according to the manufacturer’s protocol. The results were reported as sample-to-positive (S:P) ratios and the responses to temperature, time, and treatment were analyzed as continuous data, ie, no “cut-off” value was established or used in this study.
Immunoglobulin isotype quantification
All samples were assayed for IgA, IgG, and IgM antibodies using isotype-specific total swine antibody quantification assays following the procedures provided by the manufacturer (Bethyl Laboratories, Inc, Montgomery, Texas). Prior to testing, the samples were heat-inactivated at 56°C for 10 minutes. Preliminary assays were performed to determine dilution range required to quantify each immunoglobulin class. Each sample was serially diluted (1:20 to 1:160 for IgM and 1:50 to 1:400 for IgG and IgA) in diluent provided by the manufacturer in 96-well round-bottom plates. Samples were then transferred to the kit ELISA plates and the assays were completed. The optical density (OD) response was used to estimate the concentration of immunoglobulin in each sample using the linear portion of the standard curve, with appropriate adjustment for sample dilution. Samples that were nonresponsive at the initial dilutions were re-assayed at lower dilutions (1:2 to 1:20 for IgM and 1:2 to 1:50 for IgG and IgA). By definition, samples that were nonresponsive at the lowest dilution, ie, 1:2, contained ≤ 5 ng per mL of the respective antibody isotype.
Measurement of pH
The pH of one of the three replicates collected at each sampling point was measured using a pH meter calibrated using commercially available standards of pH 4, 7, and 10 (Sigma-Aldrich, St Louis, Missouri). The pH probe was washed in deionized water and blotted dry between measurements. Calibration of the pH meter was verified before and after testing samples.
Statistical methods
All analyses were performed using commercial statistical software (JMP 7.0.2; SAS Institute Inc, Cary, New Jersey). Initially, the results of each assay were examined for the effects of temperature and treatment using analysis of variance (ANOVA). Temperature, antimicrobial treatment, and temperature × antimicrobial treatment were denoted as fixed effects in the model. Thereafter, planned orthogonal comparisons by Student t test were used to identify significant differences among treatments and temperatures using the least squares means of the assay results. For clarity in graphical presentation, data were combined if a statistically significant difference (P < .05) was not detected between storage temperatures.
Results
Standard plate count
Statistical analysis of main effects (marginal means) showed that both temperature and antimicrobial treatment had significant effects on the level of bacteria detected by the SPC (Table 1). Two temperature groups were identified on the basis of SPC results. In order of increasing mean SPC (Figure 1), the two groups were (-20°C, 4°C, and 10°C) < (20°C and 30°C). Significant differences were detected among treatments, but chlorhexidine treatment produced the lowest SPC results at all temperatures.
Table 1: Bacteria in oral fluid samples tested by standard plate count (log10 cells/mL) showing main effects of temperature and antimicrobial treatment*
* To a pool of swine oral fluid (4 L) obtained from 16-week-old commercial finisher pigs were added a type 2 porcine reproductive and respiratory syndrome virus (PRRSV) isolate and concentrated anti-PRRSV antibody produced by hyperimmunizing 13 pigs.21 Antimicrobial agents (chlorhexidine digluconate and isothiazolinone) were added to samples of oral fluids at concentrations of 0.01% and 0.003% (3 ppm), respectively. For testing, 2.5-mL volumes of each antimicrobial treatment were held at each of five temperatures (-20°C, 4°C, 10°C, 20°C, and 30°C). Three replicates from each antimicrobial treatment and temperature combination were removed at 0, 12, 24, 48, 72, 144, 216, and 288 hours and held at -80°C until tested. Each value represents treatment-group mean. abcdef Values in cells with no common superscript are significantly different (P < .05; pairwise comparisons of least squares means using Student t tests) NA = not applicable |
Figure 1: To test the effect of treatment by storage temperature and time, 2.5-mL volumes of oral fluid from each antimicrobial treatment (described in Table 1) were aliquoted into 5-mL tubes and held at each of five temperatures (-20°C, 4°C, 10°C, 20°C, and 30°C). Three tubes from each antimicrobial treatment and temperature combination were removed at specific times (0, 12, 24, 48, 72, 144, 216, and 288 hours) and tested. The figure shows total number of bacteria (log10 cells per mL) in oral fluid by temperature and storage time quantified using a standard plate count (SPC). Statistical analysis (ANOVA) identified two temperature groups in terms of SPC: (-20°C, 4°C, 10°C) < (20°C and 30°C). Panels display the means of samples stored at (A), 30°C; (B), 20°C; and (C), -20°C; 4°C, and 10°C. 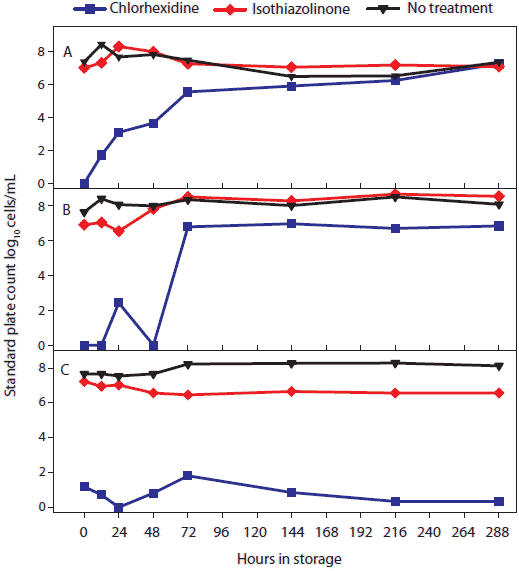 |
PRRS virus real-time RT-PCR
Statistical analysis of main effects (marginal means) showed that temperature, but not antimicrobial treatment, had a significant effect on the stability of PCR-detectable PRRSV (Table 2). Three temperature groups were identified on the basis of PRRSV qRT-PCR results (Figure 2). In order of declining concentrations of virus, the three groups were (-20°C, 4°C, and 10°C) > (20°C) > (30°C). Concentration of PRRSV in chlorhexidine-treated samples was significantly lower than in other treatments at -20°C, 4°C, and 10°C and significantly higher than in other treatments at 30°C.
Table 2: Quantitative reverse transcriptase-polymerase
chain reaction (log10 genomic copies per mL) on oral fluid
* Oral-fluid samples described in Table 1. Each value represents treatment group mean. abcde Values in cells with no common superscript are significantly different (P < .05; pairwise comparisons of least squares means using Student t tests) NA = not applicable |
Figure 2: For the oral-fluid samples described in Table 1, results of porcine reproductive and respiratory syndrome virus (PRRSV) quantitative reverse transcriptase-polymerase chain reaction (qRT-PCR; log10 genomic copies/mL) over time by temperature and storage time. Statistical analysis (ANOVA) identified three temperature groups in order of virus stability: (-20°C, 4°C, and 10°C) > (20°C) > (30°C). Panels display the means of samples stored at (A), 30°C; (B), 20°C; and (C), -20°C, 4°C, and 10°C. 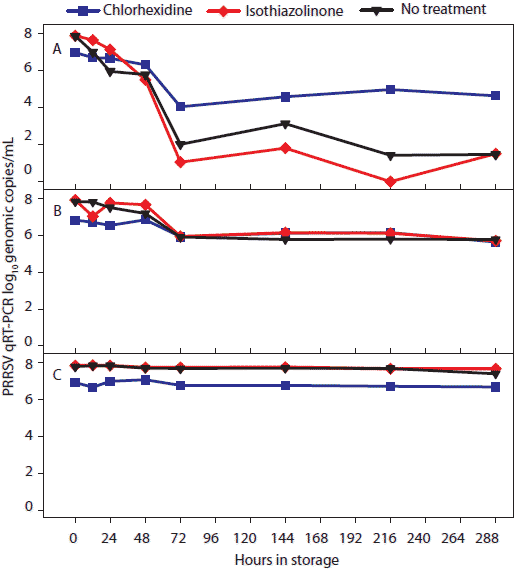 |
PRRS ELISA
Statistical analysis of main effects (marginal means) showed that both temperature and antimicrobial treatment had significant effects on the stability of ELISA-detectable anti-PRRSV antibody (Table 3). Three temperature groups were identified on the basis of PRRS ELISA results (Figure 3). In order of declining mean ELISA S:P ratios, the three groups were (-20°C, 4°C, and 10°C) > (20°C) > (30°C). Mean ELISA S:P ratios in chlorhexidine-treated samples were significantly higher than in other treatments at 30°C.
Table 3: ELISA-detectable PRRSV-specific antibody* in oral-fluid samples† showing main effects of temperature and antimicrobial treatment
* Sample-to-positive (S:P) values. † Oral-fluid samples described in Table 1. Each value represents treatment-group mean. abcd Values in cells with no common superscript are significantly different (P < .05; pairwise comparisons of least squares means using Student’s t tests) ELISA = enzyme-linked immunosorbent assay; PRRSV = porcine reproductive and respiratory syndrome virus; NA = not applicable |
Figure 3: For the oral-fluid samples described in Table1, PRRSV ELISA (sample-to-positive [S:P] ratios) over time by temperature and storage time. Statistical analysis (ANOVA) identified three temperature groups in order of declining ELISA-detectable antibody stability: (-20°C, 4°C, and 10°C) > (20°C) > (30°C). Panels display the ELISA S:P means of samples stored at (A), 30°C; (B), 20°C; and (C), -20°C, 4°C, and 10°C. 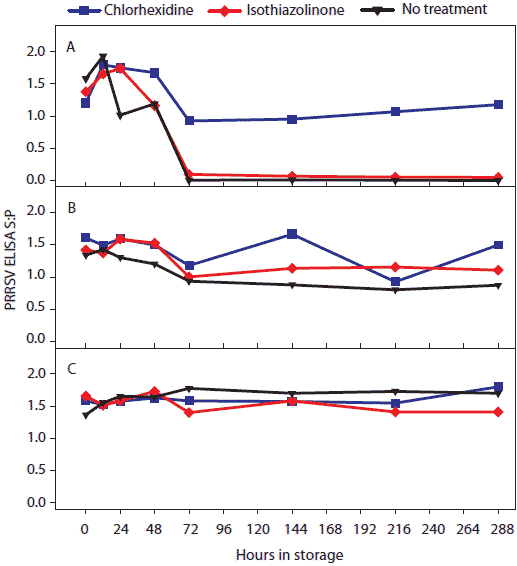 |
Total swine immunoglobulin isotype quantification ELISAs
Concentration (log10 ng per mL) of total swine IgG, IgA, and IgM antibody in oral-fluid samples by time, temperature, and treatment are shown in Figures 4, 5, and 6, respectively. For IgG and IgA, two temperature groups were identified on the basis of statistically significant differences: (-20°C, 4°C, 10°C, and 20°C) > (30°C). At 30°C, higher levels of IgG and IgA were measured in chlorhexidine- and isothiazolinone-treated samples over time relative to no treatment. For IgM, no significant difference was found among results by temperature or treatment.
Figure 4: For the oral-fluid samples described in Table 1, total swine IgG (log10 ng/mL) over time and storage temperature. Statistical analysis (ANOVA) identified two temperature groups in order of declining IgG antibody stability: (-20°C, 4°C, 10°C, and 20°C) > (30°C). Panels display the means of samples stored at (A), 30°C and (B), -20°C, 4°C, 10°C, and 20°C. 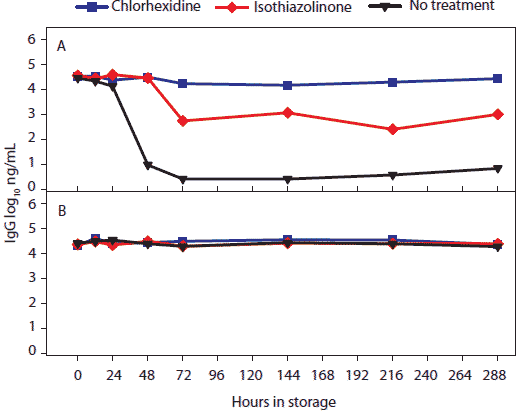 |
Figure 5: For the oral-fluid samples described in Table 1, total swine IgA (log10 ng/mL) over time and storage temperature. Statistical analysis (ANOVA) identified two temperature groups in order of declining IgA antibody stability: (-20°C, 4°C, 10°C, and 20°C) > (30°C). Panels display the means of samples stored at (A), 30°C and (B), -20°C, 4°C, 10°C, and 20°C. 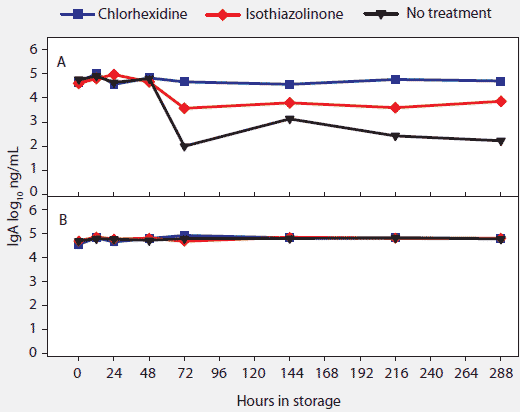 |
Figure 6: For the oral-fluid samples described in Table 1, total swine IgM (log10 ng/mL) over time and storage temperature. There were no statistical differences among storage temperatures. 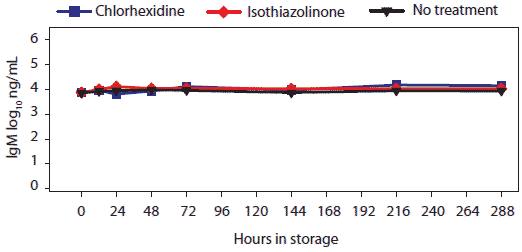 |
Measurement of pH
Statistical analysis of main effects (marginal means) showed that both temperature and antimicrobial treatment had significant effects on pH levels (Table 4). Three temperature groups were identified on the basis of pH levels: (-20°C, 4°C, and 10°C) > (20°C) > (30°C).
Table 4: pH of oral-fluid samples showing main effects of temperature and antimicrobial treatment*
* Oral-fluid samples described in Table 1. Each value represents treatment-group mean. abcdefg Values in cells with no common superscript are significantly different (P < .05; pairwise comparisons of least squares means using Student t tests) NA = not applicable
|
Discussion
Oral fluid has been shown to be a convenient diagnostic specimen for the detection of infectious agents and antibodies.6-8,10,11 The present study addressed the question of PRRSV and anti-PRRSV antibody stability in oral fluid as a function of time and temperature, both with and without antimicrobial preservatives. Stability was evaluated over a 12-day observation period to test extreme sample handling and storage on diagnostic results. The samples (pool) used in this study were collected from commercial finishing pigs in a manner consistent with previously reported methods of sample collection,7 thus representing a level of environmental contamination typical of field samples.
Standard plate-count data showed that bacterial proliferation was reduced by chlorhexidine at all temperatures tested. The concentration of isothiazolinone tested in this study did not produce a measurable effect on bacterial proliferation. Importantly, untreated samples contained concentrations of PCR-detectable PRRSV RNA and ELISA-detectable anti-PRRSV antibody at levels equal to or greater than those in preserved samples, when held at temperatures ≤ 10°C. Although this research did not investigate the effects of various levels of bacterial contamination in oral-fluid samples, preservatives were not required to protect the stability of diagnostic targets if the cold-chain was maintained.
There are few publications with which to compare these data. A search of the literature yielded no prior reports on the stability of PCR-detectable viral or bacterial agents by time and temperature in oral fluids. However, our data are in agreement with published data reporting that the concentration of antibodies in human oral fluids declined as a function of increasing time and higher temperature.23,25,26
Implications
- PRRS virus RNA and anti-PRRSV antibodies are relatively resistant to degradation in oral fluids.
- Use of antimicrobials is not necessary for preservation of diagnostic targets if appropriate handling, storage, and transport protocols are implemented.
- Appropriate specimen-handling protocols, ie, prompt freezing or refrigeration at 4°C, will maintain the integrity of PRRSV RNA and anti-PRRSV antibodies in oral-fluid samples collected for diagnostic testing.
Acknowledgements
We thank Dr Fernando A. Osorio (University of Nebraska-Lincoln) for generously providing concentrated anti-PRRSV antibody. This project was funded in part by an Advanced PRRS Research Award provided by Boehringer Ingelheim Vetmedica, Inc, St Joseph, Missouri.
References
1. Tabak LA. A revolution in biomedical assessment: the development of salivary diagnostics.J Dent Educ. 2001;65:1335–1339.
2. Tabak LA. Point-of-care diagnostics enter the mouth. Ann N Y Acad Sci. 2007;1098:7–14.
3. Streckfus CF, Bigler LR. Saliva as a diagnostic fluid. Oral Dis. 2002;8:69–76.
4. Rothman RE, Kalish B. Update on emerging infections: news from the Centers for Disease Control and Prevention. False-positive oral fluid rapid HIV tests – New York City, 2005-2008. Ann Emerg Med. 2009;53:151–156.
5. Bartington SE, Peckham C, Brown D, Joshi H, Dezateux C. Feasibility of collecting oral fluid samples in the home setting to determine seroprevalence of infections in a large-scale cohort of preschool-aged children. Epidemiol Infect. 2009;137:211–213.
6. Allan G, Ellis J. Porcine circoviruses: a review. J Vet Diagn Invest. 2000;12:3–14.
7. Prickett J, Kim W, Simer R, Yoon KJ, Zimmerman J. Surveillance of commercial growing pigs for PRRSV and PCV2 infections using pen-based oral fluid samples: A pilot study. J Swine Heath Prod. 2008;16:86–91.
8. Prickett J, Simer R, Christopher-Hennings J, Yoon KJ, Evans RB, Zimmerman JJ. Detection of porcine reproductive and respiratory syndrome virus infection in porcine oral fluid samples: a longitudinal study under experimental conditions. J Vet Diagn Invest. 2008;20:156–163.
9. Wills R, Zimmerman J, Yoon K, Swenson SL, Hoffman LJ, McGinley MJ, Hill HT, Platt KB. Porcine reproductive and respiratory syndrome virus: routes of excretion. Vet Microbiol. 1997;57:69–81.
10. DeBuysscher EV, Berman DT. Secretory immune response in intestinal mucosa and salivary gland after experimental infection of pigs with transmissible gastroenteritis virus. Am J Vet Res. 1980;41:1214–1220.
11. Loftager MK, Eriksen L, Nielsen R. Antibodies against Actinobacillus pleuropneumoniae serotype 2 in mucosal secretions and sera of infected pigs as demonstrated by an enzyme-linked immunosorbent assay. Res Vet Sci. 1993;4:57–62.
12. Loftager MK, Eriksen L, Nielsen R. Mucosal and serum IgA antibodies in pigs following infection with Actinobacillus pleuropneumoniae. Adv Exp Med Biol. 1995;371B:935–938.
13. Callan JR, Hartmann FA, West SEH, Hinshaw VA. Cleavage of influenza A virus hemagglutinin by swine respiratory bacterial proteases. J Virol. 1997;71:7579–7585.
14. Campo J, Perea MA, del Romero J, Cano J, Hernando V, Bascones A. Oral transmission of HIV, reality or fiction? An update. Oral Dis. 2006;12:219–228.
15. Deng MY, Cliver DO. Antiviral effects of bacteria isolated from manure. Microbiol Ecol. 1995;30:43–54.
16. Humphrey SP, Williamson RT. A review of saliva: Normal composition, flow, and function. J Prosthet Dent. 2001;85:162–169.
17. Shugars DC. Endogenous mucosal antiviral factors of the oral cavity. J Infect Dis. 1999;179:431–435.
18. Ryan MH, Petrone D, Nemeth JF, Barnathan E, Björck L, Jordan RE. Proteolysis of purified IgGs by human and bacterial enzymes in vitro and the detection of specific proteolytic fragments of endogenous IgG in rheumatoid synovial fluid. Mol Immunol. 2008;45:1837–1846.
19. National Pork Board. PQA Plus™ Producer Certification Book. Available at: http://www.pork.org/Producers/PQA/PQAPlusEdBook.pdf. Accessed 27 April 2010.
20. Yoon KJ, Zimmerman JJ, Swenson SL, McGinley MJ, Eernisse KA, Brevik A, Rhinehart LL, Frey ML, Hill HT, Platt KB. Characterization of the humoral immune response to porcine reproductive and respiratory syndrome (PRRS) virus infection. J Vet Diagn Invest. 1995;7:305–312.
21. Osorio FA, Galeota JA, Nelson E, Brodersen B, Doster A, Wills R, Zuckermann F, Laegreid WW. Passive transfer of virus-specific antibodies confers protection against reproductive failure induced by a virulent strain of porcine reproductive and respiratory syndrome virus and establishes sterilizing immunity. Virology. 2002;302:9–20.
22. McDonnell G, Russell A. Antiseptics and disinfectants: Activity, action, and resistance. Clin Microbiol Rev. 1999;12:147–179.
23. Morris M, Cohen B, Andrews N, Brown D. Stability of total and rubella-specific IgG in oral fluid samples: the effect of time and temperature. J Immunol Methods. 2002;266:111–116.
24. Quinn PJ, Carter ME, Markey BK, Carter GR, eds. Essential equipment and reagents for a veterinary diagnostic microbiology laboratory. In: Clinical Veterinary Microbiology. Edinburgh, Scotland: Mosby; 1994:61–65.
25. Thwe M, Frerichs RR, Oo KY, Zan E, Eskes N. Stability of saliva for measuring HIV in the tropics. J Trop Pediatr. 1999;45:296–299.
26. Gaudette D, North L, Hindahl M, Griffin K, Klimkow N, Thieme T. Stability of clinically significant antibodies in saliva and oral fluid. J Clin Immunoassay. 1994;17:171–175.