Woods AL, McDowell EJ, Holtkamp D, et al. Reproductive failure associated with porcine parvovirus and possible porcine circovirus type 2 co-infection. J Swine Health Prod. 2009;17(4):210–216
| Case report | Peer reviewed |
Cite as: Woods AL, McDowell EJ, Holtkamp D, et al. Reproductive failure associated with porcine parvovirus and possible porcine circovirus type 2 co-infection. J Swine Health Prod. 2009;17(4):210–216.
Also available as a PDF.
SummaryA 2400-sow multi-site commercial herd experienced an outbreak of reproductive failure characterized by a dramatic increase in mummified fetuses, primarily in primiparous animals. Multiple samples of mummified and stillborn fetuses, placenta, and weak liveborn pigs were submitted to the diagnostic laboratory. Diagnostic testing for porcine parvovirus (PPV) in tissue sections was positive by virus isolation and direct fluorescent antibody. Antibodies to PPV were detected by indirect fluorescent antibody (IFA) and competitive enzyme-linked immunosorbent assay. Porcine circovirus type 2 (PCV2) was also diagnosed by polymerase chain reaction, and antibodies were detected by IFA. Mummified fetuses in gilt litters rose acutely from 3.9% to 31.5%, but in multiparous animals during the same time period, the rate rose only slightly, from 3.2% to 4.2%. At its peak, the mummified-fetus rate in gilt litters reached 61.0%. Mid- to late-term gilt abortions also increased significantly during the affected time period. Although all animals were vaccinated for PPV during the isolation-acclimation period, it still became an apparent cause of severe reproductive failure. The role of PCV2 in this case is unclear, but PCV2 may have contributed to the severity of the outbreak as a co-infection or by interfering with the effectiveness of the PPV vaccine. | ResumenUn hato comercial de sitios múltiples de 2400 hembras experimentó un brote de falla reproductiva caracterizado por un incremento dramático en fetos momificados, principalmente en hembras primíparas. Se enviaron múltiples muestras de fetos nacidos muertos y momificados, placenta, y cerdos débiles nacidos vivos al laboratorio de diagnóstico. Las pruebas diagnósticas en busca de parvovirus porcino (PPV por sus siglas en inglés) en secciones de tejido fueron positivas al aislamiento viral y a la prueba directa de anticuerpos fluorescentes. Los anticuerpos contra PPV se detectaron por medio de la prueba indirecta de anticuerpos fluorescentes (IFA por sus siglas en inglés) y enzimoinmunoanálisis de adsorción. También se diagnosticó circovirus porcino tipo 2 (PCV2 por sus siglas en inglés) por medio de la reacción en cadena de la polimerasa y se detectaron anticuerpos por medio de IFA. Los fetos momificados en camadas de primerizas se elevaron de manera importante de 3.9% a 31.5%; sin embargo, durante el mismo periodo en hembras multíparas, la tasa se elevó ligeramente de 3.2% a 4.2%. En el pico del problema, la tasa de fetos momificados en camadas de primerizas alcanzó el 61.0%. Los abortos de primerizas de medio a finales del término también aumentaron significativamente durante el periodo de afección. Aunque se vacunaron todos los animales contra PPV durante el periodo de aislamiento-aclimatación, éste pareció ser la causa aparente de la severa falla reproductiva. El papel del PCV2, en este caso, es confuso, pero el PCV2 pudo haber contribuido a la severidad del brote como una coinfección ó al interferir con la eficiencia de la vacuna del PPV. | ResuméUn troupeau commercial de 2400 truies sur sites multiples a été aux prises avec des problèmes reproducteurs caractérisés par une augmentation dramatique de fÅ“tus momifiés, principalement chez les animaux primipares. Des échantillons multiples de fÅ“tus momifiés et mort-nés, du placenta, et des porcelets nés vivants mais faibles ont été soumis au laboratoire de diagnostic. La présence de parvovirus porcin (PPV) a été mise en évidence dans des sections de tissus par isolement viral et immunofluorescence directe. Des anticorps contre le PPV ont été détectés par immunofluorescence indirecte (IFA) et une épreuve immuno-enzymatique compétitive. Le circovirus porcin de type 2 (PCV2) a également été diagnostiqué par réaction d’amplification en chaîne par la polymérase et des anticorps détectés par IFA. La quantité de fÅ“tus momifiés dans les portées de cochettes a augmenté drastiquement de 3.9% à 31.5%, alors que durant la même période chez les animaux multipares, le taux n’augmenta que légèrement passant de 3.2% à 4.2%. À son maximum, le taux de fœtus momifiés atteignit 61.0% chez les cochettes. Chez ces dernières, les avortements de la mi- à la fin-gestation ont également augmenté de manière significative durant cet épisode. Bien que tous les animaux aient été vaccinés contre le PPV durant la période d’isolement-acclimatation, il est apparu évident que ce virus a causé de sérieux problèmes de reproduction. Le rôle du PCV2 dans le cas présent n’est pas clair, mais il pourrait avoir contribué à la sévérité de cette poussée de cas comme agent co-infectant ou en interférant avec l’efficacité du vaccin contre le PPV. |
Keywords: swine, porcine parvovirus, reproductive
failure, porcine circovirus type 2, PCV2, reproduction
Search the AASV web site
for pages with similar keywords.
Received: January 9, 2009
Accepted: March 5, 2009
Reproductive failure in swine refers to irregular returns to estrus due to failure of implantation, abortions, or increased numbers of nonviable piglets at farrowing, resulting in decreased litter sizes.1 The most common viral agents associated with reproductive failure are porcine reproductive and respiratory syndrome virus (PRRSV), porcine parvovirus (PPV), pseudorabies virus (PRV), and porcine circovirus type 2 (PCV2).1
Porcine parvovirus is ubiquitous in swine herds across the world.1,2 Because the virus is extremely stable in the environment, it is likely that pigs in infected herds are repeatedly exposed.2 Clinical signs associated with PPV infection are limited primarily to maternal reproductive failure, predominantly in gilts, because second- and subsequent-parity females are more likely to have become actively immune.2,3 The goal of managing PPV is to create protective immunity through natural exposure, vaccination, or both before breeding. Challenges to controlling PPV are the wide variability in levels of passive antibodies and the long duration of passive antibody decay, which may not drop below protective levels until pigs are 3 to 6 months of age. This may interfere with development of long-term active immunity. Thus, maternal-antibody interference prevents some gilts from being effectively immunized against PPV until shortly before or even after breeding.2,3
Clinical signs of PPV infection in the breeding herd are decreased abdominal girth in sows diagnosed pregnant, increased incidence of mummified fetuses, and increased number of irregular returns to estrus.2,3 Macroscopic changes in fetuses under 70 days of gestation include stunted growth, congestion and leakage of blood into tissues, increased vascularization over the surface of the fetus, accumulation of fluids in body cavities, and death with subsequent dehydration (mummification).2,3 Meningoencephalitis with perivascular cuffs of lymphocytes, plasma cells, and histiocytes is a microscopic lesion that may be present with fetal PPV infections.2
Infection of a naive breeding-age female commonly results in viremia and shedding of the virus for approximately 2 weeks after exposure.2,3 The virus can cross the placenta and infect the conceptus 10 to 14 days after maternal exposure2,3 and further infects other conceptuses in the litter via intrauterine spread. Thus, death of fetuses can occur at various stages of development within an infected litter. If a dam is exposed to PPV on or before day 56 of gestation, there are two possible sequelae. First, the virus may cross the placenta and infect an embryo (day 10 to 30 of gestation) causing death and resorption of the embryo and resorption of associated fluids. Second, the virus may cross the placenta and infect a fetus causing death and dehydration resulting in mummification. If the virus infects the dam after day 56 of gestation, the fetus will likely be infected between day 70 and term, when it is sufficiently immunocompetent to mount a protective immune response, and will survive in utero.2
One clinical manifestation of PCV2 in a mature animal is reproductive failure. Reproductive failure associated with vertical transmission of PCV2 occurs primarily in gilts. The virus crosses the placenta, infecting fetuses and causing increases in mid- to late-term abortions, mummified fetuses, stillborn pigs, weak and nonviable piglets at birth, and failure to farrow after being diagnosed pregnant.4-6 Occasionally, dilated cardiomyopathy and hepatomegaly with secondary ascites occur in fetuses. Microscopic fetal lesions include nonsuppurative and necrotizing myocarditis with fibrosis and mineralization.4-6 Reported reproductive failure associated with PCV2 has most commonly been preceded by a change in source or housing of replacement gilts prior to their introduction into the breeding herd.4-11 Subclinical PCV2 infections are responsible for decreased efficacy of a modified-live PRRS vaccine in growing pigs;12 however, similar effects have not been evaluated with other vaccines or in adult animals.
Herd history and description
The affected herd was a 2400-sow multi-site commercial pork-production operation. The main site consisted of a breeding barn, three gestation barns, a gilt breeding and gestation barn, a one-room boar stud, 14 farrowing rooms, and three finisher barns. After a 60-day period in an off-site isolation barn approximately 1 km away, all gilts entered the designated gilt barn on the main site for breeding and gestation. Although gilts were not exposed to the remainder of the sow herd until farrowing, no specific biosecurity protocols were in place for traffic between the gilt barn and the sow herd. A nursery-finish facility located 320 m from the main site and connected by a common driveway housed approximately half of the pigs produced from this sow farm. The remainder of the pigs were placed in off-site wean-to-finish barns or in a contract nursery-finish site.
The PRRS-positive sow herd had experienced no recent clinical signs of PRRS. Incoming gilts in the isolation barn were injected with serum containing live PRRSV.
At the time of the outbreak, all replacement gilts were raised on the main site, grown to maturity at the nursery-finish site near the sow farm, and moved to the isolation barn at approximately 6 months of age. At 3 and 6 weeks of age, gilts were vaccinated for PCV2 (Circumvent PCV; Intervet, Inc, Millsboro, Delaware) and for Mycoplasma hyopneumoniae (M+Pac; Schering-Plough Animal Health, Omaha, Nebraska). At 2 and 6 weeks post arrival at the isolation-acclimatization facility, gilts were vaccinated with a commercially available PPV, Leptospira, and Erysipelothrix (PLE) vaccine, a bivalent autogenous swine influenza virus vaccine containing H3N2 and H1N2 strains (MVP Laboratories, Omaha, Nebraska), and Enterisol Ileitis (Boehringer Ingelheim Vetmedica, Inc, St Joseph, Missouri). Prior to November 2007, the PLE vaccine had been FarrowSure Plus (Pfizer, Inc, Pfizer Animal Health, New York, New York). In November 2007, a change was made to Parvo Shield L5E (Novartis Animal Health US, Inc, Greensboro, North Carolina).
Beginning in the spring of 2008, replacement gilts were purchased rather than being raised on the farm, with the first shipment of purchased gilts arriving at the isolation barn on April 15, 2008 and moving into the gilt breeding and gestation barn at the sow farm June 10, 2008 (Figure 1). Gilts tested negative for PRRSV by polymerase chain reaction (PCR) after 6 weeks in isolation. A new group of boars was purchased April 28, 2008 and remained in a separate off-site isolation facility for 2 months, entering the boar stud after testing PCR-negative for PRRSV.
Figure 1: Timeline of events related to an investigation of reproductive failure in a 2400-sow commercial herd co-infected with porcine parvovirus (PPV) and porcine circovirus type 2 (PCV2).
|
Case description
An increase in stillborn and late-term mummified fetuses occurred in early April 2008, particularly in first-parity litters. Several stillborn and mummified fetuses (approximately 65 days to full term), as well as placentas, were submitted to the Indiana Animal Disease Diagnostic Laboratory (ADDL) at Purdue University (West Lafayette, Indiana) April 11, 2008. Diagnostic testing for a full array of swine reproductive pathogens was conducted. Tissues were negative for PRRSV and PCV2 by PCR and virus isolation (VI). Fetal sera were negative for antibodies against PPRSV (enzyme-linked immunosorbent assay; ELISA), PRV (competitive ELISA; cELISA), Brucella abortus (buffered acidified plate antigen [BAPA] serum agglutination), and seven common Leptospira interrogans serovars (microagglutination). However, fetal sera tested positive for antibodies to PCV2 (indirect fluorescent antibody; IFA). Thus, the fetuses must have been infected at > 70 days of gestation, when a fully competent fetal immune system, capable of responding to pathogen exposure, has developed.
Since the first diagnostic submission was inconclusive, and increased stillborn- and mummified-fetus rates were still occurring, additional fetuses and placentas (55 days gestation to full term) from two litters were submitted to ADDL April 25, 2008. Results were again negative for PRRSV in fetal tissues (PCR, VI) and negative for Leptospira interrogans serovars via fluorescent antibody test on a tissue section (FATS). Tissues were negative for PCV2 by FATS and for PPV by VI, but tissues and thoracic fluid were positive for PCV2 by PCR (Figure 1).
After an acute spike in the number of mummified fetuses the last week of May, additional fetuses and placentas were submitted to ADDL, ranging from approximately 80 days gestation to full term. While fetal sera were positive for PPV antibodies (cELISA), sera were negative for PCV2 antibodies (IFA) and pooled tissues were negative for PCV2 by PCR. Diagnostic tests were also negative for PRRSV (PCR, VI, and serum ELISA) and Leptospira interrogans serovars (microagglutination). No viral particles were detected on negatively stained tissues examined by electron microscopy.
Before a full diagnostic report was received on the third
submission, all bred and non-bred gilts in the breeding and
gestation barn were vaccinated for PCV2 with Ingelvac CircoFlex
(Boehringer Ingelheim Vetmedica, Inc, St Joseph, Missouri)
(Figure 1). Vaccination efforts focused solely on PCV2, the
pathogen initially identified, and on gilts only, as clinical signs
were predominantly in first-parity litters.
After the third diagnostic submission confirmed that fetuses were seropositive for PPV, another set of fetuses (approximately 55 days gestation to full term), weak liveborn piglets, and placenta from PCV2-vaccinated gilts were submitted to ADDL on June 26, 2008. Fetal sera were positive for PCV2 (IFA) and fetal tissues were positive for PCV2 by PCR (“weak” positive) and fluorescent antibody. Myocardial tissue showed no histological lesions and was negative by immunohistochemistry (IHC) for PCV2. Fetal tissues were positive for PPV (VI and FATS), and fetal sera were positive for antibodies to PPV (cELISA). Again, PRRSV was not detected in fetal tissues by VI or PCR and sera were negative for Leptospira interrogans serovars (microagglutination). Sera from weak liveborn pigs were positive for PRRSV antibodies (ELISA), as was expected in a PRRS-positive stable herd in samples from pigs that had suckled colostrum.
Gross lesions in fetuses from all submissions consisted of excessive serosanguineous fluid in all body cavities, hepatomegaly, and subcutaneous edema. Histopathological lesions included pulmonary congestion and edema, hepatic congestion, lymphocytic perivacular cuffing in the brain, splenic congestion, and renal congestion. Microscopic placental lesions included mineralization and intracytoplasmic trophoblastic inclusions. These gross and histopathological lesions are consistent with those described for parvovirus abortions.2
After determining that PPV was associated with the reproductive failure on this farm, the entire breeding herd, including all gilts, sows, and boars, was vaccinated with FarrowSure Gold (Pfizer, Inc, Pfizer Animal Health, New York, New York) on July 2, 2008. Changes in gilt acclimatization protocols concerning PPV vaccination and exposure were implemented. Gilts in isolation were vaccinated at an older age than previously, in order to reduce the possibility of maternal antibody interfering with vaccination. Much emphasis was also placed on adequate PPV exposure through feedback of feces, placentas, and mummified fetuses from the sow farm to gilts in the isolation barn.
In an attempt to understand the PPV immunity in incoming replacement gilts, sera from 30 of the initial group of purchased gilts were tested for PPV by hemagglutination inhibition (HI) at the Iowa State University Veterinary Diagnostic Laboratory (ISU VDL), Ames, Iowa. Testing on this initial group of gilts was performed approximately 4 weeks after vaccination with a single dose of Parvo Shield L5E. These gilts had extremely high HI titers (1:1024 to >1:16,384), much higher than expected as a result of vaccination, suggesting a natural PPV infection.13 To gain a fuller understanding of the PPV status of purchased gilts coming from the source farm, blood samples were collected from 20 animals in the next incoming group of gilts on the day of delivery. Again, HI tests were performed at ISU VDL and titers were very high (1:1024 to 1:8192), suggesting natural infection.
During the period when reproductive failure was occurring in the sow herd, two boars exhibited orchitis and diminished semen quality, and two additional boars exhibited diminished semen quality. Since both PPV and PCV2 can be shed in semen,1,14,15 semen and serum samples were submitted to ADDL. Semen was PCR-negative for both PPV and PCV2, but all boars were seropositive for both PPV (cELISA) and PCV2 (IFA). These boars had been vaccinated for both PCV2 and PPV approximately 18 and 6 months prior to testing, respectively. Sera were negative for B abortus antibodies (BAPA serum agglutination).
As the clinical problems progressed, the farm staff noted decreased abdominal girth in numerous gilts that had been diagnosed pregnant. These gilts did not appear to be pregnant on their expected farrowing date. Some farrowed an entire litter of mummified fetuses near their expected farrowing date, while others did not farrow until up to 3 weeks after their expected farrowing date, with litters of only a few mummified fetuses.
The reproductive failure affecting this herd had a dramatic impact on farrowing productivity. PigCHAMP Care 3000 (PigCHAMP, Inc, Ames, Iowa) production data in 2008 indicated a herd average mummified fetus rate of 3.3% between January 1 and May 26. In contrast, between May 26 and July 26, the herd rate was 7.7%. During this period, the rate in gilts rose acutely from 3.9% to 31.5% (Figure 2), while in multiparous sows, it rose only slightly from 3.2% to 4.2%. The percentage of mummified fetuses peaked the week of June 23 (week 26 of 2008), when 61.0% of pigs from parity-one litters were mummified. Many parity-one females farrowed entire litters of mummified fetuses.
Figure 2: Statistical process control charting demonstrating the dramatic spike in percent mummified fetuses seen exclusively in gilt litters in a 2400-sow commercial herd co-infected with porcine parvovirus (PPV) and porcine circovirus type 2 (PCV2). P1 = first parity; P2+ = second and higher parities. 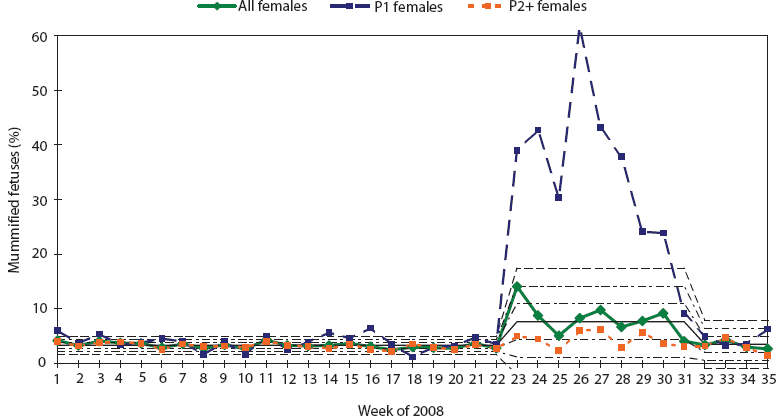 |
Numbers of total-born and liveborn pigs for parity-one females decreased from 11.6 and 10.2 pigs per litter, respectively, for the period of January 1 to May 26, to 9.7 and 5.9 pigs per litter, respectively, for the period of May 26 to July 28 (weeks 21 to 31 of 2008). Figure 3 shows the decrease in total-born pigs in parity-one females, which may indicate resorption of embryos infected before day 30 in utero. In multiparous females, production data showed little effect on mummification.
Figure 3: Statistical process control charting of total
born per litter in the herd described in Figure 2 shows the
decrease in total born in gilt litters (P1) indicating absorption
of embryos before 30 days gestation and a marked decrease in litter
size. 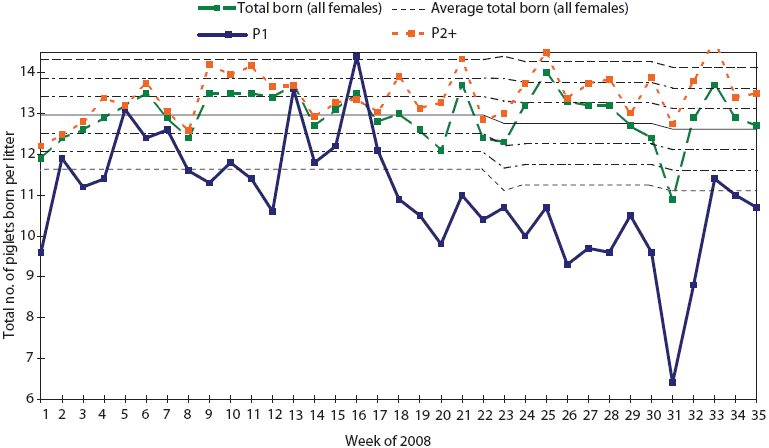 |
PigCHAMP Care 3000 production data for 2008 indicated 77 mid- to late-term abortions between January 1 and August 4, predominantly in bred gilts. The number of abortions increased from normal levels starting May 26 (Figure 4), following a trend similar to that of the mummified fetus rate.
Figure 4: Mid- to late-term abortions between January 1 and August 2 (weeks 1 to 31 of 2008), predominantly in bred gilts, in the herd described in Figure 2. 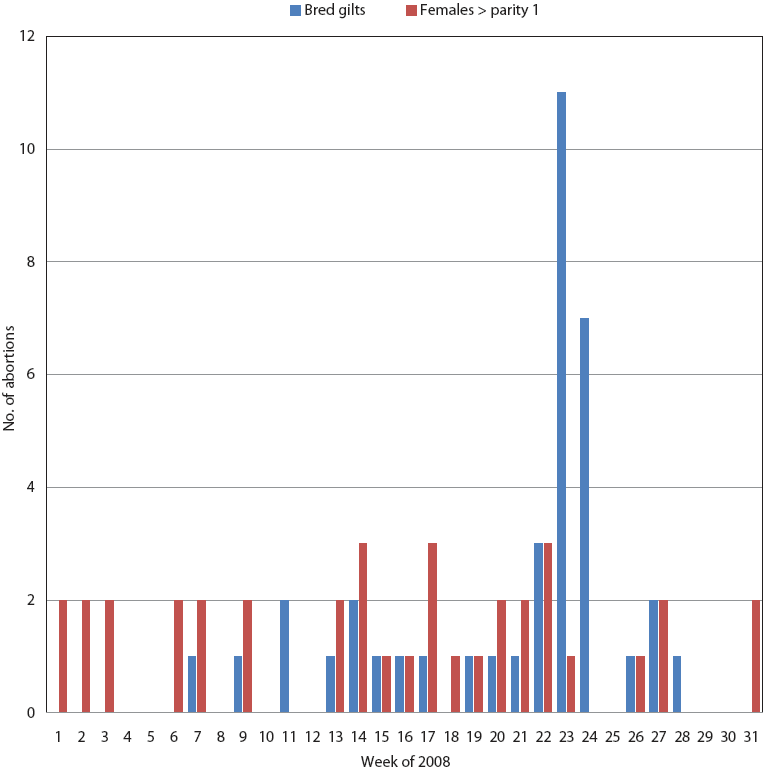 |
Discussion
Reports of reproductive failure due to PPV have diminished over the past several years, but recent reports have generally been in high-health-status herds or farms undergoing a PRRSV-elimination process.16 In contrast, reproductive failure due to PCV2 has been reported in newly-established gilt herds or herds undergoing a source or facilities change.4-11 None of these scenarios were involved in this case. Although this herd did undergo a gilt-source change, clinical signs were observed in the sow herd before the purchased replacement gilts entered the herd.
Maternal antibodies to PPV are long-lasting and can often interfere with vaccination.2 In this case, since the serological status of the replacement gilts raised on this farm was unknown at the time of vaccination, we do not know if maternal antibody interference was a factor in the development of inadequate immunity. The affected animals were replacement gilts raised on the farm. The purchased replacement gilts, which demonstrated very high PPV HI titers, indicating natural exposure and adequate protection, entered the herd after the outbreak and never exhibited reproductive failure.
Vaccination compliance is always a consideration in a case such as this, although the manager giving all PLE vaccinations on this farm was typically very diligent in his work. Vaccine failure must also be considered, as this farm changed PLE vaccines in November 2007. The gilts in isolation during this time would have been bred starting in January 2008 and farrowed starting in May 2008, the approximate period when clinical signs were first observed in the sow farm. Infection with PCV2 at the time of vaccination with modified-live PRRS vaccine in growing pigs can interfere with the immune response to the vaccine.12 It is plausible that a similar phenomenon may have occurred in breeding animals infected with PCV2 at the time of PPV vaccination, ie, they may not have developed adequate immunity, making them vulnerable to the PPV circulating in the sow herd.
Facility sanitation procedures may lead to poor natural exposure of gilts to endemic pathogens, which would leave them more vulnerable to infections during gestation. Strict biosecurity protocols implemented to control other viruses, multi-site production, and segregation of age groups within the herd may have decreased the probability of natural exposure to PPV. Commercially available disinfectants in the formaldehyde, paraformaldehyde, gluteraldehyde, and hypochlorite classes are expected to be effective at inactivating parvoviruses.17 Many of these disinfectants are commonly used in production systems today to inactivate other viruses (eg, PRRSV and PCV2). Retrospectively, it is impossible to know exactly why gilts in this herd apparently had inadequate immunity to PPV and exhibited reproductive failure.
Porcine circovirus type 2, which has previously been implicated as the sole cause of reproductive failure,9 was present in tissue submissions from this case. The PCR result was reported as a “weak” positive. Diffuse nonsuppurative and necrotizing myocarditis with fibrosis and mineralization, usually observed in cases of PCV2-associated reproductive failure,4-11 were not observed. The role of PCV2 in this case is uncertain, but subclinical PCV2 infection may have acted synergistically with PPV to increase the severity of the outbreak. Alternatively, subclinical PCV2 infection or immunosuppression may have decreased the efficacy of the PPV vaccine, as has been demonstrated in growing pigs vaccinated with PRRS vaccine at the time of PCV2 infection.12
Co-infections of PCV2 and PPV under experimental and natural conditions have resulted in porcine circovirus associated diseases in growing pigs.18-20 Co-infections of PPV and PCV2 in utero have also been known to enhance the lesions seen in aborted piglets over those observed with PCV2 infection alone.21,22 Thus, the synergistic effects of PPV and PCV2 may have contributed to the unusually high percentage of mummified fetuses. The high percentage of mid- to late-term abortions in this case is commonly observed in PCV2 infections, but not in PPV infections. Although the exact role of PCV2 cannot be definitively determined in this case, it was consistently identified by diagnostic testing of tissues and serum, indicating exposure.
The role of semen and venereal spread of PCV2 and PPV must also be considered in this case. Both PPV and PCV2 may be shed sporadically in the semen of infected animals.2,14 Gilts in this herd were not in direct contact with multiparous animals, but they were apparently exposed to PPV and PCV2 quickly and very uniformly. It is plausible that the infection spread via a venereal route through semen from the on-site boar stud. Semen was not tested for PPV and PCV2 until several months after the affected animals had been bred. Even though semen tested negative for both pathogens, these agents are spread intermittently,2,14 and may have been shed during the problematic time period. It is uncertain whether reproductive failure can be caused by PPV-infected semen, although there is strong circumstantial evidence that this may occur.2
The infectivity of PCV2-positive semen has been examined. Recently, PCV2-positive semen from artificially infected boars was infective in a swine bioassay model.15 When this semen was used to artificially inseminate naive gilts, they did not become viremic and farrowed clinically normal litters of pigs.15 However, when naive gilts were inseminated with semen deliberately spiked with PCV2, the gilts became viremic, seroconverted to PCV2, and farrowed litters of mostly mummified fetuses. In these fetuses, myocardial samples were IHC-positive for PCV2.23 It is unknown how much PCV2 may be shed in semen from a natural infection and whether this amount is sufficient to induce reproductive failure under field conditions. Regardless, venereal transmission must be considered in cases such as this.
Implications
- Vaccination protocols need to include PPV, a ubiquitous organism that is still a significant cause of reproductive failure.
- While testing replacement gilts for PPV prior to breeding-herd entry might identify poor immunity before reproductive problems occur, this is not financially justifiable for most herds.
- As infection of fetuses with PPV may be only 10% to 50%, multiple fetuses per litter, multiple submissions, or both may be required for diagnostic testing when reproductive failure occurs.
- The role of PCV2 as a co-factor in reproductive failure is unknown, but deserves further investigation.
References
1. Almond GW, Flowers WL, Batista L, D’Allaire S. Diseases of the reproductive system. In: Straw BE, Zimmerman JJ, D’Allaire S, Taylor DJ, eds. Diseases of Swine. 9th ed. Ames, Iowa: Blackwell Publishing; 2006:113–147.
2. Mengeling WL. Porcine parvovirus. In: Straw BE, Zimmerman JJ, D’Allaire S, Taylor DJ, eds. Diseases of Swine. 9th ed. Ames, Iowa: Blackwell Publishing; 2006:373–385.
3. Mengeling WL. Porcine parvovirus. In: Pensaert MB, ed. Virus Infections of Porcines. New York, New York: Elsevier Science; 1989:83–93.
4. Mauch C, Bilkei G. Porcine circovirus (PCV) associated losses in pregnant gilts. Pig J. 2004;53:69–74.
*5. Sanford SE. PCV2 related reproductive failure in startup herds. Proc 17th IPVS. Ames, Iowa. 2002;1:171.
6. Opriessnig T, Meng X, Halbur P. Porcine circovirus type 2-associated disease: Update on current terminology, clinical manifestations, pathogenesis, diagnosis, and intervention strategies. J Vet Diagn Invest. 2007;19:591–615.
7. Ladekjær-Mikkelsen J, Storgaard T, Bøtner A, Allan G, McNeilly F. Transplacental infection with PCV2 associated with reproductive failure in a gilt. Vet Rec. 2001;148:759–760.
8. O’Connor B, Gauvreau H, West K, Bogden J, Ayroud M, Clark EG, Konoby C, Allan G, Ellis JA. Multiple porcine circovirus 2-associated abortions and reproductive failure in a multisite swine production unit. Can Vet J. 2001;42:551–553.
9. Pittman JS. Reproductive failure associated with porcine circovirus type 2 in gilts. J Swine Health Prod. 2008;16:144–148.
10. West KH, Bystrom JM, Wojnarowicz C, Shantz N, Jacobson M, Allan GM, Haines DM, Clark EG, Krakowka S, McNeilly F, Konoby C, Martin K, Ellis JA. Myocarditis and abortion associated with intrauterine infection of sows with porcine circovirus 2. J Vet Diagn Invest. 1999;11:530–532.
*11. Josephson G, Charbonneau G. Case report of reproductive problem in a new startup operation. J Swine Health Prod. 2001;9:258–259.
12. Opriessnig T, McKeown NE, Harmon KL, Meng XJ, Halbur PG. Porcine circovirus type 2 infection decreases the efficacy of a modified live porcine reproductive and respiratory syndrome virus vaccine. Clin Vacc Immunol. 2006;13;923–929.
*13. Goyal SM. Porcine parvoviral serology. Proc Allen D. Leman Swine Conf. St Paul, Minnesota. 1994;63–64.
14. Larochelle R, Bielanski A, Muller P, Magar R. PCR detection and evidence of shedding of porcine circovirus type 2 in boar semen. J Clin Microbiology. 2000;38:4629–4632.
*15. Madson D, Opreissnig T, Kuster C, Pal N, Ramamoorthy S, Meng XJ, Halbur P. Shedding of porcine circovirus type 2 by boars and the role of PCV-2 in semen transmission. Proc AASV. San Diego, California. 2008;129–135.
*16. Bower B. Porcine parvovirus: A field investigation into vaccine failure. Allen D. Leman Swine Conf. St Paul, Minnesota. 2001;105–107.
17. The Antimicrobial Spectrum of Disinfectants. The Center for Food Security and Public Health. Available at: http://www.cfsph.iastate.edu/BRM/resources/Disinfectants/AntimicrobialSpectrumDisinfectants1207.pdf. Accessed 12 Mar 2009.
18. Ellis JA, Brantanich A, Clark EG, Allen G, Meehan B, Haines DM, Harding J, West KH, Krakowka S, Konoby C, Hassard L, Martin K, McNeilly F. Coinfection by porcine circoviruses and porcine parvovirus in pigs with naturally acquired postweaning multisystemic wasting syndrome. J Vet Diagn Inv. 2000;12:21–27.
19. Krakowka S, Ellis JA, Meehan B, Kennedy S, McNeilly F, Allan G. Viral wasting syndrome of swine: experimental reproduction of postweaning multisystemic wasting syndrome in gnotobiotic swine by coinfection with porcine circovirus 2 and porcine parvovirus. Vet Pathol. 2000;37:254–263.
20. Allan GM, Kennedy S, McNeilly F, Foster JC, Ellis JA, Krakowka SJ, Meehan BM, Adair BM. Experimental reproduction of severe wasting disease by co-infection of pigs with porcine circovirus and porcine parvovirus. J Comp Path. 1999;121:1–11.
*21. Altherr B, Zimmermann P, Etschmann B, Ritzmann M, Heinritzi M, Selbitz HJ, Truyen U. Detection of porcine Circovirus type 2 (PCV2) and porcine parvovirus (PPV) in aborted fetuses. 4th Int Symp Emerg Re-emerg Pig Dis. Rome, Italy. 2003;218–219.
22. Pescador CA, Bandarra PM, Castro LA, Antoniassi NAB, Ravazzolo AP, Sonne L, Cruz CEF, Dreimeier D. Co-infection by porcine Circovirus type 2 and porcine parvovirus in aborted fetuses and stillborn piglets in southern Brazil. Pesquisa Veterinária Brasileira. 2007;27:425–429.
*23. Madson D, Opriessnig T, Patterson A, Ramamoorthy S, Pal N, Halbur P. PCV2 and semen contamination – practical implications. Proc Iowa State Univ Swine Dis Conf. Ames, Iowa. 2008;77–80.
* Non-refereed references.