Campbell TA, Garcia MR, Miller LA, et al. Immunocontraception in male feral swine treated with a recombinant gonadotropin-releasing hormone vaccine. J Swine Health Prod. 2010;18(3):118–124
| Original research | Peer reviewed |
Cite as: Campbell TA, Garcia MR, Miller LA, et al. Immunocontraception in male feral swine treated with a recombinant gonadotropin-releasing hormone vaccine. J Swine Health Prod. 2010;18(3):118–124.
Also available as a PDF.
SummaryObjective: To determine if a recombinant gonadotropin-releasing hormone (GnRH) vaccine is a potential immunocontraceptive agent for juvenile male feral swine. Materials and methods: At the beginning of the trial (Day 0) we treated animals in Treatment One with a single injection of a sham vaccine containing 1 mL of a buffer-adjuvant emulsion (adjuvant: AdjuVac; National Wildlife Research Center, Fort Collins, Colorado). Treatment Two received 1000 μg of a recombinant GnRH (rGnRH) vaccine (IMX294; Imaxio, Lyon, France). Treatment Three received 500 μg of a rGnRH vaccine. Treatment Four received 1000 μg of a GnRH vaccine (GonaCon; National Wildlife Research Center, Fort Collins, Colorado). On Day 90, Treatment Three received an additional 500-μg boost treatment. All vaccines were emulsified with AdjuVac and injected intramuscularly into the rump. On Day 180, we performed necropsies on swine and compared mass of testes, percent normal seminiferous tubules, numbers of spermatogonia, spermatocytes, and spermatids, serum testosterone levels, and anti-GnRH antibody titers among treatments. Results: As expected, a single dose of GonaCon vaccine reduced testes mass, serum testosterone, and percent normal tubules, and restricted sperm development at each stage. These reductions in reproductive development were associated with elevated GnRH antibodies. The single injection of rGnRH was not as effective in reducing these reproductive parameters; however, the two-dose injection of rGnRH was as effective as the single injection of GonaCon. Implication: Further research and development is needed into oral immunocontraceptive vaccines and oral delivery systems. | ResumenObjetivo: Determinar si la vacuna recombinante que libera la hormona gonadotropina (GnRH por sus siglas en inglés) es un agente inmunoanticonceptivo potencial para cerdos machos juveniles silvestres. Materiales y métodos: Al inicio de la prueba (Día 0) tratamos a los animales en el Tratamiento Uno con una inyección única de una vacuna placebo que contenía 1 mL de emulsión buffer-adyuvante (adyuvante: AdjuVac; National Wildlife Research Center, Fort Collins, Colorado). El Tratamiento Dos recibió 1000 μg de una vacuna (IMX294; Imaxio, Lyon, France) recombinante GnRH (rGnRH). El Tratamiento Tres recibió 500 μg de una vacuna rGnRH. El Tratamiento Cuatro recibió 1000 μg de una vacuna GnRH (GonaCon; National Wildlife Research Center, Fort Collins, Colorado). En el Día 90, el Tratamiento Tres recibió 500 μg de tratamiento adicional de refuerzo. Todas las vacunas se emulsificaron con AdjuVac, y se inyectaron intramuscularmente en la pata trasera. En el Día 180, se realizó una necropsia a todos los cerdos y se compararon la masa testicular, porcentaje de túbulos seminíferos normales, número de espermatogonia, espermatocitos y espermátidos, niveles de testosterona en suero, y títulos de anticuerpos anti-GnRH entre los tratamientos. Resultados: Como se esperaba, una dosis única de la vacuna GonaCon redujo la masa testicular, la testosterona en suero, y el porcentaje de túbulos normales, y restringió el desarrollo de esperma en cada etapa. Estas reducciones en el desarrollo reproductivo fueron asociadas a la presencia de anticuerpos GnRH elevados. La inyección única de rGnRH no fue efectiva para reducir estos parámetros reproductivos; sin embargo, la inyección de rGnRH de dos dosis fue tan efectiva como la inyección única de GonaCon. Implicacion: Se necesita más investigación y desarrollo de las vacunas inmunoanticonceptivas orales y los sistemas de distribución oral. | ResuméObjectif: Déterminer si un vaccin recombinant dirigé contre la gonadolibérine (GnRH) est un agent immuno-contraceptif potentiel pour les porcs féraux mâles juvéniles. Matériels et méthodes: Au début de l’expérience (Jour 0) les animaux du groupe Traitement Un ont reçu une injection unique d’un faux vaccin contenant 1 mL d’une émulsion tampon-adjuvant (adjuvant: AdjuVac; National Wildlife Research Center, Fort Collins, Colorado). Le groupe de Traitement Deux a reçu 1000 μg d’un vaccin recombinant GnRH (rGnRH) (IMX294; Imaxio, Lyon, France). Le groupe de Traitement Trois a reçu 500 μg d’un vaccin rGnRH. Le groupe de Traitement Quatre a reçu 1000 μg d’un vaccin GnRH (GonaCon; National Wildlife Research Center, Fort Collins, Colorado). Au jour 90, le groupe de Traitement Trois a reçu un traitement de rappel de 500 μg. Tous les vaccins étaient émulsifiés avec de l’AdjuVac et injecté par voie intramusculaire dans la fesse. Au Jour 180, des nécropsies ont été effectuées sur les porcs et on a comparé la masse des testicules, le pourcentage de tubules séminifères normaux, le nombre de spermatogonie, de spermatocytes, et de spermatides, les niveaux de testostérone sérique, et les titres d’anticorps anti-GnRH parmi les groupes de traitement. Résultats: Tel qu’attendu, une dose unique de vaccin GonaCon a réduit la masse des testicules, la testostérone sérique, et le pourcentage de tubules normaux, de même qu’il a restreint le développement du sperme à tous les stades. À ces réductions dans le développement reproducteur étaient associés des titres d’anticorps élevés contre la GnRH. L’injection unique de rGnRH n’était pas aussi efficace pour réduire ces paramètres reproducteurs; toutefois, l’administration de deux doses de rGnRH était aussi efficace que l’injection unique de GonaCon. Implication: De la recherche et du développement supplémentaire sont requis en ce qui regarde les vaccins immuno-contraceptifs et les systèmes de livraison oraux. |
Keywords: swine, feral, fertility control,
gonadotropin-releasing hormone, immunocontraception
Search the AASV web site
for pages with similar keywords.
Received: August 25, 2009
Accepted: December 1, 2009
Feral swine (Sus scrofa) (ie, escaped or released domestic swine, European wild boar, their hybrids, and their offspring) occur throughout the United States, where in the absence of native predators they have become the most fecund free-ranging ungulate.1 Agricultural damages, not including those associated with domestic swine biosecurity and disease-abatement strategies, have been estimated at $800 million annually.2 Technologies that are available to stabilize population growth in the United States are trapping (eg, cage, box, corral, and snare) with euthanasia, and shooting (eg, ground, aerial, with dogs, and at night).3 Technologies that are being developed worldwide include toxicants and fertility-control agents.4-6 The fertility-control agent that has received most attention in the literature for feral swine is an immunocontraceptive vaccine that incorporates gonadotrophin-releasing hormone (GnRH). This vaccine functions by disrupting the production of GnRH, which regulates other sex hormones, such as luteinizing hormone and follicle-stimulating hormone. The reduction in these two stimulating hormones results in a reduction in testosterone and estrogen.7
The National Wildlife Research Center (NWRC; Fort Collins, Colorado) has developed and extensively tested a GnRH immunocontraceptive vaccine entitled “GonaCon” containing the adjuvant AdjuVac (NWRC; Fort Collins, Colorado). The GonaCon product (ie, a single-injection mollusk-GnRH-AdjuVac vaccine7) has been evaluated in cats,8 white-tailed deer (Odocoileus virginianus),9-12 bison (Bison bison),13 mares,14 elk (Cervus elaphus),15 and feral swine.5,6,16,17 Results from GonaCon trials have been highly favorable. The NWRC has also tested a recombinant GnRH (rGnRH) developed by Dr G. Talwar (New Delhi, India).18 This rGnRH vaccine, along with a positive control of GonaCon, was tested in female domestic swine at Pennsylvania State University.17 The rGnRH was evaluated as a one- and two-injection vaccine. The two-injection rGnRH vaccine was successful in reducing fertility in female domestic swine.
A major obstacle to field application of immunocontraceptive vaccines for free-ranging feral swine is the requisite that animals are treated through injection, rather than orally. For example, the large molecular weight of GonaCon prevents absorption in the gut,17 thereby inhibiting effectiveness when administered orally. Another challenge with the GonaCon vaccine is the high cost of formulation.17 Imaxio (Lyon, France) has developed a rGnRH vaccine, IMX294, that is expressed in Escherichia coli. This recombinant is a small 7-kDa peptide with a GnRH molecule fused at the N terminal. After extraction from E coli, the 7-kDa peptide combines by two cystine linkages to form a cylindrical heptamer of approximately 50 kDa. Conceptually, this recombinant should be small enough to enable absorption in the gut and inexpensive to produce in large quantities. The potential for oral delivery is also true with the Talwar recombinant; however, it is important to evaluate these vaccines by injection first to insure that these recombinants will function under standard treatments. Therefore, the Talwar recombinant was tested as an injectable in female swine,17 and the Imaxio’s recombinant as an injectable contraceptive for male feral swine was evaluated herein.
Our objectives were to determine if the small rGnRH vaccine is a potential immunocontraceptive or immunocastration agent for juvenile male feral swine. Specifically, after vaccination, we compared testicular mass, anti-GnRH antibody titers, serum testosterone, seminiferous tubule morphology, and spermatogenesis cells among several GnRH vaccine treatments in a captive setting. Given the successes in France, we hypothesized that Imaxio’s rGnRH vaccine, IMX294, would alter reproductive parameters in juvenile male feral swine.
Materials and methods
All capture, handling, and housing procedures for feral swine were approved by the Texas A&M University-Kingsville Animal Care and Use Committee.
Animals and housing
Our trial was conducted at the Caesar Kleberg Wildlife Research Institute, Captive Wildlife Research Facility at Texas A&M University-Kingsville from April to October 2008. We used 24 captured male feral swine of unknown ancestry, < 3 months old at the beginning of the trial, as study subjects. Pelleted commercial swine feed (USDA Pig; Lyssy and Eckels, Poth, Texas) was available ad libitum throughout the duration of the trial, as well as water and shade. We used four 2000-m2 enclosures without bedding. We followed Texas Animal Health Commission regulations and university policies during trapping, transport, housing, and disposal of animals.
Treatments
We randomly assigned animals to four treatment groups. Treatment One was a negative control or sham treatment (n = 6). Treatment Two was one dose of Imaxio’s rGnRH product (n = 6). Treatment Three was two doses of Imaxio’s rGnRH product (n = 6 to 2 weeks and n = 3 thereafter). Treatment Four was a positive control, the GonaCon product (n = 6). On the first day of the trial (Day 0), we treated animals in Treatment One with 1 mL of a buffer-AdjuVac emulsion. Animals in Treatment Two received 1000 μg of the rGnRH vaccine (recombinant emulsified with AdjuVac), animals in Treatment Three received a prime of 500 μg rGnRH followed in 90 days by a 500-μg boost of rGnRH, and animals in Treatment Four received 1000 μg of GonaCon (GnRH peptide conjugated to mollusk protein used in a emulsion form with AdjuVac as used in GonaCon-blue17). All treatments were administered intramuscularly in the rump.
Data collection
On Days 0, 30, 60, 90, 120, 150, and 180 of our trial, we weighed animals and collected 5 mL of whole blood via femoral venipuncture. On Day 180, we humanely euthanized all feral swine following AVMA Guidelines on Euthanasia,19 at which time we performed necropsies to recover and weigh testes on an electronic scale accurate to 0.1 g (PL601-S; Mettler Toledo, Columbus, Ohio). We report testicular mass as the sum of both testes. We centrifuged, recovered, and stored serum from whole blood collected on Days 0, 30, 60, 90, 120, 150, and 180 of our trial. We tested serum for anti-GnRH antibodies with an enzyme-linked immunosorbent assay. We used a 96-well plate prepared by adding 100 ng of bovine serum albumin-GnRH antigen to each well and blocking with phosphate-buffered saline-2% powdered milk.5 We tested serum for testosterone with a Coat-a-Tube radioimmunoassay test kit (Diagnostic Products, Los Angeles, California).
Histochemistry
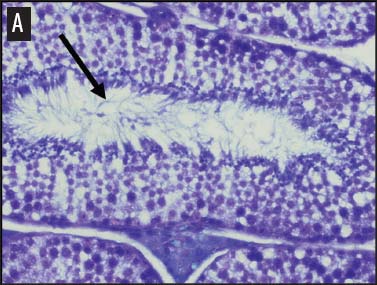
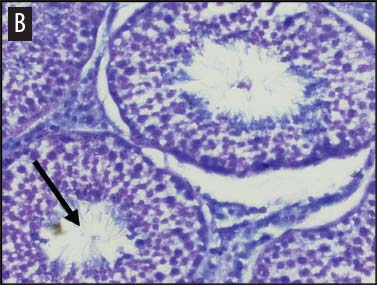
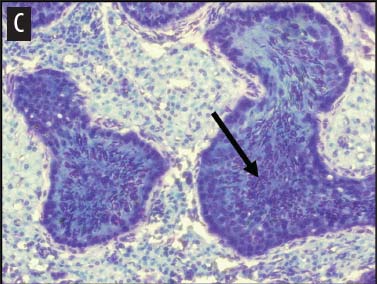
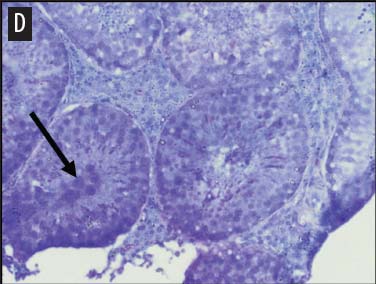
We placed testicular tissue (approximately 1 mg) immediately in ice-cold 10% formalin solution (Sigma Aldrich, St Louis, Missouri). We gently rocked the tissue for 24 hours and then washed tissues twice for 1 hour each in 40%, 60%, 80%, 95%, and 100% ethanol, rocking at 4°C. We transferred tissues from 100% ethanol to xylene for 1 hour and then repeated for 1 hour in fresh xylene. We placed the tissue from xylene treatment into a paraffin bath (TissuePrep; Fisher Scientific, Pittsburgh, Pennsylvania) at 60°C for 2 hours and then embedded tissues in a cassette mold. We sectioned tissues (6-μm thickness) and placed on slides (ProbeOn Plus slides; Fisher Scientific), with two serial sections per slide. Following the sectioning process, we deparaffinized tissues and rehydrated them through a graded series of ethanol baths (100%, 100%, 95%, 95%, and 70%) for 2 minutes each. We stained tissue sections with hematoxylin and dipped them in a graded series of ethanol baths (79%, 95%, 100%) for 15 seconds each and then xylene for 1 minute. We dried and mounted sections with Permount (Fisher Scientific) for analysis. We quantified abnormal versus normal seminiferous tubules from six randomly chosen areas of 13.3 × 104 mm2 per slide (Figure 1). We classified abnormal tubules as tubules that were closed, occluded, or did not exhibit any stage of spermatogenesis. We did not determine specific stages of spermatogenesis in normal tubules, because the presence of spermiation indicated that spermatogenesis was occurring. We counted spermatogonia, spermatocytes, and spermatids in two randomly selected tubules per slide (selected using gridded slides and a random-number generator). We identified spermatogonia as the cells approximately 7 mm in diameter located next to the basal lamina of the tubules, containing round, condensed, pale-staining nuclei. Total spermatogonia included both type A (cells that either divide to produce copies of themselves or divide by mitosis to produce type B cells) and B (divide to produce primary spermatocytes).20 We identified spermatocytes as the cells that were luminal to the spermatogonia, dark staining, with large nuclei, and larger in diameter than spermatogonia (approximately 8 to 16 μm). Total spermatocytes included both primary and secondary (secondary spermatocytes are short-lived).21 Categorized spermatids included those that were in the process of spermiation or had completed spermiation (ie, small condensed chromatin in elongated nuclei with very little cytoplasm).21
Figure 1: Representative histological images at 20× magnification from slides of juvenile male feral swine testes in Kingsville, Texas, in 2008 (hematoxylin stain). A: negative control (n = 6); B: one-dose recombinant GnRH (rGnRH) vaccine (n = 6); C: two-dose rGnRH vaccine (n = 3); D: GnRH vaccine (GonaCon; National Wildlife Research Center, Fort Collins, Colorado; n = 6). Arrows indicate tubules. On Day 0, negative controls were treated with a single injection of a sham vaccine (1 mL of a buffer-AdjuVac emulsion (AdjuVac is the adjuvant in GonaCon); the one-dose rGnRH vaccine treatment received 1000 μg of a rGnRH vaccine (IMX294; Imaxio, Lyon, France); the two-dose rGnRH vaccine treatment group received 500 μg of rGnRH vaccine (and an additional 500-μg dose on Day 90), and the GonaCon treatment received 1000 μg of GonaCon. All vaccines were injected intramuscularly into the rump. All animals were euthanized on Day 180.
|
Data analysis
We compared mass of testes, percent normal tubules, number of spermatogonia, number of spermatocytes, and number of spermatids among treatments with a one-factor unbalanced analysis of variance (SAS Version 9.1; SAS Institute, Inc, Cary, North Carolina). Percentage data were square-root transformed prior to analysis.22 For this model, we considered treatment as the main effect. We compared monthly serum testosterone levels and anti-GnRH antibody titers with an unbalanced split-plot repeated measures ANOVA. For this model, we considered month as the within-subject factor and treatment as the between-subject factor.23 When appropriate, we performed Tukey’s honestly significant difference as a multiple-range test. We reported means with standard errors and used a Type I error rate of 10% for all analyses.
Results
We removed three feral swine from Treatment Three (two-dose rGnRH) ≤ 2 weeks after our trial began. Two animals were found dead of unknown causes and one animal was missing and presumably escaped. These findings illustrate the challenges of housing and maintaining juvenile (< 3 months old) feral swine in captivity.
During our trial, we found feral swine body mass increased by 141%, 155%, 198%, and 188% for Treatments One to Four, respectively, with final mean body weights ranging from 25 to 41 kg. We determined that serum testosterone increased over time (F6,102 = 26.78; P < .001) and that feral swine in the control treatment displayed higher serum testosterone levels and feral swine in the GonaCon treatment exhibited lower serum testosterone levels than the other treatments (F3,17 = 22.37; P < .001, Figure 2). Similarly, we found anti-GnRH antibody titers differed by treatment (F3,17 = 17.33; P < .001, Figure 3) and that animals in different treatments displayed different titers across months (F15,85 = 3.55; P < .001). Specifically, no anti-GnRH antibodies were observed in the control group feral swine, swine in the GonaCon treatment displayed decreasing antibodies over time, and animals in the two-dose rGnRH treatment exhibited a spike 4 months after vaccination, associated with administration of the second vaccine dose.
Figure 2: Mean ± SE monthly serum testosterone of juvenile male feral swine by treatment. Treatments were a negative control, a one-dose rGnRH vaccine, a two-dose rGnRH vaccine, and a GonaCon treatment, as described in Figure 1. Swine in the control treatment displayed higher serum testosterone levels and swine in the GonaCon treatment exhibited lower serum testosterone levels than the other treatments (F3,17 = 22.37; P < .001; unbalanced split-plot repeated measures ANOVA with P < .10 considered statistically significant). 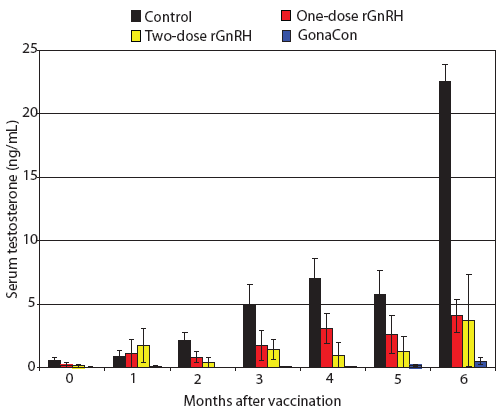 |
Figure 3: Mean ± SE monthly anti-GnRH antibody titers of juvenile male feral swine. Treatments were a negative control (n = 6), a one-dose rGnRH vaccine (n = 6), a two-dose rGnRH vaccine (n = 3), and a GonaCon treatment (n = 6), as described in Figure 1. Titers differed by treatment (F3,17 = 17.33; P < .001; unbalanced split-plot repeated measures ANOVA, with P < .10 considered statistically significant). 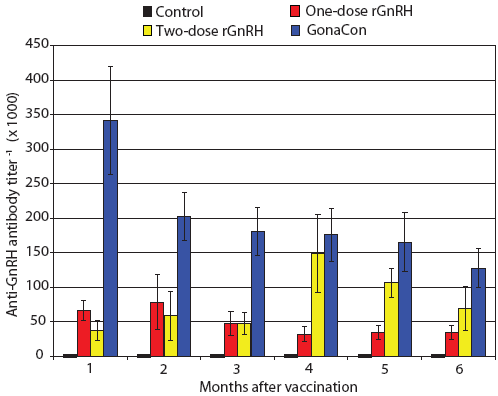 |
We observed that mass of testes differed by treatment (F3,17 = 8.18; P < .01), with testicular mass greatest in the control animals (Figure 4). The percentage of normal tubules varied by treatment (F3,17 = 2.45; P < .10), with feral swine in the control treatment displaying a greater percent of normal tubules than animals in the two-dose rGnRH treatment (Figure 5). We determined that the number of spermatogonia differed by treatment (F3,17 = 9.53; P < .01), with swine in the control and one-dose rGnRH treatments exhibiting more spermatogonia than the two-dose rGnRH and GonaCon treatments (Figure 5). We observed similar differences and relationships for the number of spermatocytes (F3,17 = 8.21; P < .01) and the number of spermatids (F3,17 = 7.83; P < .01, Figure 5).
Figure 4: Mean ± SE testes mass of juvenile male feral swine. Treatments were a negative control (sham-vaccinated; n = 6), a one-dose rGnRH vaccine (n = 6), a two-dose rGnRH vaccine (n = 3), and a GonaCon treatment (n = 6), as described in Figure 1. Testes mass was greater in the negative control treatment (F3,17 = 8.18; P < .01; one-factor unbalanced analysis of variance with P < .10 considered statistically significant). 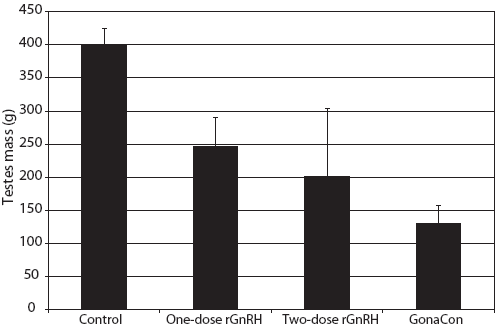 |
Figure 5: Mean ± SE percent normal seminiferous tubules in six randomly chosen areas of 13.3 × 104 mm2 per slide (left axis) and numbers of spermatogonia, spermatocytes, and spermatids (right axis) in two randomly selected tubules per slide in tissues from juvenile male feral swine. Treatments included a negative control (sham-vaccinated; n = 6); a one-dose rGnRH vaccine (n = 6); a two-dose rGnRH vaccine (n = 3); and a GonaCon treatment (n = 6), as described in Figure 1. Percentage data were square-root transformed prior to analysis. Parameters were compared using a one-factor unbalanced analysis of variance with P < .10 considered statistically significant. 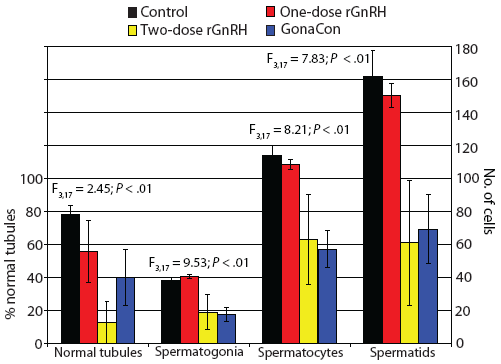 |
Discussion
Our negative control (Treatment One) functioned as expected. Specifically, serum testosterone increased steadily over time until our final measurement, 6 months after administering the treatment, when it spiked sharply, no anti-GnRH antibodies were detected, testes mass was greater than in other treatments, a greater percent of normal tubules were found, and sperm were more developed than in most of the other treatments. We surmise that the increase in serum testosterone 6 months after administering the treatment was associated with the onset of sexual maturity and may be related to animals reaching a critical body mass. For example, feral swine in the negative control group of a different study had peak serum testosterone of approximately 12 ng per mL, or half our values, yet animals in that study weighed ≤ 32 kg,5 whereas animals in our study weighed ≤ 41 kg.
Our positive control (Treatment Four), GonaCon treatment, was effective at altering reproductive parameters in feral swine. This was expected, given prior findings of lower testis mass, lower serum testosterone, and higher anti-GnRH antibody titers within feral swine administered a single dose of 1000 μg of GonaCon.5 Our observed anti-GnRH antibody titers ranged from 341 during the first month post vaccination to 128 six months after vaccination, and were generally greater than those reported by others at 12 weeks and 36 weeks post vaccination.5 It is apparent that anti-GnRH antibody titers peak shortly after vaccination and that this spike was not captured in the 12-week measurements of Killian et al (2006).5 Other parameters that were lower in feral swine in the GonaCon treatment were numbers of spermatogonia, spermacytes, and spermatids.
Our one-dose GnRH vaccine treatment did induce an immunogenic response, as evidenced by the presence of anti-GnRH antibodies and lower serum testosterone. However, the immunogenic response was weak, and percent normal tubules and numbers of spermatogonia, spermacytes, and spermatids did not differ from those of the control treatment. Furthermore, anti-GnRH antibodies during May, 1 month after vaccination, were five times less than those in the GonaCon treatment, indicating marginal immunogenicity.
Our two-dose rGnRH vaccine treatment induced a strong immunogenic response after administration of the second dose on Day 90. Therefore, by the end of our study, the reproductive parameters of feral swine in this treatment were similar to those in the GonaCon treatment. For example, serum testosterone, anti-GnRH antibodies, and numbers of spermatogonia, spermacytes, and spermatids did not differ from the GonaCon treatment for data collected from 4 to 6 months after vaccination. We conclude that the two-dose rGnRH vaccine was successful at altering reproductive parameters in juvenile male feral swine. Consequently, we believe that this product is a potential immunocontraceptive agent for feral swine. Future areas of research on this product should include studies involving female feral swine, challenge studies with conception opportunities, and studies including physiology and behavior of animals similar to Massei et al (2008).6 Furthermore, the ultimate goal for the field application of immunocontraceptive vaccines for free-ranging feral swine is oral delivery. Considerable development needs to occur with recombinant GnRH vaccines before they can be delivered orally. An alternative application of this product is to reduce the testosterone production of juvenile male swine, thereby reducing “boar taint” in situations where physical castration is not possible.24 Under this scenario, delivery of the vaccine through injection may be appropriate.
Implications
- This rGnRH vaccine is a potential immunocontraceptive agent for feral or domestic swine.
- Future areas of research should include studies involving domestic swine and female feral swine, and studies including physiology and behavior of animals.
- Further research and development is needed into oral immunocontraceptive vaccines and oral-delivery systems.
Acknowledgements
We are grateful to E. Aguirre, C. Coots, O. Fitzsimmons, R. Kaiser, D. Krueger, K. Kubala, V. Myers, N. Perry, S. Rabe, and D. Sanders for field assistance. Financial support was provided by the United States Department of Agriculture, Animal and Plant Health Inspection Service, Wildlife Services, National Wildlife Research Center. We thank the Caesar Kleberg Wildlife Research Institute at Texas A&M University-Kingsville for providing logistical support. Our mention of commercial products herein is for identification purposes and does not constitute endorsement or censure by the United States Department of Agriculture.
References
1. Sweeney JR, Sweeney JM, Sweeney SW. Feral hog Sus scrofa. In: Feldhamer FA, Thompson BC, and Chapman JA, eds. Wild Mammals of North America. 2nd ed. Baltimore, Maryland: Johns Hopkins University Press; 2003:1164–1179.
2. Pimentel D, Zuniga R, Morrison D. Update on the environmental economic costs associated with alien-invasive species in the United States. Ecol Econ. 2005;52:273–288.
3. Campbell TA, Long DB. Feral swine damage and damage management in forested ecosystems. For Ecol Manage. 2009;257:2319–2326.
4. Cowled BD, Elsworth P, Lapidge SJ. Additional toxins for feral pig (Sus scrofa) control: identifying and testing Achilles’ heels. Wildl Res. 2008;35:651–662.
5. Killian G, Miller L, Rhyan J, Doten H. Immunocontraception of Florida feral swine with a single-dose GnRH vaccine. Am J Reprod Immunol. 2006;55:378–384.
6. Massei G, Cowan DP, Coats J, Gladwell F, Lane JE, Miller LA. Effect of the GnRH vaccine GonaCon on the fertility, physiology and behaviour of wild boar. Wildl Res. 2008;35:540–547.
*7. Miller L, Rhyan J, Killian G. Evaluation of GnRH contraceptive vaccine using domestic swine as a model for feral hogs. Proc Wildl Damage Manage Conf. 2003;10:120–127.
8. Levy JK, Miller LA, Crawford PC, Ritchey JW,
Ross MK, Fagerstone KA. GnRH immunocontraception of male cats.
Theriogenology. 2004;62:1116–1130.
9. Curtis PD, Richmond ME, Miller LA, Quimby FW. Physiological effects of gonadotropin-releasing hormone immunocontraception on white-tailed deer. Human-Wildl Confl. 2008;2:68–79.
10. Miller LA, Gionfriddo JP, Fagerstone KA, Rhyan JC, Killian GJ. The single-shot GnRH immunocontraceptive vaccine (GonaCon) in white-tailed deer: comparison of several GnRH preparations. Am J Reprod Immuno. 2008;60:214–223.
11. Miller LA, Gionfriddo JP, Rhyan JC, Fagerstone KA, Wagner DC, Killian GJ. GnRH Immunocontraception of male and female white-tailed deer fawns. Human-Wildl Confl. 2008;2:93–101.
12. Gionfriddo JP, Eisemann JD, Sullivan KJ, Healey RS, Miller LA, Fagerstone KA, Engeman RM, Yoder CA. Field test of a single-injection gonadotrophin-releasing hormone immunocontraceptive vaccine in female white-tailed deer. Wildl Res. 2009;36:177–184.
13. Miller LA, Rhyan JC, Drew M. Contraception of bison by GnRH
vaccine: a possible means of decreasing transmission of brucellosis
in bison.
J Wildl Dis. 2004;40:725–730.
14. Killian G, Thain D, Diehl NK, Rhyan J, Miller L. Four-year contraception rates of mares treated with single-injection porcine zona pellucida and GnRH vaccines and intrauterine devices. Wildl Res. 2008;35:531–539.
15. Killian G, Kreeger TJ, Rhyan J, Fagerstone K, Miller L. Observations on the use of GonaCon™ in captive female elk (Cervus elaphus). J Wildl Dis. 2009;45:184–188.
*16. Killian G, Miller L, Rhyan J, Dees T, Perry D, Doten H. Evaluation of GnRH contraceptive vaccine in captive feral swine in Florida. Proc Wildl Damage Manage Conf. 2003;10:128–133.
*17. Miller LA, Talwar GP, Killian GJ. Contraceptive effect of a recombinant GnRH vaccine in adult female pigs. Proc Vert Pest Conf. 2006;22:106–109. Available at: http://168.68.129.70/wildlife_damage/nwrc/publications/06pubs/miller064.pdf. Accessed 10 February 2010.
18. Talwar GP, Raina K, Gupta JC, Ray R, Wadhwa S, Ali MM. A recombinant luteinizing-hormone-releasing-hormone immunogen bioeffective in causing prostatic atrophy. Vaccine. 2004;22:3713–3721.
19. AVMA Guidelines on Euthanasia. June 2007. Available at: http://www.avma.org/issues/animal_welfare/euthanasia.pdf. Accessed 22 December 2009.
20. Pinart E, Sancho S, Briz MD, Bonet S, Garcia N, Badia E. Ultrastructural study of boar seminiferous epithelium: changes in cryptorchidism. J Morphol. 2000;244:190–202.
21. Junqueira LC, Carneiro J, Kelley RO. The male reproductive system. In: Junqueira LC, Carneiro J, eds. Basic Histology. 7th ed. New York, New York: Appleton and Lange; 1992:423–426.
22. Steel RGD, Torrie JH. Principles and Procedures of Statistics: A Biometrical Approach. New York, New York: McGraw-Hill; 1980:633.
23. von Ende CN. Repeated-measures analysis: growth and other time dependent measures. In: Scheiner SM, Gurevitch J, eds. Design and Analysis of Ecological Field Experiments. New York, New York: Oxford University Press; 1993:113–137.
24. Schmoll F, Kauffold J, Pfützner A, Baumgartner J, Brock F, Grodzycki M, Andrews S. Growth performance and carcass traits of boars raised in Germany and either surgically castrated or vaccinated against gonadotropin-releasing hormone. J Swine Health Prod. 2009;17:250–255.
* Non-refereed references.