Neumann E, Simpson S, Wagner J, et al. Longitudinal field study of the effect of a commercial porcine circovirus type 2 vaccine on postweaning mortality in New Zealand farms. J Swine Health Prod. 2009;17(4):204–209
| Original research | Peer reviewed |
Cite as: Neumann E, Simpson S, Wagner J, et al. Longitudinal field study of the effect of a commercial porcine circovirus type 2 vaccine on postweaning mortality in New Zealand farms. J Swine Health Prod. 2009;17(4):204–209.
Also available as a PDF.
SummaryObjective: To evaluate the efficacy of a commercial porcine circovirus type 2 (PCV2) vaccine in reducing postweaning mortality in a multi-site field setting. Materials and methods: In 2007, a PCV2 vaccine recommended for use in piglets ≥ 4 weeks of age was made available in New Zealand for control of porcine circovirus associated disease (PCVAD). The effectiveness of vaccination in reducing mortality attributed to the disease was determined on nine farms that met the New Zealand case definition for postweaning multisystemic wasting syndrome (PMWS), a specific clinical expression of PCVAD. During 2006 and 2007, mortality data were collected from 138 groups of pigs affected by PMWS but not vaccinated and 67 groups affected by PMWS but immunized with PCV2 vaccine. Historical data were available from 130 groups on the study farms prior to the appearance of PMWS. Results: Average postweaning mortality prior to the appearance of PMWS was 2.86%. After farms were affected by PMWS, postweaning mortality averaged 10.38%, a response consistent with other reports of the disease. Subsequent to instituting use of PCV2 vaccine on these affected farms, mean postweaning mortality was 5.02%. Implications: Use of PCV2 vaccine is effective in reducing the level of postweaning mortality on farms affected with PMWS, although it may be unable to return mortality to levels seen prior to occurrence of the disease. | ResumenObjetivo: Evaluar la eficacia deuna vacuna comercial contra circovirus porcino tipo 2 (PCV2 por sus siglas en inglés) para reducir la mortalidad post destete en un escenario de campo de sitios múltiples. Materiales y métodos: En 2007, una vacuna de PCV2 recomendada para su uso en lechones ≥ 4 semanas de edad para el control de la enfermedad asociada al circovirus porcino (PCVAD) se puso a disposición en Nueva Zelanda. La eficacia de la vacunación para reducir la mortalidad atribuida a la enfermedad se determinó en nueve granjas que cumplían con la definición de Nueva Zelanda de caso de síndrome de desgaste multisistémico post destete (PMWS por sus siglas en inglés), una expresión clínica específica del PCVAD. Durante 2006 y 2007, se recolectaron los datos de mortalidad de 138 grupos de cerdos afectados por el PMWS pero no vacunados, y 67 grupos afectados por el PMWS pero inmunizados con la vacuna contra PCV2. Se contaba también con datos históricos de 130 grupos en las granjas de estudio, antes de la aparición del PMWS. Resultados: La mortalidad post destete promedio, antes de la aparición del PMWS, fue de 2.86%. Después de que las granjas fueron afectadas por el PMWS, la mortalidad post destete promedió 10.38%, parámetro consistente con otros reportes de la enfermedad. Después del establecimiento del uso de la vacuna contra PCV2 en las granjas afectadas, la mortalidad media post destete fue de 5.02%. Implicaciones: El uso de la vacuna contra PCV2 es eficaz para reducir el nivel de mortalidad post destete en granjas afectadas con PMWS, aunque puede ser incapaz de regresar la mortalidad a los niveles vistos antes de la incidencia de la enfermedad. | ResuméObjectif: Évaluer l’efficacité d’un vaccin commercial contre le circovirus porcin de type 2 (PCV2) à réduire la mortalité en post-sevrage dans un environnement à sites multiples. Matériels et méthodes: En 2007, un vaccin PCV2 avec utilisation recommandée chez des porcelets âgés de ≥ 4 semaines était disponible en Nouvelle-Zélande pour limiter la maladie associée au circovirus porcin (PCVAD). L’efficacité de la vaccination à réduire la mortalité attribuable à cette maladie a été déterminée sur neuf fermes qui rencontraient la définition néo-zélandaise du syndrome de dépérissement multi-systémique en post-sevrage (PMWS), une expression clinique spécifique du PCVAD. Pendant les années 2006 et 2007, les données de mortalité ont été colligées pour 138 groupes de porcs affectés par PMWS mais non vaccinés et 67 groupes affectés par PMWS mais immunisés avec le vaccin PCV2. Des données historiques précédant l’apparition de PMWS étaient disponibles pour 130 groupes sur les fermes faisant partie de l’étude. Résultats: La mortalité moyenne en post-sevrage avant l’apparition de PMWS était de 2.86%. Après que les fermes aient été affectées par le PMWS, la mortalité en post-sevrage dans les groupes était en moyenne de 10.38%, une observation en lien avec d’autres rapports de la maladie. Suite à la mise en place du vaccin PCV2 dans les fermes affectées par la maladie, la mortalité moyenne en post-sevrage était de 5.02%. Implications: L’utilisation du vaccin PCV2 est efficace pour réduire le niveau de mortalité en post-sevrage sur les fermes affectées par PMWS, bien qu’il puisse être impossible de revenir à des taux de mortalité observés avant l’apparition de la maladie. |
Keywords: swine, postweaning multisystemic wasting
syndrome, porcine circovirus type 2, porcine circovirus associated
disease, vaccine
Search the AASV web site
for pages with similar keywords.
Received: November 26, 2008
Accepted: February 3, 2009
Porcine circovirus associated disease (PCVAD), an important cause of morbidity and mortality across much of the world, is a result of infection with porcine circovirus type 2 (PCV2). However, additional infectious and noninfectious cofactors seem to be important in the severity and variability of clinical signs that are displayed on affected farms. Extensive descriptions of PCVAD and characteristics of the virus have been published.1 New Zealand has recognized the presence of PCV2 in pigs since at least 2003.2 While infection with the agent has been associated with various clinical signs, the current situation in New Zealand seems to most closely follow early descriptions of a specific manifestation of PCVAD called postweaning multisystemic wasting syndrome (PMWS). The first outbreak of PMWS in New Zealand occurred in the North Island in 2003, but between-farm spread was effectively limited through an industry-funded depopulation program of affected farms.3 A second outbreak of the disease occurred in the South Island early in 2006, and has been previously reported.4 According to recently published genotyping criteria, the PCV2 associated with 2006 and 2007 PMWS cases in New Zealand were genotype 1.4,5 The clinical presentation of PMWS in New Zealand was similar to that reported by other countries, despite New Zealand’s freedom from porcine reproductive and respiratory syndrome virus (PRRSV), classical swine fever virus, pseudorabies virus, and enteric Coronaviruses (porcine epidemic diarrhea and transmissible gastroenteritis).
Until vaccines became available, control strategies for PMWS in New Zealand focused primarily on implementation of good on-farm production practices, purchasing only pigs or replacement stock from farms known to be unaffected by the disease, and control of pathogens acting concomitantly with or secondary to PCV2.
Extensive research into development of PCV2 vaccines has occurred, and many techniques have been used to construct suitable vaccines for protection against PCVAD, including inactivated whole virus,6-8 chimeric PCV1-PCV2 virus,9 a DNA vaccine,10 and viral vector-based constructs expressing PCV2 capsid protein.11-14 After several years of accumulated experimental and field evidence, four commercial PCV2 vaccines became available in 2006 and 2007 and were variably distributed in parts of Europe, North America, and New Zealand. The four vaccines represented unique development efforts and resulted in products for use in breeding herds or young pigs, provided either one- or two-dose regimens, and delivered either subunit, chimeric whole-virus, or inactivated whole-virus preparations. One of these PCV2 vaccines was made available in New Zealand through a special-use importation permit in April 2007, and a study was implemented to determine the efficacy of the vaccine under field conditions.
Vaccine research that has already been published presented uniform agreement that the immune response directed against epitopes in the PCV2 capsid protein provides protection against PCVAD. However, as commercial registered PCV2 vaccines only became available in 2006, just recently have the results of field-based efficacy studies become available. Three peer-reviewed field studies of PCV2 vaccines were identified.12,15,16 In one study,12 a numerical but nonsignificant difference in postweaning mortality of 2.04% was detected between vaccinated and unvaccinated groups, but on average, pigs in the vaccinated group gained significantly more per day (18 g more per day, P < .001) than those in the unvaccinated group. In the two remaining studies,15,16 vaccinated groups had significantly less postweaning mortality (ADG; approximately 50% lower mortality, P < .01 in both studies) and greater average daily gains (greater by 40 to 50 g per day, P < .001 in both studies). All three of these studies were conducted on single-farm sites. Additional reports on the performance of PCV2 vaccines have been presented in abstract form at various veterinary conferences in 2006 through 2008.17-23 Improvements in postweaning mortality of 3 to 7 percentage points and increases in ADG of 20 to 30 g per day were typical.
The present study was designed to measure the effectiveness of PCV2 vaccination in reducing postweaning mortality on farms known to be affected by PMWS. This field study was conducted on multiple farms using a longitudinal design to compare historical mortality data accumulated from farms affected with PMWS with mortality data collected after PCV2 vaccination had been instituted. Historical mortality data was also available from some study farms to make a further assessment of the ability of the vaccine to return mortality to pre-PMWS levels.
Materials and methods
Description of farms and their enrolment
The PMWS outbreak region in New Zealand in 2006 involved both independent farms and farms that were epidemiologically related through routine inter-farm movement of pigs (integrated production). Veterinarians who had been involved in the outbreak were contacted before the PCV2 vaccine was made available for use in New Zealand and were asked to assist in collection of mortality data from client farms that they expected would use the vaccine. All producers using the vaccine were invited to participate in the study, but only those who could provide group-level postweaning mortality data were enrolled.
All study farms were located in the South Island, and were of typical commercial health status for New Zealand farms. With regard to specific pathogens suspected of contributing to the severity of PMWS, all farms in this study were Mycoplasma hyopneumoniae-positive (without vaccination), free of both systemic and enteric forms of salmonellosis, and free of swine influenza virus. Both outdoor- and indoor-housed herds were included in the study. The average age of pigs at weaning ranged from 28 to 30 days on each farm. Three farm-status categories were identified and applied to study groups for this longitudinal study: historical groups that had been reared prior to when the farm was diagnosed with PMWS (pre-PMWS), historical groups that had been reared after the farm had been diagnosed with PMWS (PMWS-affected), and groups that were established and reared after PMWS had been diagnosed on the farm but were vaccinated with PCV2 vaccine (PCV2-vaccinated) and then observed until slaughter.
A study group was defined as a group of pigs originating from a single-source farm (breeding-farrowing farm) and housed and managed as a cohort group. Specifically, a study group comprised pigs weaned in the same 7-day period, relocated to different production facilities as an intact group, and reared together in the same farm facility. On some individual farms, multiple study groups were maintained concurrently. In these instances, all groups on a given farm were of the same status (pre-PMWS, PMWS-affected, or PCV2-vaccinated). Pigs in the PCV2-vaccinated groups were vaccinated intramuscularly (IM) at 4 weeks of age with a single 2-mL dose of PCV2 vaccine (Suvaxyn PCV2 One Dose; Fort Dodge Animal Health, Fort Dodge, Iowa), according to the manufacturer’s instructions.
Vaccination began in study groups in April 2007, and data were collected from that point through December 2007. Total postweaning mortality data were collected throughout the growing period until pigs were sold to slaughter at approximately 22 weeks of age. All farms in this study met the New Zealand case definition for PMWS,3 a definition similar to that published by the European Union Sixth Framework Programme in 2005.24
Statistical analysis
Data for this study were stored in Microsoft Excel (Microsoft Corporation, Seattle, Washington) and statistical analyses were completed in R:A Language and Environment for Statistical Computing (epiR, pwr, nlme and base packages; R Foundation for Statistical Computing version 2.70; Vienna, Austria).
Total postweaning mortality was calculated by combining the nursery and finishing mortality data accumulated by each group of pigs prior to slaughter. Frequently, a single breeding farm contributed weaned pigs to multiple nursery-finisher groups, but in no case did nursery-finisher groups include pigs from multiple breeding farms. The identities of source farms (breeding farms) and finisher farms were maintained as separate variables in the statistical analysis. Total postweaning mortality was calculated as the sum of nursery deaths and finishing deaths divided by the starting inventory for the entire group at weaning. Death loss for each group was recorded by the producer; no effort was made to distinguish cause of death. On the basis of estimates of postweaning mortality from farms in New Zealand affected by PMWS,4 a sample size of 72 groups representing each farm-status category (pre-PMWS, PMWS-affected, and PCV2-vaccinated) would result in 80% power of detecting a 2% difference in mortality (pooled SD estimated at 4.88%) among group-status categories.
Mortality data were square-root-transformed prior to analysis in order to achieve a more normal distribution of values. Analysis of variance (ANOVA) was used to determine if mortality and starting-group inventory were different among pre-PMWS, PMWS-affected, and PCV2-vaccinated groups. A Tukey’s honestly significant difference comparison was utilized to identify significant differences among the three farm-status categories. Further analysis using a mixed regression model was performed to determine the effect of starting-group inventory, vaccination status, and PMWS status on group mortality. The source farm and finishing farm were included in the model as random effects and again, group mortality was square-root-transformed prior to analysis.
Results
Three farrow-to-finish farms, one farrow-to-wean farm, and five finishing-only farms were enrolled. The finishing-only farms received pigs at 28 to 30 days of age and raised them to 85 kg live weight or 22 weeks of age. When groups were established on the finishing-only farms, mortality that had occurred in the group during the nursery period at the source farm was recorded in order to determine the total postweaning mortality for the entire growing period (4 to 22 weeks of age). Mortality data were collected from 130 pre-PMWS groups, 138 PMWS-affected groups, and 67 PCV2-vaccinated groups (Figure 1).
Figure 1: Box-and-whisker plot of average postweaning mortality on New Zealand farms before the occurrence of postweaning multisystemic wasting syndrome (PMWS) (pre-PMWS; 130 groups), during the period when PMWS was occurring but pigs were not vaccinated against porcine circovirus type 2 (PCV2) (PMWS-affected; 138 groups), and during the period when PMWS was occurring but after PCV2 vaccination had been instituted (PCV2-vaccinated; 67 groups). 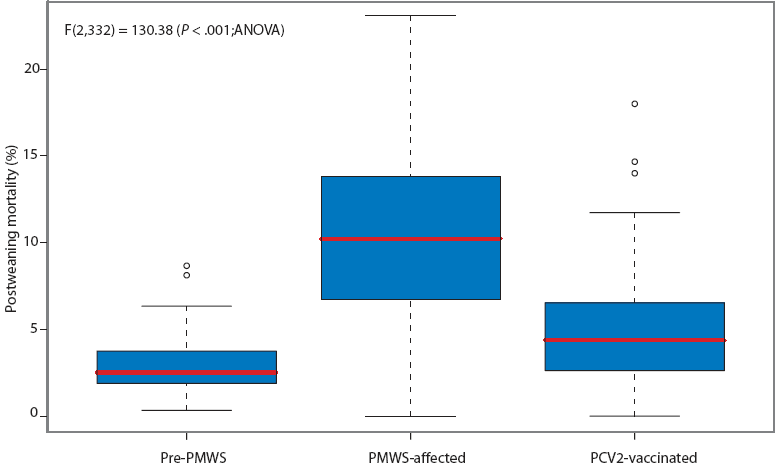 |
Mean starting pig inventory (± SD) was 382 ± 115 for pre-PMWS groups, 265 ± 65 for PMWS-affected groups, and 211 ± 103 for PCV2-vaccinated groups. Pairwise differences in mean starting inventories among the three farm-status categories were significant (P < .05). The longitudinal study design prevented control of the size of each group’s starting inventory because group-level housing facilities varied both within and between farms. The duration of the study did not allow for each group-level study unit to be repeated over time, which would have provided a more balanced starting inventory variable across farm-status categories. However, within a farm, no changes in pig-flow pattern nor physical changes to the farm’s facilities were instituted during the pre-PMWS, PMWS-affected, or PCV2-vaccinated study periods. Table 1 provides the number of groups and farm sources represented in each of the study periods.
Table 1: Number of study groups stratified by farm status (related to PMWS and PCV2 vaccination) and type of source farm from which groups originated in a study to determine effect of PCV2 vaccination on wean-to-finish mortality in nine New Zealand swine herds*
* Three study periods were identified: pre-PMWS, before herds were infected with PCV2; PMWS-affected, when herds were infected with PCV2 and were showing clinical signs of PMWS; and PCV2-vaccinated, when 4-week-old pigs were vaccinated with a PCV2 vaccine in herds infected with PCV2 and showing clinical signs of PMWS. Group integrity was maintained from weaning to slaughter. A total of three farrow-to-finish, one farrow-to-wean, and five finishing-pig-only farms were included in the study. † Groups originating from weaned-pig producer went to multiple different finishing sites. PMWS = postweaning multisystemic wasting syndrome; PCV2 = porcine circovirus type 2 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||
Mortality (± SD) was 2.86% ± 1.50%, 10.38% ± 5.05%, and 5.02% ± 3.62% in pre-PMWS, PMWS-affected, and PCV2-vaccinated groups, respectively. The overall result of the one-way ANOVA used to test differences in mortality was significant, ie, F(2,332) = 130.38 (P < .001). A Tukey’s comparison found that significant differences (P < .05) existed among the three status categories. A post-hoc analysis indicated the statistical power of the final experiment exceeded 99% (eta-squared = 0.50 for 67 groups in each of three status categories).
In the mixed model regression analysis, the effect of PMWS and of PCV2 vaccination on mortality were each significant at P < .001. Starting inventory differed significantly among study groups at P < .01. The final model retained all three variables and is described as predicted postweaning mortality = 0.1017 + 0.1569*(PMWS status)(P < .001) – 0.0926*(PCV2 vaccination status)(P < .001) + 0.0001*(starting inventory)(P < .01).
Discussion
The results of the current study indicate that the PCV2 vaccine was effective in reducing postweaning mortality of groups of pigs affected by PMWS. Postweaning mortality in vaccinated groups was 5.37 percentage points less than in historical cohort groups affected by PMWS, a reduction of 52%. This effect is consistent with PCV2 vaccine performance reported in other studies. Experimental reproduction of PMWS in a laboratory setting remains a challenge, as most infection models rely on exposure to both PCV2 and one or more other pathogens in order to cause clinical expression of the disease in a substantial number of the pigs.25-27 This makes evaluation of PCV2 vaccines a difficult task. However, PMWS in a field setting is well-described and widespread. This field efficacy study demonstrated that postweaning mortality on PMWS-affected farms was lower after PCV2 vaccination was incorporated according to the vaccine manufacturer’s instructions. While average mortality did not return to pre-outbreak levels, mortality in vaccinated groups was significantly lower than in affected groups that were not vaccinated, suggesting that PCV2 vaccination can be a very useful tool in managing outbreaks of PMWS.
The study design presented several issues that limit broad inference beyond the study population. First, historical mortality data could not be obtained from all study farms. While the regression modeling approach accounted for the effect of source farms to the extent possible, no historical data were available for two of the four source farms. As these farms contributed no historical cohort data for the pre-PMWS period, the overall mean mortality estimates for this period, compared to those for either the PMWS-affected or PCV2-vaccinated period, may be biased. However, as pig source was included as a random effect in the regression modeling approach, bias introduced into the estimated effects of PMWS or PCV2 vaccination on postweaning mortality was minimized to the extent possible. All farms in the study contributed PMWS-affected and PCV2-vaccinated data to the study, thus allowing comparison between these groups without bias. The primary objective of the study was to determine the effect of PCV2 vaccination on postweaning mortality in PMWS-affected herds. The comparison made between pre-PMWS and PMWS-affected groups helped to describe the severity of PMWS on the study farms – the mortalities reported in this study are similar to those reported in a previous description of the disease in New Zealand.4
Second, the effect of management factors as contributors to the severity of PMWS has been previously recognized,28 but an analysis of these factors was not included in this study of vaccine efficacy. The presence of these risk factors on the study farms may have contributed to the severity of PMWS that was reported, and elimination of these factors might have contributed to the eventual control of the disease (as measured by postweaning mortality), creating a possibility that the effect of vaccination on mortality was overestimated. The limited number of farms in the study prevented any meaningful analysis of these factors, aside from the uniform absence of several diseases known to contribute to the severity of PMWS (PRRSV, swine influenza, and salmonellosis) and the fact mycoplasmal pneumonia was known to occur on all study farms. Group size was included in the analysis, and while it had a statistically significant effect on mortality (larger groups were predicted to have higher mortality), the magnitude of this effect suggested it had only limited influence on the predicted final postweaning mortality. To date, all farms in the study have continued to use the PCV2 vaccine. Development of criteria or protocols for determining when and if vaccination should be discontinued remains an important area of future research.
Third, mortality recording was potentially biased, as farmers self-reported the mortality data that was analyzed. To minimize data integrity issues, study investigators made two site visits to all farms in the study and were in contact with farm managers by telephone and e-mail at approximately 60-day intervals during the study. Cause of mortality was not distinguished. An assumption was made that the extent of mortality unrelated to PMWS remained stable prior to and after the occurrence of PMWS, and that increases in mortality were most likely related to PMWS. While disease pressure on swine farms only rarely remains static, the New Zealand swine industry does have the benefit of minimal exposure to highly contagious viral diseases known to be transmitted by aerosol or through local area spread mechanisms. Bacterial pathogens, including Actinobacillus pleuropneumoniae, Lawsonia intracellularis, and Haemophilus parasuis, are typical of the important pathogens in the industry for which direct contact (rather than aerosol or local spread) are the key means of transmission. No farms in the current study changed their source of replacement females, semen, or weaned pigs (all weaned pigs were directly moved from farm to farm) during the pre-PMWS, PMWS-affected, and PCV2-vaccinated study periods. Given the disease profile of the industry and the absence of atypical pig movements onto the study farms, a decision was made to assume that changes in postweaning mortality during the study periods were most likely due to the occurrence of PMWS or PCV2 vaccination. As the exact mechanism of PCV2 in causing mortality has yet to be described (and experimental PCV2-infection models confirm the limited ability of PCV2 alone to cause mortality), utilizing crude mortality as an outcome variable provided the best measure of vaccine efficacy. Given our current limited knowledge of PCV2 pathogenesis and its subsequent mechanism of causing death, attributing cause of death to one endemic pathogen versus another in the context of this field trial had limited value. Much of the New Zealand pig industry operates under a continuous-flow production style. Keeping an accurate record of mortality, growth rates, or feed conversion efficiency is difficult in a continuous-flow setting, and for this reason, several herds that used the PCV2 vaccine were not enrolled in the study. All producers using the vaccine were considered for enrolment in the study, but only those who demonstrated reliable, group-level recordkeeping systems were enrolled. It must be considered that producers for whom good recordkeeping was a normal part of farm management may also have had better than average overall management and husbandry skills, and this might introduce a potential bias into this study. The degree to which this bias actually occurred is unknown.
The extent to which study farms were already recovering from the most acute and severe peak of the PMWS outbreak by the time PCV2 vaccination was applied is unknown. Each farm in the study was first diagnosed with the disease between January and April of 2006, 12 to 16 months prior to instituting PCV2 vaccination. Previous reports of PMWS suggested that the epidemic stage of the disease peaks at 46 to 159 days (95% CI, 122-195 days).29 However, in the same study, mortality on only four of 45 affected herds returned to normal or below normal levels, suggesting the clinical affects of PMWS are long-lasting. All farmers in the current study voluntarily chose to institute PCV2 vaccination at their own expense, suggesting that the clinical effects of PMWS on their farms were substantial and ongoing.
No farms in New Zealand were using PCV2 vaccines unless they had also been previously diagnosed with clinical PCVAD, so no opportunity was available to evaluate the effect of PCV2 vaccine in negative-control pigs. Similarly, at the time this study was initiated, anecdotal evidence was accumulating in other countries supporting the efficacy of PCV2 vaccines, and no study participants were willing to leave unvaccinated groups on their farms as negative controls. Indeed, given the severity of the PMWS outbreak in 2006 in New Zealand,4 affected producers were anxious to try any strategy available to reduce the ongoing production losses on their farms.
The results of this study support other peer-reviewed and anecdotal reports of the efficacy of PCV2 vaccines in controlling PMWS and other PCVAD. In pigs 7 to 12 weeks of age, PMWS remains the most prevalent clinical expression of PCVAD in the pig industry in New Zealand. This is contrary to evidence from the United States, whereby PCV2 was most commonly identified as a constituent of porcine respiratory disease complex during the mid-finishing phase.1 However, PMWS in nursery pigs remains an important manifestation of PCVAD in parts of Europe, with lesser significance in North America.
Implications
- Under the conditions of this study, PCV2 vaccine administered to 4-week-old pigs on farms known to be affected by PMWS significantly reduces postweaning mortality.
- Vaccination with PCV2 vaccine may be unable to return mortality to levels seen prior to occurrence of PMWS.
Acknowledgements
The authors would like to thank Pacificvet Ltd and Fort Dodge Animal Health for financial support. Additionally, the participation and support of Selwyn Dobbinson, Bruce Welch, Steve Lucas, and Ian McIntosh in accumulating data from study farms were appreciated. Finally, cooperation of the owners of the farms involved in the study is gratefully acknowledged.
References
1. Opriessnig T, Meng XJ, Halbur PG. Porcine circovirus type 2 associated disease: update on current terminology, clinical manifestations, pathogenesis, diagnosis, and intervention strategies. J Vet Diagn Invest. 2007;19:591–615.
2. Tham K, Hansen M. Detection of porcine circovirus types 1 and 2 in abattoir-slaughtered pigs in New Zealand. Surveillance. 2003;30:3–5.
3. Stone M. Response to first diagnosis of post-weaning multisystemic wasting syndrome. Surveillance. 2004;31:8–14.
4. Neumann EJ, Dobbinson SS, Welch EB, Morris RS. Descriptive summary of an outbreak of porcine post-weaning multisystemic wasting syndrome (PMWS) in New Zealand. N Z Vet J. 2007;55:346–352.
5. Grau-Roma L, Crisci E, Sibila M, Lopez-Soria S, Nofrarias M, Cortey M, Fraile L, Olvera A, Segales J. A proposal on porcine circovirus type 2 (PCV2) genotype definition and their relation with postweaning multisystemic wasting syndrome (PMWS) occurrence. Vet Microbiol. 2008;128:23–35.
*6. Pogranichniy R, Yoon KJ, Yaeger MJ, Vaughn EM, Harmon KL, Stammers R, Roof M. Possible prevention of PMWS using inactivated PCV2 vaccine in CDCD pigs. Proc IPVS Cong. Hamburg, Germany. 2004;55.
*7. Charreyre C, Beseme S, Brun A, Bublot M, Lapostolle B, Sierra P, Vaganay A. Protection of piglets against a PCV2 experimental challenge by vaccinating gilts with an inactivated adjuvanted PCV2 vaccine, Circovac: serological, clinical and pathological aspects. Proc IPVS Cong. Copenhagen, Denmark. 2006;176.
*8. Charreyre C, Beseme S, Brun A, Boblot B, Joisel F, Lapostolle B, Sierra P, Vaganay A. Vaccination strategies for the control of porcine circoviral diseases in pigs. Proc Int Conf Anim Circoviruses Assoc Dis. Belfast, Ireland. 2005;26–30.
9. Fenaux M, Opriessnig T, Halbur PG, Elvinger F, Meng XJ. A chimeric porcine circovirus (PCV) with the immunogenic capsid gene of the pathogenic PCV type 2 (PCV2) cloned into the genomic backbone of the nonpathogenic PCV1 induces protective immunity against PCV2 infection in pigs. J Virol. 2004;78:6297–6303.
10. Blanchard P, Mahé D, Cariolet R, Keranflec’h A, Baudouard MA, Cordioli P, Albina E, Jestin A. Protection of swine against post-weaning multisystemic wasting syndrome (PMWS) by porcine circovirus type 2 (PCV2) proteins. Vaccine. 2003;21:4565–4575.
11. Fort M, Sibila M, Allepuz A, Mateu E, Roerink F, Segales J. Porcine circovirus type 2 (PCV2) vaccination of conventional pigs prevents viremia against PCV2 isolates of different genotypes and geographic origins. Vaccine. 2008;26:1063–1071.
12. Fachinger V, Bischoff R, Jedidia SB, Saalmuller A, Elbers K. The effect of vaccination against porcine circovirus type 2 in pigs suffering from porcine respiratory disease complex. Vaccine. 2008;26:1488–1499.
13. Song Y, Jin M, Zhang S, Xu X, Xiao S, Cao S, Chen H. Generation and immunogenicity of a recombinant pseudorabies virus expressing cap protein of porcine circovirus type 2. Vet Microbiol. 2007;119:97–104.
14. Wang X, Jiang P, Li Y, Jiang W, Dong X. Protection of pigs against post-weaning multisystemic wasting syndrome by a recombinant adenovirus expressing the capsid protein of porcine circovirus type 2. Vet Microbiol. 2007;121:215–224.
15. Horlen KP, Dritz SS, Nietfeld JC, Henry SC, Hesse RA, Oberst R, Hays M, Anderson J, Rowland RR. A field evaluation of mortality rate and growth performance in pigs vaccinated against porcine circovirus type 2. JAVMA. 2008;232:906–912.
16. Kixmoller M, Ritzmann M, Eddicks M, Saalmuller A, Elbers K, Fachinger V. Reduction of PMWS-associated clinical signs and co-infections by vaccination against PCV2. Vaccine. 2008;26:3443–3451.
*17. Herin JB, Fily B, Longo S, Joisel F. Field results of the use of Circovac: a sow PCV2 vaccine in France under provisional license. Proc Int Symp Emerg Re-emerg Pig Dis. Krakow, Poland. 2007;125.
*18. Joisel F, Brune A, Schade E, Longo S, Charreyre C. Vaccination of sows and gilts against PCV2 diseases with CIRCOVAC (Merial): Field experience in Europe. Proc AASV. Orlando, Florida. 2007;311–314.
*19. de Grau AF, Jorgensen J, Thacker B, Francisco C, Wilson W, Schlueter R, Eggen A. Field performance of a conditionally licensed vaccine: Canadian experience. Proc AASV. Orlando, Florida. 2007;159–161.
*20. Desrosiers R, Clark E, Tremblay D, Tremblay R, Polson D. Preliminary results with Ingelvac CircoFLEX to protect multiple ages of Quebec pigs against PCVAD. Proc AASV. Orlando, Florida. 2007;143–145.
*21. Feldmann L, Cline G, Wilt V, Diaz E. Effects of porcine circovirus type 2 (PCV2) vaccination on mortality and average daily gain. Proc AASV. San Diego, California. 2008;317–319.
*22. Thacker B, Wilson W, Francisco C, Schlueter R. Circumvent PCV vaccine: Performance evaluation and serological studies update. Proc AASV. San Diego, California. 2008;153–156.
*23. von Richthofen I, Woolfenden N, Lischewski A, Strachan WD. Field efficacy study of a PCV2 vaccine in three week old piglets in the United Kingdom. Proc Int Symp Emerg Re-emerg Pig Dis. Krakow, Poland. 2007;122.
*24. Control of porcine circovirus diseases (PCVDs): Towards improved food quality and safety. PMWS case definition (Herd level) October 2005. Available at: http://www.pcvd.org/documents/Belfast_Presentations_PCVD/Final_pmws_case_definition_EU_October_2005.doc. Accessed 24 March 2009.
25. Opriessnig T, Fenaux M, Thomas P, Hoogland MJ, Rothschild MF, Meng XJ, Halbur PG. Evidence of breed-dependent differences in susceptibility to porcine circovirus type-2-associated disease and lesions. Vet Pathol. 2006;43:281–293.
26. Opriessnig T, Yu S, Gallup JM, Evans RB, Fenaux M, Pallares F, Thacker EL, Brockus CW, Ackermann MR, Thomas P, Meng XJ, Halbur PG. Effect of vaccination with selective bacterins on conventional pigs infected with type 2 porcine circovirus. Vet Pathol. 2003;40:521–529.
27. Opriessnig T, Patterson AR, Madson DM, Pal N, Halbur PG. Comparison of efficacy of commercial one dose and two dose PCV2 vaccines using a mixed PRRSV-PCV2-SIV clinical infection model 2-3-months post vaccination. Vaccine. 2009;27:1002–1007.
28. Elbers AR, de Jong MF, Wellenberg GJ. [Risk factors for clinical signs of PMWS and PDNS in pigs in The Netherlands: a case-control study]. Tijdschr Diergeneeskd. 2006;131:318–325.
29. Gresham A, Cook AJ, Thomson JR, Kennedy S. Survey of veterinary practitioners on PMWS and PDNS in the UK. Vet Rec. 2003;153:400–403.
*Non-refereed references.