Lewis CRG, Torremorell M, Bishop SC. Effects of porcine reproductive and respiratory syndrome virus infection on the performance of commercial sows and gilts of different parities and genetic lines. J Swine Health Prod. 2009;17(3):140–147
| Original research | Peer reviewed |
Cite as: Lewis CRG, Torremorell M, Bishop SC. Effects of porcine reproductive and respiratory syndrome virus infection on the performance of commercial sows and gilts of different parities and genetic lines. J Swine Health Prod. 2009;17(3):140–147.
Also available as a PDF.
SummaryObjectives: To examine the parity and genetic-line differences and trends for major sow reproductive traits during an outbreak of porcine reproductive and respiratory syndrome (PRRS) and to determine whether specific parities or genetic lines are more severely affected by PRRS virus. Materials and methods: Reproductive performance data (gestation length, total piglets in utero, total piglets born alive, total piglets born dead, total mummified piglets born, total stillborn, and total weaned) were obtained from a commercial herd in China (8098 litters from 1820 sows) from differing genetic lines (Landrace, Large White, Pietrain, Meishan, Duroc composite, and various crosses). The dataset was partitioned into a baseline and a disease phase. Statistical analysis compared performance differences between these two phases for sows of different parities and lines. Results: PRRS caused significant production losses, impacting most reproductive traits. However, the impacts were greater in early parities, eg, the numbers of mummified piglets per litter were greater for sows of lower parities (1 through 5) than older sows (parities 6 through 11), for which there were no differences between disease and baseline data. Line differences and interactions were also detected, highlighting a greater impact of PRRS virus on the Meishan line than on their European counterparts. Implications: Parity should be considered when examining host genetic resistance to PRRS virus and when designing management strategies. The Meishan line may be more susceptible to reproductive PRRS. The use of the Meishan-type line to impact fecundity traits in a disease situation should be reconsidered. | ResumenObjetivos: Examinar las diferencias de paridad y línea genética, y las tendencias de los parámetros reproductivos más importantes de las hembras durante un brote del síndrome reproductivo y respiratorio porcino (PRRS por sus siglas en inglés) y determinar si ciertas paridades ó las líneas genéticas son más severamente afectadas por el virus del PRRS. Materiales y métodos: Se obtuvieron los datos del desempeño reproductivo (duración de la gestación, total de lechones en el útero, total de lechones nacidos vivos, total de lechones nacidos muertos, total de lechones nacidos momificados, total de nacidos muertos, y total de destetados) de un hato comercial en China (8098 camadas de 1820 hembras) de líneas genéticas diferentes (Landrace, Large White, Pietrain, Meishan, Duroc, y varias cruzas). La base de datos se dividió en información de base y una fase de enfermedad. El análisis estadístico comparó las diferencias de desempeño entre las dos fases en hembras de diferentes paridades y líneas. Resultados: El PRRS causó pérdidas significativas de producción, impactando a la mayoría de los parámetros reproductivos. Sin embargo, los impactos fueron mayores en paridades jóvenes, ej, el número de lechones momificados por camada fueron mayores en las hembras de paridades jóvenes (1 a 5) comparados con las hembras adultas (paridades 6 a 11), para las cuales no hubo diferencias entre la información de base y la de enfermedad. También se detectaron interacciones y diferencias de línea, destacando un impacto mayor del virus de PRRS en la línea Meishan que en sus equivalentes europeas. Implicaciones: La paridad debería ser considerada al examinar la resistencia genética del huésped al virus de PRRS y al planear estrategias de manejo. La línea Meishan puede ser más susceptible al PRRS reproductivo. En una situación de enfermedad se debe reconsiderar el uso de la línea tipo Meishan para mejorar los parámetros de fecundidad. | ResuméObjectifs: Examiner les différences de parité et de lignée génétique et les tendances pour les caractères de reproduction principaux des truies lors d’un épisode de syndrome reproducteur et respiratoire porcin (PRRS) et déterminer si des parités ou des lignées génétiques spécifiques sont affectées plus sévèrement que d’autres par le virus du PRRS. Matériels et méthodes: Les relevés des performances de reproduction (durée de la gestation, nombre de porcelets in utero, nombre de porcelets nés vivants, nombre de porcelets mort-nés, nombre de porcelets nés momifiés, et le nombre de porcelets sevrés) ont été obtenus d’un troupeau commercial en Chine (8098 portées provenant de 1820 truies) de différentes lignées génétiques (Landrace, Large White, Pietrain, Meishan, Duroc composite, et plusieurs croisements). La base de données a été séparée en deux phases: niveau de base et phase de maladie. Les analyses statistiques ont comparé les différences de performance entre ces deux phases pour des truies de différentes parités et lignées. Résultats: Le PRRS a causé des pertes de production significatives, influençant la plupart des caractéristiques de reproduction. Toutefois, les impacts étaient plus grands sur les animaux de parité moins élevée, eg, le nombre de porcelets momifiés par portée était plus élevé pour les truies de parité moins élevé (1 à 5) que les truies plus âgées (parités 6 à 11), pour lesquelles il n’y avait pas de différence entre les données de base et celles de la phase de maladie. Des différences de lignée et des interactions ont également été détectées, faisant ressortir un plus grand impact du virus PRRS sur la lignée Meishan comparativement aux lignées européennes. Implications: La parité des animaux devrait être considérée lors de l’évaluation de la résistance génétique de l’hôte envers le virus PRRS et lors de l’élaboration de stratégies de gestion. La lignée Meishan pourrait être plus susceptible aux problèmes reproducteurs associés au PRRS. L’utilisation de la lignée de type Meishan pour influencer les caractères de fécondité dans une situation de maladie devrait être reconsidée. |
Keywords: swine, porcine reproductive and respiratory syndrome,
parity, breed, PRRS
Search the AASV web site
for pages with similar keywords.
Received: March 9, 2008
Accepted: August 26, 2008
Porcine reproductive and respiratory syndrome (PRRS) is a viral disease that has major effects on the reproductive performance of sows and gilts.1 Many studies have sought to understand the complex interaction between the pathogen and the host2,3 with a view to developing new control strategies. More recently, evidence of host genetic variation for disease resistance or tolerance has been presented by various authors, as reviewed by Lewis et al.4
This study utilizes a dataset from which the genetic parameters for traits associated with PRRS virus (PRRSV) tolerance were estimated.5 However, in the study of Lewis et al,5 little attention was paid to management factors that the stockman has immediate control over, and this study fills that gap. As PRRS is such an economically important disease to swine populations globally, it is important that on-farm management practices are appropriate and efficient when PRRSV is present. Improvements in straightforward management procedures may reduce many of the disease-associated costs. From a commercial-production perspective, an interaction between disease impact and sow parity could influence many on-farm management decisions, such as deciding when to cull sows. A parity effect can have major implications, as associated costs from “empty days” (days not pregnant) and smaller litter sizes (owing to fewer piglets born alive in higher parities) mean that fixed sow costs will be spread over fewer piglets, and ongoing maintenance costs of the sow will increase. These cost issues are increasingly important as overall farm incomes decline and gross margins per sow are reduced. This study seeks to examine the influence of sow parity and the broader effects of genetic line on reproductive traits during a PRRS outbreak. Because the study was conducted in a commercial herd, other unknown agents or management factors might also have contributed to the observed traits.
Materials and methods
Datasets
The data used were collected from a commercial multiplication herd (farrow-to-finish and multi-site production practices) located in China. Highly trained stockpeople continually recorded the main herd descriptors and reproductive traits, ie, gestation length, total piglets born alive, total piglets born dead, total piglets in utero (measured as total piglets born alive plus total piglets born dead), total mummified piglets, total stillborn, pre-weaning mortality, and total weaned. The available data, collected over 10 years, spanned two PRRS outbreaks. Data were edited to exclude animals from genetic lines that included few sows or zero observations over many variables. The resultant dataset after editing consisted of 8098 litters from 1820 sows and from 11 distinct genetic lines, including purebred Landrace, Large White, Pietrain, Duroc composite, Meishan, and various crosses among these named breeds. Full pedigree information (five generations) was also available for every animal, for a total of 4104 animals in the pedigree. This dataset was originally utilized for genetic-parameter estimation, and the full description of the data handling is reported by Lewis et al.5
Individual litters in the dataset were assigned to two treatment groups, baseline and disease. Ideally, this would have been performed by individually testing the sows to determine if they had faced a PRRS virus challenge and had become infected. This was not possible in a retrospective study. Two methods were used to partition the data into the baseline and disease phases.
The first, termed threshold-threshold, was used to create the disease dataset. This method was based on trends in the underlying performance using a threshold cut-off point for a related production trait, separating periods of high disease impacts and normal production levels. The relevant trait of interest in this case was number of mummified piglets per litter, as increases in this trait are commonly associated with PRRS outbreaks.6 When the 30-day rolling average for this trait was above the approximate 99% confidence threshold (defined as μ + 3σ, where m and s are the baseline mean and standard deviation of the raw data, respectively), a division was made (thus defining the start of the outbreak), and subsequent litters were allocated to the threshold-threshold dataset. The end date for the epidemic was defined when the 30-day rolling average fell below the threshold (thus returning to normal bounds).
A date-threshold method was used to create the baseline dataset. This dataset was defined using dates determined by on-farm random monthly serological testing of samples collected from 30 breeding sows. Samples were tested using the HerdChek 2XR PRRS ELISA (Idexx Laboratories, Westbrook, Maine), with a sensitively of 97.4% and a specificity of 99.6%.7 Clinical signs of PRRS were also monitored by herd veterinarians. Positive ELISA and clinical results were confirmed by additional testing using both serology (HerdChek ELISA) and polymerase chain reaction (PCR). The cutoff point for the date-threshold dataset was defined by identifying the first day of the month of a confirmed outbreak (determined by the random ELISA testing) and then moving back 5 full months to account for a complete gestation period, a standard lactation period, sow dry time (period between lactation and gestation), and the approximate period of 10 days for seroconversion of the sow post infection.8 Data were then removed to eliminate any “gray” period where the infectious status of the herd may have been unknown. The end of the disease phase (and thus the start of the next baseline phase as the data shows the herd returning to previous production bounds) was defined using the threshold approach as described (trait m + 3s).
Management during PRRS outbreaks
PRRS outbreaks during the period of study occurred in 2002 and 2006 and lasted 5 and 10 months, respectively. The disease dataset for analysis consisted of the pooled data from these two disease periods, and the baseline dataset was collated from all baseline periods. After the first PRRS outbreak, the herd was managed to return it to PRRS-negative status by stopping introduction of replacement animals for a period of time and waiting for the remaining sows to return to PRRS-negative status (defined both by clinical signs and ELISA-negative results). The management techniques utilized are fully described by McCaw9 and Torremorell et al.10 The reproductive traits observed during these PRRS outbreaks are considered to be influenced by PRRSV and not by another pathogen on the basis of diagnostic test results, clinical presentation, and the health history of the herd. The herd was under a well-established health program during both outbreaks. A veterinarian visited the farm bimonthly and herd-health status was monitored and assessed. The herd was monitored clinically for a number of diseases, with help of production parameters to identify variation from usual production levels, and sample tissues from affected animals were submitted to the diagnostic laboratory on an as-needed basis. The herd was also tested bimonthly using the HerdChek 2XR PRRS ELISA. A conventional vaccination protocol was established to meet Chinese market demands, including vaccines for foot-and-mouth disease, classical swine fever, pseudorabies, porcine parvovirus, Escherichia coli, several pathogens that cause pneumonia, and Japanese encephalitis. PRRS virus vaccines were not used.
Statistical analysis
Table 1 shows the distribution of the litters born for each parity used for our analysis. This consisted of 6355 litters of the total dataset due to missing data in the parameters used for mean estimation. The statistical analysis was performed using SAS (SAS Institute, Cary, North Carolina). A mixed model was fitted using disease status, parity, and sow line (plus two- and three-way interactions) as fixed effects, and individual sow and sow sire were fitted as random effects to account for the repeated measure of litter and underlying genetic effects. To comply with the assumptions of the mixed-model methodology, ie, that the trait residuals should be normally distributed, the traits of total piglets born dead, mummified, and stillborn were transformed by log(trait + 1) prior to statistical analyses, although the reported least squares (LS) means values were back-transformed to the observed scale. A further analysis was undertaken whereby we examined all interactions in the same model as before, but added an effect of European versus Chinese origin with Landrace, Large White, Pietrain, and composites classed as “European” origin, and Meishan and composite lines containing at least 25% Meishan genotypes classified as “Chinese” origin. Seasonality was also explored as a fixed effect; however, no significant effects were demonstrated.
Table 1: Numbers of litters within each parity in datasets* derived from 10 years of data from a commercial multiplication herd in China
* Data collected over a period that spanned two outbreaks of porcine reproductive and respiratory syndrome (PRRS). Data was partitioned into baseline and disease phases, with disease-phase data including two outbreaks of PRRS, which were defined by criteria based on numbers of mummified piglets and results of monthly serological testing (HerdChek 2XR PRRS ELISA; Idexx Laboratories, Westbrook, Maine).The dataset consisted of 6355 litters from 11 distinct genetic lines, including purebred Landrace, Large White, Pietrain, Duroc composite, Meishan, and various crosses among these named breeds. |
||||||||||||||||||||||||||||||||||||||||
Results
The results show that the PRRS outbreaks had a dramatic influence on production traits, as seen from the performance-trait LS means for the disease and baseline data subsets (Table 2) and the associated P values describing the significance of the difference. Most traits analyzed were significantly different in the baseline and disease phases, independent of parity. However, there were significant differences among parities for all traits, and genetic lines also differed for many of the traits, as would be expected due to the different selection histories for the different lines (ie, for different lines, selection pressures for multiple traits differed).
Table 2: Overall least squares (LS) means values (back-transformed) for baseline- and disease-phase data from a commercial multiplier herd during a 10-year period,* and fixed effect P values for reproductive traits when baseline and disease periods were compared in a mixed-model analysis
* Data and herd described in Table 1. † Data transformed by log(trait +1) for analysis. Total born dead = total mummified + total stillborn. ‡ Values back-transformed (for the transformed traits) to show actual values. Negative value, which effectively did not differ from zero, was due to use of LS means. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The results focused on in this study are the significant interactions between disease phase and parity for most traits. These interactions suggest that sows of different parities are affected differently by PRRSV. Also, the significant interactions between disease phase and genetic line indicate that different lines are also affected differently by PRRSV.
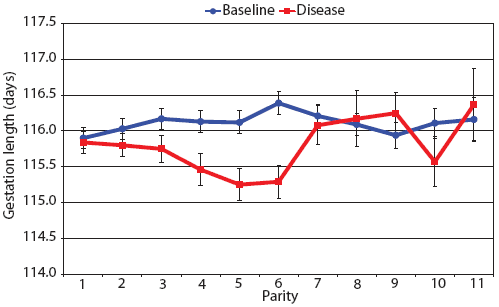
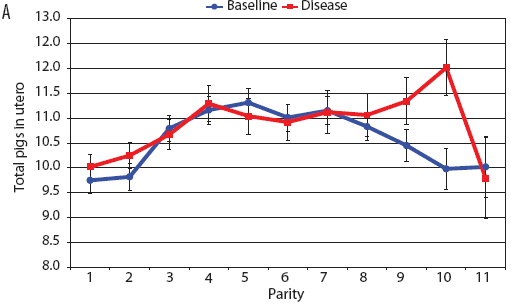
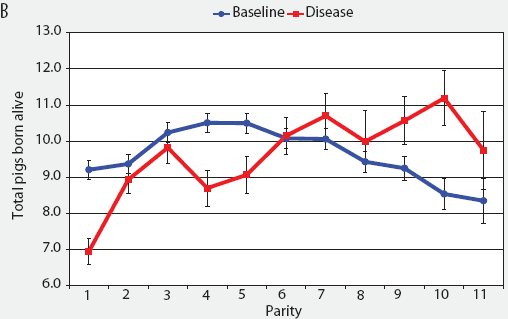
Consider the parity-by-disease phase interaction and its impact on reproductive performance, starting with gestation length and the total number of piglets in utero. Gestation-length effects are shown in Figure 1, which shows the mean gestation length by parity. Although the parity-by-disease-phase interaction was significant, the effects were small and no clear pattern was apparent. This is echoed in Figure 2A, where the total number of piglets in utero by parity was not strongly affected by disease phase. However, the total number of piglets born alive (Figure 2B) was markedly affected by both parity and disease phase, with differences due to disease phase being large in early parities and subsequently declining. In late parities, disease phase had no impact on the numbers of piglets born alive.
| Figure 1: Least squares means with standard error bars
for gestation length by parity for 6355 litters described in Table
1.
|
| Figure 2: Least squares means with standard error bars
for (A) total piglets in utero per litter (total piglets born alive
plus total piglets born dead) and (B) piglets born alive per litter
by parity for baseline- and disease-phase data (6355 litters)
described in Table 1.
|
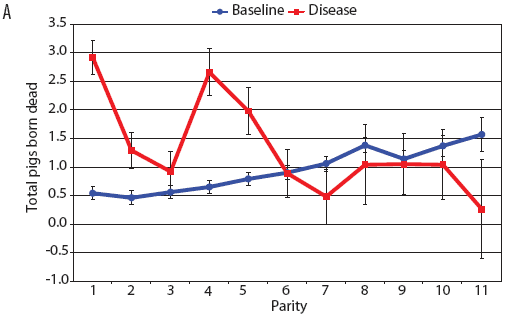
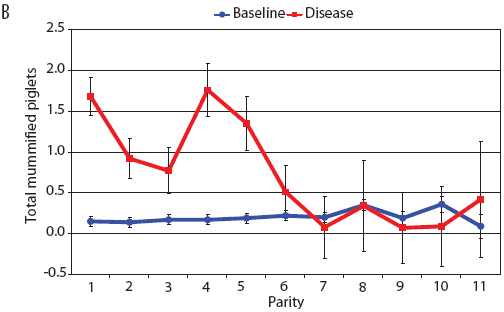
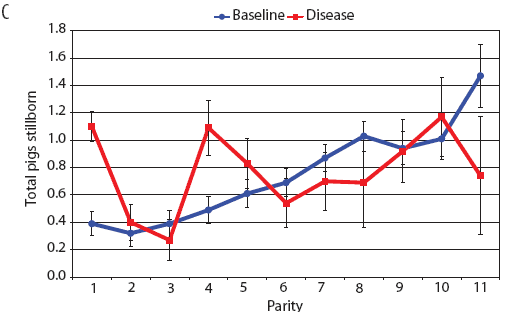
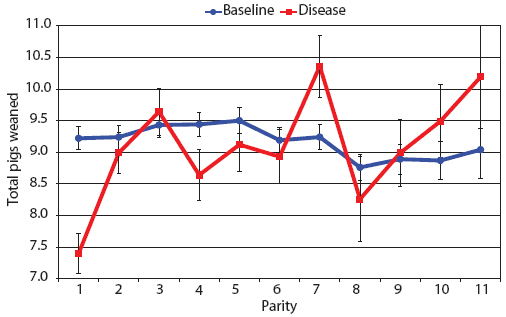
Examination of piglet deaths at parturition explained results seen for piglets born alive. Figure 3A shows the transformed mean total number of piglets born dead per litter. In the baseline data, the total piglets born dead steadily increased with parity. In the disease-phase data, the numbers of piglets born dead were high in early parities, but in late parities they were little different from those in baseline sows. When these data were dissected further in Figures 3B and 3C, showing transformed total mummified piglets per litter and transformed total stillborn piglets per litter, respectively, the data shows that the results for the total piglets born dead were due mainly to the number of mummified piglets per litter. PRRS had a large effect on the number of mummified piglets per litter in early parities (1 to 5), but the effects in higher parities (6 to 11) were small. Importantly, this result is not simply explicable by differences in litter size in older sows in the disease-phase dataset, ie, the same number of mummified piglets just being a larger proportion total piglets in utero (Figure 2A). The numbers of stillbirths in the disease-phase data generally followed the baseline trend (Figure 3C), although there were slightly more stillborn piglets in some of the early parities. The net impact in terms of the number of piglets weaned is shown in Figure 4. The number of piglets weaned was almost constant across parity in baseline sows, but was markedly affected by parity in the disease phase. Low-parity sows were badly affected by the PRRS outbreak; however, this effect was less prominent in older sows. These effects were more likely to be due to fewer piglets born alive to gilts (Figure 2B) and more piglets born dead (Figure 3A) than to greater post-parturition losses. It is worth noting that this difference was smaller for piglets weaned than piglets born alive. Analysis of piglets that died during lactation (ie, the number of piglets per litter that died before weaning) showed no significant differences between the baseline and disease data (P = .35 when litters including fostered piglets were removed).
| Figure 3: Least squares means (transformed) with standard
error bars for (A) piglets born dead per litter, (B) mummified
piglets per litter, and (C) stillborn piglets per litter by parity
for baseline- and disease-phase data (6355 litters) described in
Table 1.
|
| Figure 4: Least squares means with standard error bars
for total piglets weaned per litter by parity for baseline- and
disease-phase data (6355 litters) described in Table 1.
|
There was also an overall effect of genetic line and a significant interaction between line and disease status for nearly all traits. The line difference was essentially a difference between lines of European and Chinese origin. Analyses comparing the lines of European origin and the lines of Chinese origin showed that PRRS had a dramatic effect on the lines of Chinese origin (Table 3). For example, in the baseline data, the Chinese-origin lines were more prolific than their European-origin counterparts, with total piglets born alive per litter of 12.39 and 10.46 for these two groups, respectively. However in the presence of clinical PRRS, this advantage entirely eroded, with the mean values of 8.41 and 8.88 piglets born alive per litter in Chinese-origin and European-origin lines, respectively.
Table 3: Trait P values and overall least squares
means values (± SE) for baseline- and disease-phase data for pigs
of
* Data and herd described in Table 1. Breeds of European origin included Landrace, Large White, Pietrain, Duroc composite, and various crosses. Chinese origin included Meishan and crosses including more than 25% Meishan. † Negative values, which effectively do not differ from zero, are due to use of LS means. ‡ Mixed-model analysis. § Total born dead = total mummified + total stillborn. |
The final interaction (genetic line by parity by disease) was fitted to investigate whether the parity-by-disease relationships were consistent across lines. The three-way interaction was significant for all traits (Table 2), indicating that some of the line effects differed with parity. The Meishan breed differed from the other breeds most significantly, suffering the greatest disease impact. However, all lines seem to cope better with the disease in later parities.
Discussion
The mean and standard deviation values of the baseline data for all traits were well within the bounds of what is expected in commercial herds11,12 (and also PIC commercial data). These results suggested that the method of partitioning the data was effective and that these data should be representative of production on a typical commercial farm.
In terms of this study area within PRRS, there are currently few published data on the role of sow parity on reproductive performance when a commercial herd is exposed to PRRSV. This is an important question as, with the presence of PRRSV in a herd, one may consider altering the parity distribution of the sows within the herd in order to better manage the disease. Parity should also be considered when looking at the host-pathogen interaction, as apparent genetic variation in the host could well be due to these parity differences. Parity effects may arise because, as the animal ages, its immunocompetence and ability to respond to pathogens may change. It is possible that the parity effect observed in this study might be due to the older animals in the second outbreak having an increased tolerance to infection because of “pre-exposure” to PRRSV during the first outbreak. However, after further examination, this was found not to be the case, as the effect persisted even after these animals’ data were removed. In addition, although older parity animals were less affected by PRRSV, from a practical point of view, this observation may have limited application, since a balanced parity distribution is needed in order to maximize whole-herd productivity.
Previous work by Spooner and Roberts13 examining the interaction between PRRSV and parity did not find any significant parity effects in terms of the Idexx HerdChek PRRS 2XR ELISA or serum neutralization test results; however, no performance data from these animals were included. Our results suggest that when reproductive disease in pigs at a commercial level is investigated, the influence of parity on the impacts of disease, and hence on production traits, should be considered.
It is noted that our findings focus on fecundity and not fertility traits. We acknowledge that the examination of fertility traits (such as abortions and returns to estrus) would be interesting and valuable to industry. However, in the dataset analyzed, these traits were not recorded in a sufficiently precise manner to provide robust results (ie, as the dataset comprised “litter” information, a sow that aborted or failed to become pregnant was not included in these data). It should also be noted that further analysis of traits such as pre-weaning mortality would be beneficial in terms of a PRRS outbreak. Piglet mortality during a PRRS outbreak is related to loss of piglets that are nonviable or of low viability at birth as a consequence of in utero viremia, loss of piglets as a consequence of the effect of PRRS on the appetite and milk production of lactating sows, and loss of piglets related to reduced viability resulting from viremia acquired peri- or post-farrowing with subsequent increased susceptibility to secondary disease agents. These are very interesting outcomes of PRRS; however, we did not have access to all of the phenotypes required to fully dissect this complex trait of pre-weaning mortality. More specific trait recording, for example, cause of piglet death (eg, poor viability or crushing), would be required to detect possible effects of PRRS on piglet survival.
When it comes to application of the main findings, these data produce dilemmas. It would be beneficial in terms of numbers of mummified piglets to shift the demographics of a herd to an older population. This, however, is very difficult to implement and may incur other age-related issues and slower genetic gain. Clearly, gilts need intensive management in the presence of disease to avoid high piglet mortality (mummified and stillborn piglets) and associated poor weaning numbers. It could be suggested that separate housing (eg, through separate air spaces and also the use of air filtration), customized nutrition, and new husbandry practices (eg, fewer gilts per stockperson in the farrowing house, reducing cross-fostering) may help to protect gilts and increase productivity. This recommendation is similar to currently utilized “parity-segregation” systems and reduced fostering. However, the lower-parity herd in such a system includes only the first or second parities and the high-parity herds include the remaining parities. Some advantages to PRRSV control have been shown with this type of system.9 However, the guidelines in the parity-segregation system may not be sufficient to make an economic impact, as this current study suggests the cut-off for differential management of sows should be about parity 4 to 5. This is certainly an area of future research. This study also suggests that gilts (ie, parity-one sows) are probably the best model for research into the pathogenesis of the PRRSV and the study of host-virus interactions, as they are possibly more susceptible than older sows and show a greater phenotypic impact of disease.
It was interesting that no real effect of seasonality was seen in these data. The explanation for this may be that fecundity traits were the primary focus of the study, while the main affects of seasonality are seen in traits associated with reproductive-service success (such as returns to estrus).
The European lines suffered less of a decline in production due to PRRS than the pure Meishan line, although all genetic lines did experience a drop in productivity. Not only does this serve as another strand of evidence establishing that there is host genetic variation to PRRSV resistance or tolerance, it also creates an interesting dilemma in terms of commercial swine breeding. Generally, pig breeding companies have utilized the Meishan-type Chinese lines for their prolificacy attributes in breeding programs; however, this comes at a cost in terms of lower growth rates and a reduction in leanness. This single study that included a single commercial herd found that in the presence of PRRSV, the benefits of using the Chinese lines are eroded. This wisdom needs to be re-examined in future research.
Implications
- Under the conditions of this study, a PRRS outbreak has a significant detrimental effect on reproductive performance, seen most clearly by an increase in the number of mummified piglets born and the subsequent reduction in total piglets weaned.
- Under the conditions of this study, the parity of the sow affects performance during a PRRS outbreak, with impacts being larger in low-parity sows.
- In this herd, the Meishan genetic line appears to suffer more from PRRS outbreaks than their European counterparts.
- The methodology used here for partitioning data into disease and baseline datasets could be extended to other diseases to examine other disease impacts.
Acknowledgements
This work was financially supported by the Biotechnology and Biological Sciences Research Council (BBSRC – EGA16307) and Genus plc. The authors would also like to thank Dr Dave McLaren and the reviewers for their critical reading and insightful input into this paper.
References
1. Neumann EJ, Kliebenstein JB, Johnson CD, Mabry JW, Bush EJ, Seitzinger AH, Green AL, Zimmerman JJ. Assessment of the economic impact of porcine reproductive and respiratory syndrome on swine production in the United States. JAVMA. 2005;227:385–392.
2. Drew TW. A review of evidence for immunosuppression due to porcine reproductive and respiratory syndrome virus. Vet Res. 2000;31:27–39.
3. Lopez OJ, Osorio FA. Role of neutralizing antibodies in PRRSV protective immunity. Vet Immunol Immunopathol. 2004;102:155–163.
4. Lewis CRG, Ait-Ali T, Clapperton M, Archibald AL, Bishop SC. Genetic perspectives on host responses to porcine reproductive and respiratory syndrome (PRRS). Viral Immunol. 2007;20:343–357.
5. Lewis CRG, Torremorell M, Galina L, Bishop S. Genetic parameters for performance traits in commercial sows estimated before and after an outbreak of porcine reproductive and respiratory syndrome (PRRS). J Anim Sci. 2008. doi:10.2527/jas.2008–0892.
*6. Murtaugh MP, Rowland RRR. Preface. Special issue. PRRS immunology and immunopathology.Vet Immunol Immunopathol. 2004;102:105.
*7. Idexx Herdchek PRRS. Accurate screening for porcine reproductive and respiratory syndrome. 2003. Available at: http://www.idexx.com/production/swine/6417801m.pdf. Accessed 16 Feb 2009.
8. Batista L, Pijoan C, Dee S, Olin M, Molitor T, Joo HS, Xiao ZG, Murtaugh M. Virological and immunological responses to porcine reproductive and respiratory syndrome virus in a large population of gilts. Can J Vet Res. 2004;68:267–273.
9. McCaw MB. Effect of reducing crossfostering at birth on piglet mortality and performance during an acute outbreak of porcine reproductive and respiratory syndrome. Swine Health Prod. 2000;8:15–21.
10. Torremorell M, Henry S, Christensen WT. Eradication using herd closure. In: Zimmerman J, Yoon K-J, eds. PRRS Compendium. 2nd ed. Des Moines, Iowa: National Pork Board. 2003:157–160.
11. Short TH, Rothschild MF, Southwood OI, McLaren DG, de Vries A, van der Steen H, Eckardt GR, Tuggle CK, Helm J, Vaske DA, Mileham AJ, Plastow GS. Effect of the estrogen receptor locus on reproduction and production traits in four commercial pig lines. J Anim Sci. 1997;75:3138–3142.
12. Sasaki Y, Koketsu Y. Variability and repeatability in gestation length related to litter performance in female pigs on commercial farms. Theriogenology. 2007;68:123–127.
*13. Spooner C, Roberts J. Clinical investigation of the association of cross fostering and sow parity on the incidence of porcine reproductive and respiratory syndrome virus (prrsv) in pre-weaned piglets: A case report. Proc AASV. Orlando, Florida. 2003;35–37.
* Non-refereed references.